Introduction
Lung cancer is among the most lethal types of cancer
for both males and females worldwide (1). Non-small cell lung cancer (NSCLC)
accounts for ~80% of all lung cancer cases and is the most
prevalent type of lung cancer, with 1.2 million new cases reported
annually worldwide (2). At
present, the prognosis for patients with NSCLC is poor, with the
five-year overall survival rate being <15% (3). Thus, the identification of potential
molecular markers of NSCLC is required for the prediction of
survival and the development of novel therapeutic targets.
Transcription factors belong to the basic
helix-loop-helix (bHLH) family and are key regulators of cell
proliferation, differentiation and cell lineage determination, as
well as other essential processes (4). As a member of the bHLH leucine-zipper
(LZ) subgroup of bHLH proteins (5), activating enhancer-binding protein
(AP)-4 has been reported to have a role in tumor biology. The
activation of AP-4 has been reported to induce
epithelial-mesenchymal transition and enhance migration and
invasion in colorectal cancer cells. Moreover, the downregulation
of AP-4 has been found to cause mesenchymal-epithelial transition
and inhibit migration and invasion, suggesting that AP-4 may be a
novel regulator in cancer (6). It
has also been reported that high expression of AP-4 predicts poor
prognosis in hepatocellular carcinoma following curative
hepatectomy (7). However, the role
of AP-4 in NSCLC has yet to be elucidated.
The present study investigated the role of AP-4
expression in NSCLC by analyzing AP-4 expression in NSCLC and the
correlation between AP-4 expression and clinicopathological
characteristics and prognosis by using quantitative polymerase
chain reaction (qPCR) analysis, western blot analysis and
immunohistochemical staining.
Materials and methods
Patients
Patients with NSCLC who underwent radical resection
of their primary cancer at the Department of Radiation Oncology,
Jinan Military General Hospital (Jinan, China) were used in the
present study. Patients were excluded from the study if they had
previously undergone radiotherapy or chemotherapy for cancer
treatment. Two groups were included in the present study. The first
group included 42 fresh NSCLC tumor samples, which were immediately
frozen and stored in liquid nitrogen for protein and RNA extraction
following surgical resection. In addition to the first group, 240
NSCLC tissue specimens, including 139 adenocarcinomas and 101
squamous cell carcinomas, obtained from the Department of Radiation
Oncology, Jinan Military General Hospital between January 2005 and
January 2008, were used. All specimens were histologically analyzed
and classified using the World Health Organization classification
system. Detailed clinical, pathological and survival data were
available. Written informed consent was obtained from all patients
for the use of their tissues. Furthermore, the present study was
approved by the Institutional Review Board at Jinan Military
General Hospital. Patient follow-up was performed at three-month
intervals. The median follow-up period was 48 months (range, 9–66
months) for all patients. Overall survival was defined as the
period from the time of surgery to mortality.
qPCR analysis
The total RNA from frozen fresh samples was
extracted using a TRIzol® extraction kit (Invitrogen
Life Technologies, Carslbad, CA, USA) and reverse transcribed in a
25 μl reaction volume using Taqman® reverse
transcription reagents (Applied Biosystems, Foster City, CA, USA)
according to the manufacturer’s instructions. The complementary
(c)DNA was diluted and quantified using qPCR analysis using
SYBR® Green I. The primer sequences used for the qPCR
analysis were as follows: AP-4 forward, 5′-GAGGGCTCTGTAGCCTTGC-3′
and reverse, 5′-GAATCCCGCGTTGATGCTCT-3′; GAPDH forward,
5′-ACAACTTTGGTATCGTGG-3′ and reverse,5′-GCCATCACGCCACAGTTTC-3′.
Data were analyzed using the ΔΔCt method and normalized using GAPDH
expression.
