Introduction
Ovarian cancer is the leading cause of mortality
compared with all other gynecological malignancies (1,2).
Ovarian cancer is difficult to be diagnosed at the early stages.
Consequently, 80% of patients are already at the intermediate or
late stages of ovarian cancer when they present with symptoms.
Qualitative and location diagnosis at the early stages of cancer
progression is one of the key factors important in improving the
survival rate of patients (3,4).
Currently, the serum biomarkers of cancer antigen-125 (CA-125) and
recently identified human epididymis secretory protein-4 (HE4) are
used as predictors for ovarian cancer diagnosis (5–9). The
low specificity of CA-125 and HE4, however, limits their clinical
application (10,11). With the development of ultrasonic
contrast technology, scientists have begun to consider the
possibility of applying non-invasive ultrasonic molecular imaging
technology (12–14) in the diagnosis of early stage
ovarian cancer. The most critical part of this technique is to
prepare a nano-scale targeting microbubble contrast agent. Compared
with a micro-scale contrast agent, a nano-scale contrast agent has
the ability to penetrate blood vessels and to be visualized by
confocal imaging (15,16). Low echo reflecting nano-scale
contrast agents are able to penetrate the blood vessels, accumulate
around the lesion and generate a significantly enhanced signal in
the target area with an extremely low background signal (17). Non-specific microbubble contrast
agents do not specifically interact with lesion tissues, which
makes these agents unsuitable to be used for targeting. Once in the
human body, the majority of microbubbles non-specifically bind to
the hepatic sinusoid, the spleen sinus and the vascular endothelial
system (18), so that the
microbubbles are trapped in the microcirculatory system and cannot
effectively reside in the targeted tissue for a sufficient amount
of time, compromising the contrast signal. For efficient targeting
in in vivo imaging, it is crucial to develop microbubbles
that specifically accumulate and adhere to tumor lesions following
injection into the human body.
With the progress in research on tumor-specific
antigens and receptor biology, ultrasonic contrast agent targeting
of malignant tumors by conjugating tumor-specific antibodies or
ligands onto the surface of microbubbles has generated
tumor-specific targeting microbubbles (19,20).
In addition, previous studies suggested that the
luteinizing-hormone releasing hormone (LHRH) receptor is
overexpressed in ovarian cancer cells, with little to no expression
in normal tissues (21,22). Based on this evidence, it was
hypothesized that by linking LHRH antibodies onto the surface of
the nano-microbubble, LHRH nanoliposomal ultrasonic contrast agents
targeting ovarian cancer can be prepared (Fig. 1) (23). Following penetration of the
nano-scale contrast agents into the blood vessels and reaching of
their target site, the LHRH antibodies on the surface of
microbubbles bind to LHRH receptors in ovarian cancer cells, which
results in selective accumulation. This allows for long resident
time in ovarian cancer tissue and targeted ovarian cancer imaging
at the molecular level by the confocal principle. The present study
demonstrated the preparation of LHRH nanoliposomal microbubbles
(LHRH-N-Mbs) as ultrasound contrast agents and their in
vitro targeting ability of ovarian cancer cells, which provided
experimental evidence for specific ultrasound imaging of ovarian
cancer at an early stage.
Materials and methods
Materials
Dipalmitoyl phosphatidylcholine (DPPC) and
biotinylated dipalmitoyl phosphatidylethanolamine
(DSPE-PEG2000-Biotin) were ordered from Avanti Polar Lipids Inc.
(Alabaster, AL, USA). Human ovarian cancer cells (OVCAR-3), McCoy’s
5A medium and avidin were purchased from Wuhan Boster Biological
Technology, Ltd. (Wuhan, China). Biotinylated LHRH antibody and
rhodamine goat anti-rabbit immunoglobulin (Ig) G were obtained from
Beijing Biosynthesis Biotechnology Co., Ltd. (Beijing, China).
Perfluorinated propane (C3F8) was purchased
from the Medical Ultrasound Imaging Research Institute, Chongqing
University (Chongqing, China).
Cell culture
The OVCAR-3 cells were cultured in McCoy’s 5A medium
containing 10% heat-inactivated fetal calf serum at 37°C and
incubated in 5% CO2. Cells were split every 4–6 days and
experimental cells were in the log-growth phase.
Preparation of non-targeting
nanoliposomal microbubbles (N-N-Mbs)
DPPC and DSPE-PEG-Biotin were mixed in a 5 ml
plastic tube to form a suspension. Following lyophilization, 1 ml
of hydration solution [glycerin, phosphate-buffered saline (PBS)]
was added to the samples to rehydrate them and then
C3F8 gas was slowly injected into the
container to replace the air. Samples were then agitated using a
horizontal reciprocating ultrasonic mechanical vibrator for 90 sec
to form a milky white solution. Following separation at 4°C, the
bottom layer was discarded and the milky white upper layer was
collected following washing with PBS three times. The upper layer
was then added to 1 ml of PBS to obtain non-targeting nanoliposomal
microbubbles. Following radiation sterilization by
60Cogγ-ray (Sinotex CX, Shanghai, China), microbubbles
were observed by optical microscopy to detect the morphology and
particle distribution. Particle size range and the surface
potential were measured by a zeta detector (Zeta sizer 3000HSA;
Malvern Instruments Ltd., Worcestershire, England).