Western blot analysis
Frozen tumor tissues were prepared by washing twice
in cold phosphate-buffered saline (PBS). Approximately 20 mg tissue
from each fresh sample was homogenized in 0.5 ml ice-cold cell
lysis buffer (Roche Applied Science, Penzberg, Germany) containing
fresh protease and phosphate inhibitors. Lysates were then
centrifuged at 12,000 × g in a microcentrifuge at 4°C for 20 min
and the resulting supernatants were used as tissue extracts. The
extracted proteins were separated by 10% SDS-PAGE and transferred
to nitrocellulose membranes. Membranes were blocked using
Tris-buffered saline (TBS) containing 5% non-fat dried milk and
then probed with primary antibodies in PBS containing 5% bovine
serum. The following primary antibodies were used: Rabbit anti-AP-4
(Millipore Corp., Billerica, MA, USA) and mouse anti-GAPDH
(Sigma-Aldrich, St. Louis, MO, USA). Immunoreactive bands were
detected and quantified using an imaging system (Invitrogen Life
Tecnologies).
Immunohistochemical analysis
The antibodies used for western blot analysis were
also used for immunohistochemical staining. Formalin-fixed and
paraffin-embedded tissue sections (5-μm thick) were deparaffinized,
hydrated and heated in a steamer for 10 min for antigen retrieval.
Peroxidase activity was blocked using 3% H2O2
in methanol at room temperature for 10 min, followed by incubation
in 10% bovine serum albumin in TBS-Tween 20 for 30 min. Slides were
then incubated with primary antibodies against AP-4 at a 1:100
dilution for 60 min at room temperature. Subsequent to washing with
PBS, the slides were incubated with biotin-labeled secondary
antibodies for 30 min. The samples were then incubated with
streptavidin-peroxidase at a 1:40 dilution for 30 min. Samples were
stained with 0.05% 3′,3-diaminobenzidine tetrahydrochloride
prepared in 0.05 mol/l TBS (pH 7.6) containing 0.02%
H2O2, then counterstained with hematoxylin.
Formalin-fixed and paraffin-embedded lung tissues with normal
bronchial epithelia were used as a positive control. Tissue samples
which were not incubated with the primary antibodies were used as a
negative control. Immunohistochemical staining was quantified by
two independent pathologists.
Assessment of immunohistochemical
staining
Staining was quantified using a scoring method based
on the intensity and proportion of the immunohistochemically
stained cells. The proportion of positively stained tumor cells was
determined semi-quantitatively and each sample was scored as
follows: 0, <1%; 1, 1–25%; 2, 26–50%; 3, 51–75%; and 4, 76–100%.
The staining intensity of the positively stained tumor cells was
scored as follows: 0, negative; 1, weak; 2, moderate; and 3,
strong. The immunoreactive score of each tumor was calculated by
the sum of the two parameters. The immunohistochemical staining was
ultimately graded as either negative (total score, 0–1) or positive
(total score, 2–7). All stained sections were assessed by two
independent pathologists without knowledge of the
clinicopathological features.
Statistical analysis
Statistical analyses were performed using SPSS 17.0
software (SPSS, Inc., Chicago, IL, USA). Independent sample
Student’s t-tests and χ2 tests were used to analyze the
continuous and categorical variables, respectively. Survival
probability was assessed using the Kaplan-Meier estimator. The
log-rank test was used for the comparison of patient survival. The
Cox proportional hazards model was used to calculate the effect of
AP-4 expression on patient survival, with adjustments made for
clinical and histopathological parameters, including age, gender
and smoking status. P<0.05 was considered to indicate a
statistically significant difference.
Results
AP-4 mRNA and protein expression are
significantly increased in fresh NSCLC tissue
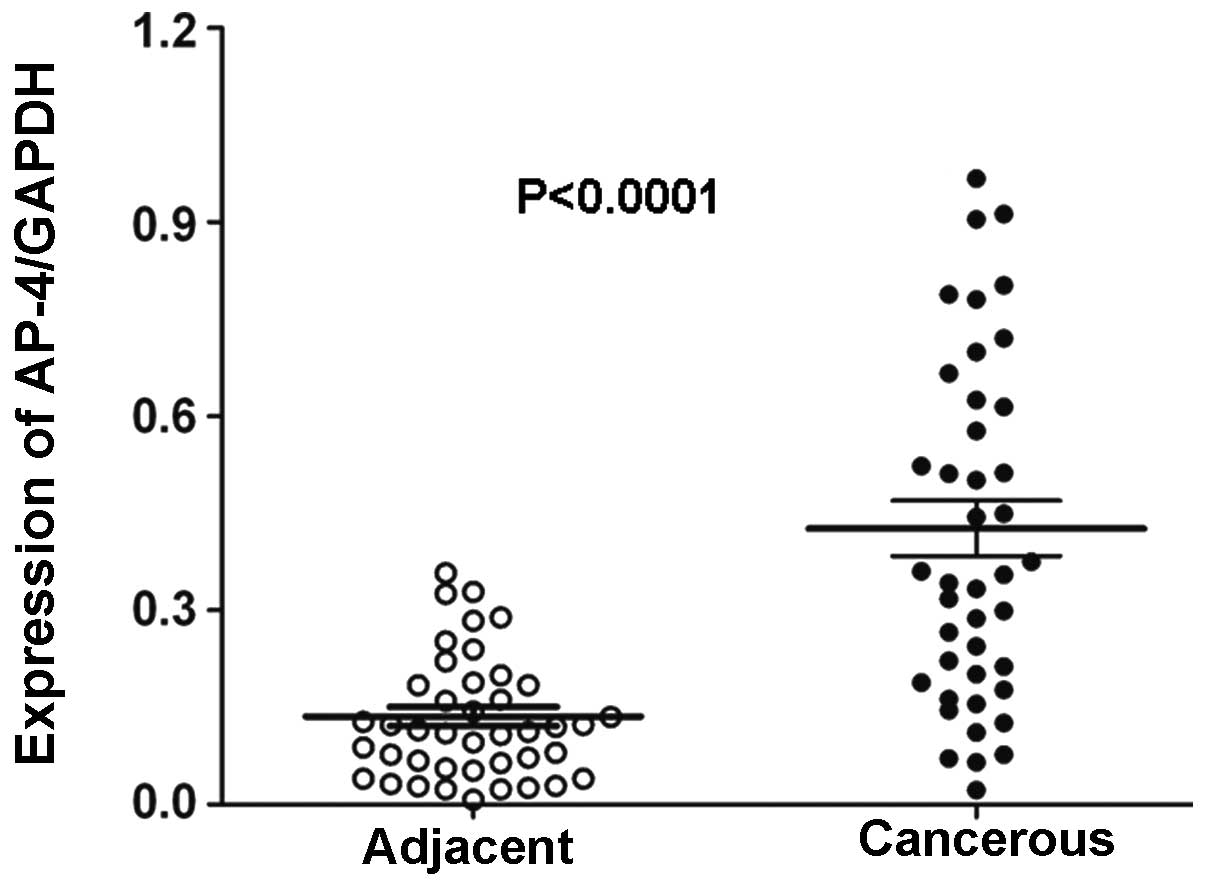
AP-4 expression was assessed using qPCR analysis in
42 fresh NSCLC samples and matched adjacent non-cancerous lung
tissues. AP-4 mRNA expression was found to be significantly higher
in the NSCLC samples compared with the adjacent noncancerous
tissues (P<0.0001; Fig. 1).
Furthermore, western blot analysis revealed that AP-4 protein
expression was significantly increased in the 42 fresh NSCLC
samples compared with the matched adjacent noncancerous tissues, as
quantified using densitometry (P<0.0001; Fig. 2).
Correlation between AP-4 expression and
clinicopathological parameters
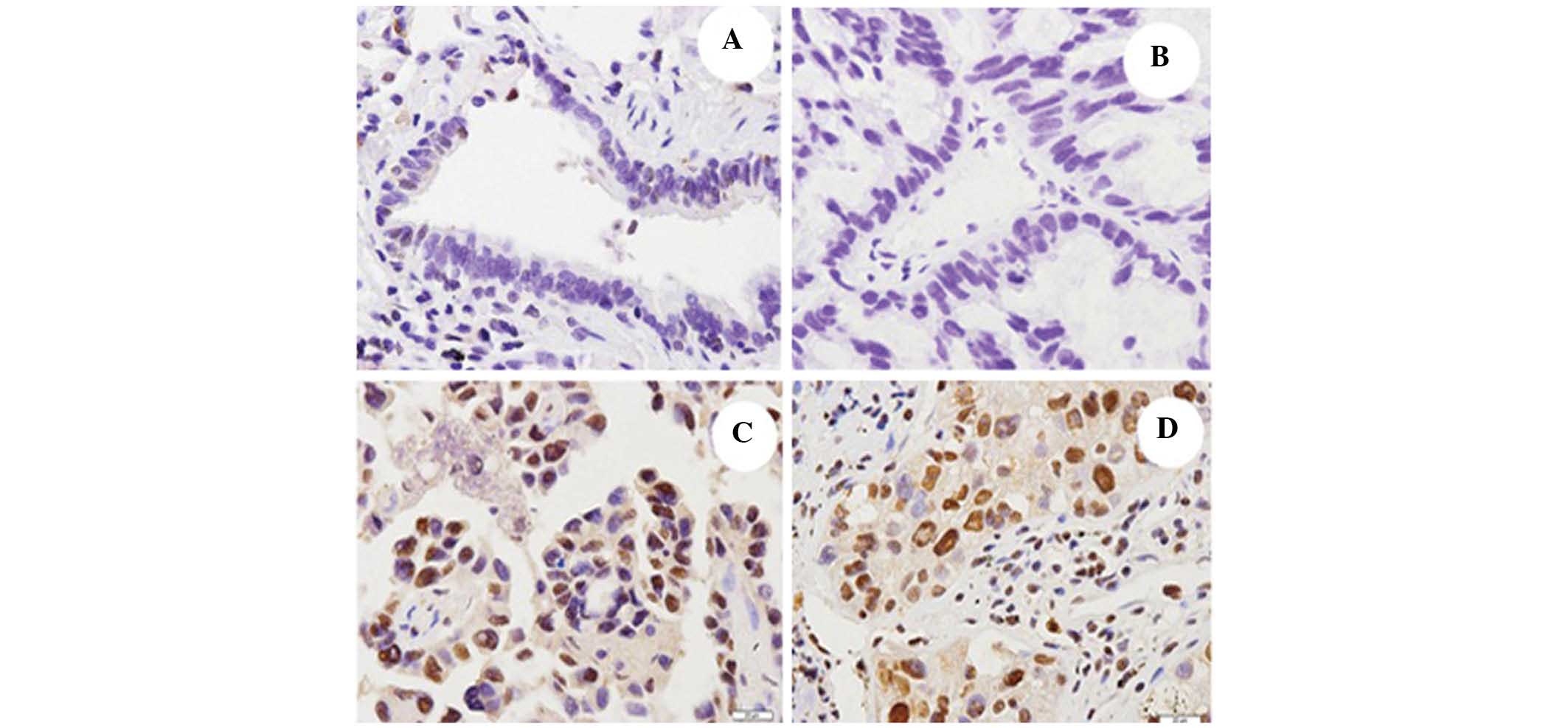
Immunohistochemical staining revealed that AP-4 was
expressed in the nuclei of the cells in the NSCLC tissues. The rate
of AP-4 expression was significantly higher in the NSCLC tissue
samples (48.3%; 116/240) compared with the adjacent noncancerous
lung tissues (5.8%; 14/240; P<0.01; Fig. 3). The correlation between AP-4
expression and clinicopathological features is shown in Table I. AP-4 expression was found to be
significantly associated with the tumor, nodes and metastasis (TNM)
stage.
 | Table IAP-4 expression and
clinicopathological features in 240 patients with non-small cell
lung cancer. |
Table I
AP-4 expression and
clinicopathological features in 240 patients with non-small cell
lung cancer.
| | AP-4 expression | |
|---|
| |
| |
|---|
| Parameter | Cases (n) | Negative | Positive | P-value |
|---|
| Gender | | | | 0.51 |
| Male | 154 | 80 | 74 | |
| Female | 86 | 44 | 42 | |
| Age | | | | 0.44 |
| <60 years | 113 | 63 | 50 | |
| ≥60 years | 127 | 61 | 66 | |
| TNM stage | | | | 0.028 |
| I | 50 | 29 | 21 | |
| II | 69 | 33 | 36 | |
| III–IV | 121 | 62 | 59 | |
| Histology | | | | 0.43 |
| Squamous cell | 101 | 49 | 52 | |
| Adenocarcinoma | 139 | 75 | 64 | |
AP-4 expression is associated with
prognosis in patients with NSCLC
Kaplan-Meier survival estimates revealed that
overall survival was significantly lower in patients with positive
AP-4 expression than in those with negative AP-4 expression
(P=0.0026; Fig. 4). Furthermore,
Cox proportional hazard multivariate analysis was used to analyze
the correlation between AP-4 expression in NSCLC tissues and other
features, including patient gender and smoking history, as well as
tumor histology, size, differentiation, metastasis status and TNM
stage. Positive AP-4 expression (hazard ratio, 2.543; 95%
confidence interval, 1.18–5.016; P=0.016) was found to be an
independent prognostic indicator in patients with NSCLC, in
addition to lymph node status and distant metastasis (Table II).
 | Table IIMutivariate analysis of clinical
features and prognosis in patients with non-small cell lung
cancer. |
Table II
Mutivariate analysis of clinical
features and prognosis in patients with non-small cell lung
cancer.
| | | | | | 95% CI for HR |
|---|
| | | | | |
|
|---|
| Parameter | B | SE | Wald | P | HR | Lower | Upper |
|---|
| Histology | −0.02 | 0.17 | 1.48 | 0.345 | 0.74 | 0.49 | 1.26 |
| Gender | 0.596 | 0.31 | 3.31 | 0.081 | 4.91 | 0.83 | 3.29 |
| Smoking | 0.445 | 0.28 | 2.69 | 0.231 | 1.72 | 0.81 | 2.91 |
| Tumor size | 0.082 | 0.22 | 0.09 | 0.816 | 1.46 | 0.59 | 1.67 |
| Position | −0.32 | 0.20 | 0.009 | 0.574 | 0.87 | 0.60 | 1.73 |
| Differentiation | −0.03 | 0.23 | 0.04 | 0.892 | 0.98 | 0.57 | 1.60 |
| TNM stage | 0.16 | 0.19 | 0.40 | 0.432 | 1.21 | 0.77 | 1.63 |
| Lymph node
status | 0.58 | 0.22 | 5.59 | 0.013 | 1.79 | 1.09 | 2.90 |
| Distant
metastasis | 1.29 | 0.26 | 19.76 | 0.001 | 3.91 | 2.04 | 7.05 |
| AP-4 expression | 1.75 | 0.49 | 11.46 | 0.016 | 2.54 | 1.18 | 5.01 |
Discussion
NSCLC is one of the leading causes of mortality
associated with cancer worldwide; therefore, improvements in the
diagnosis and treatment of NSCLC are urgently required (8). The identification of novel biomarkers
may help to guide the diagnosis and treatment of NSCLC. In the
present study, AP-4 expression was found to be increased at the
transcriptional and translational levels in fresh NSCLC samples.
Moreover, the present study analyzed the correlation between AP-4
expression and clinical outcome and clinicopathological parameters.
Positive AP-4 expression was identified in 48.3% (116/240) of NSCLC
cases. By contrast, positive AP-4 expression was only observed in
5.8% (14/240) of the matched adjacent lung tissues. Of note, a
significant correlation was identified between AP-4 expression and
poor prognosis, independent of other clinicopathological
parameters. These findings support the role for AP-4 as an oncogene
and a novel prognostic marker in NSCLC.
Previous studies have demonstrated that AP-4 is
involved in tumor biology. It was reported that AP-4 expression was
significantly correlated with the progression of colorectal cancer
and lymph node metastasis (9).
AP-4 expression was also found to be associated with the expression
of matrix metalloproteinase-9 and vascular endothelial growth
factor in advanced colorectal cancer (9). A recent study has shown that AP-4
expression is associated with clinicopathological parameters in
gastric cancer, including differentiation, lymph node metastasis,
depth of invasion, TNM stage and poor prognosis (10). Furthermore, high AP-4 expression
has been found to predict poor prognosis in hepatocellular
carcinoma following curative hepatectomy (7). Thus, AP-4 may be a molecular marker
to predict the progression and prognosis of the various types of
tumors. However, the expression and clinical significance of AP-4
in NSCLC has yet to be elucidated. Therefore, the present study
aimed to analyze the clinical significance of AP-4 expression in
NSCLC.
The present study investigated AP-4 mRNA and protein
expression in fresh NSCLC samples using qPCR and western blot
analyses. AP-4 mRNA and protein expression were observed to be
significantly increased in the tumor tissue samples compared with
the adjacent non-tumor tissue samples. Moreover, in a relatively
large number of NSCLC patients (n=240), high expression of AP-4 was
found to be significantly correlated with the TNM stage of NSCLC,
suggesting that an increase in AP-4 expression may promote tumor
growth and invasion. These findings suggested that AP-4 may have an
important role in the tumorigenesis or progression of NSCLC.
Kaplan-Meier survival analysis revealed that
patients with positive AP-4 expression had a significantly lower
overall survival than those with negative AP-4 expression.
Multivariate analysis demonstrated that AP-4 expression was an
independent prognostic factor in patients with NSCLC. These
findings suggested that AP-4 may serve as a valuable prognostic
biomarker for patients with NSCLC.
In conclusion, the present study revealed that
positive AP-4 expression in NSCLC was correlated with a more
malignant phenotype and poor prognosis in a large number of
clinical samples. Thus, AP-4 may be utilized as a valuable
prognostic biomarker for NSCLC. Translational studies of AP-4 as a
therapeutic target in NSCLC are required.
References
|
1
|
Chen YT, Feng B and Chen LB: Update of
research on drug resistance in small cell lung cancer chemotherapy.
Asian Pac J Cancer Prev. 13:3577–3581. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fossella F, Pereira JR, von Pawel J,
Pluzanska A, Gorbounova V, Kaukel E, Mattson KV, Ramlau R, Szczesna
A, Fidias P, Millward M and Belani CP: Randomized, multinational,
phase III study of docetaxel plus platinum combinations versus
vinorelbine plus cisplatin for advanced non-small-cell lung cancer:
the TAX 326 study group. J Clin Oncol. 21:3016–3024. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
4
|
Jones S: An overview of the basic
helix-loop-helix proteins. Genome Biol. 5:2262004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee SU, Song HO, Lee W, Singaravelu G, Yu
JR and Park WY: Identification and characterization of a putative
basic helix-loop-helix (bHLH) transcription factor interacting with
calcineurin in C. elegans. Mol Cells. 28:425–461.
2009.PubMed/NCBI
|
|
6
|
Jackstadt R, Röh S, Neumann J, Jung P,
Hoffmann R, Horst D, Berens C, Bornkamm GW, Kirchner T, Menssen A
and Hermeking H: AP4 is a mediator of epithelial-mesenchymal
transition and metastasis in colorectal cancer. J Exp Med.
210:1331–1350. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hu BS, Zhao G, Yu HF, Chen K, Dong JH and
Tan JW: High expression of AP-4 predicts poor prognosis for
hepatocellular carcinoma after curative hepatectomy. Tumour Biol.
34:271–276. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Reck M, Heigener DF, Mok T, Soria JC and
Rabe KF: Management of non-small-cell lung cancer: recent
developments. Lancet. 382:709–719. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cao J, Tang M, Li WL, Xie J, Du H, Tang
WB, Wang H, Chen XW, Xiao H and Li Y: Upregulation of activator
protein-4 in human colorectal cancer with metastasis. Int J Surg
Pathol. 17:16–21. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xinghua L, Bo Z, Yan G, Lei W, Changyao W,
Qi L, Lin Y, Kaixiong T, Guobin W and Jianying C: The
overexpression of AP-4 as a prognostic indicator for gastric
carcinoma. Med Oncol. 29:871–877. 2012. View Article : Google Scholar : PubMed/NCBI
|