Preparation of LHRH-N-Mbs
The prepared N-N-Mbs (100 μl; 1×108/ml)
were mixed with saturated biotin (100 μg) in an ultrasonic
agitating reaction for 30 min. Following centrifugation at 50 × g
for 5 min, the bottom layer was discarded. The upper layer was
washed with PBS three times and then collected as LHRH-N-Mbs.
Following sterilization, the same methods were applied to assess
the physicochemical properties of the LHRH-N-Mbs.
Determination of LHRH binding onto the
LHRH-N-Mb surface
Rhodamine-conjugated secondary antibodies (1:100
w/v) were added to 100 μl LHRH-N-Mb or 100 μl of N-N-Mb suspension
containing avidin. The two groups were incubated at 37°C for 30
min, washed and observed under a fluorescence microscope (CKX41;
Olympus, Tokyo, Japan) or analyzed by flow cytometry (Beckman
Coulter XL; Beckman Coulter, Miami, FL, USA) to assess the binding
rate.
In vitro targeting experiment
OVCAR-3 cells (5×104/ml) were incubated
with LHRH-N-Mb (1×108/ml) at room temperature for 30 min
prior to washing to remove free microbubbles (final volume 5 ml).
The binding between cells and LHRH-N-Mbs was observed under a light
microscope (Olympus).
Blocking experiment
OVCAR-3 cells were pre-incubated with biotinylated
LHRH antibody at room temperature (20–25°C) for 30 min prior to the
cells being incubated with LHRH-N-Mbs for another 30 min. The
binding between cells and LHRH-N-Mbs was observed using light
microscopy.
Statistical analysis
Values are expressed as the mean ± standard
deviation (SD) and analyzed by SAS 10.0 statistical software. The
Student’s t-test was employed for the comparison of two independent
samples. P<0.05 was considered to indicate a statistically
significant difference.
Results
Physicochemical properties of N-N-Mbs and
LHRH-N-Mbs
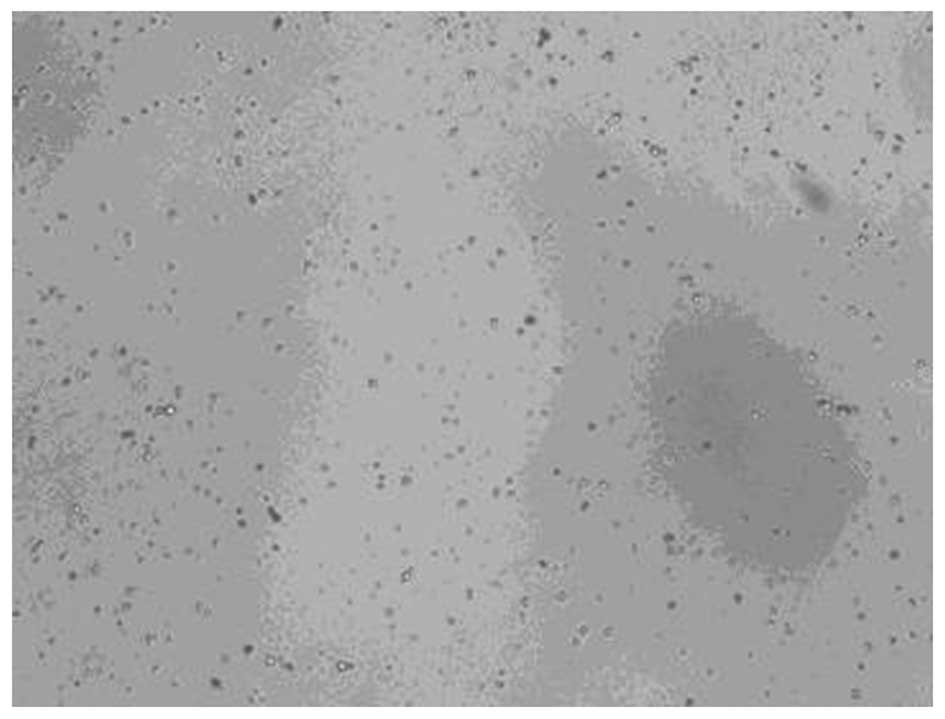
The suspensions of the microbubbles in PBS appeared
to be milky white. Light microscopy revealed that the microbubbles
were uniformly distributed with no visible aggregation. A single
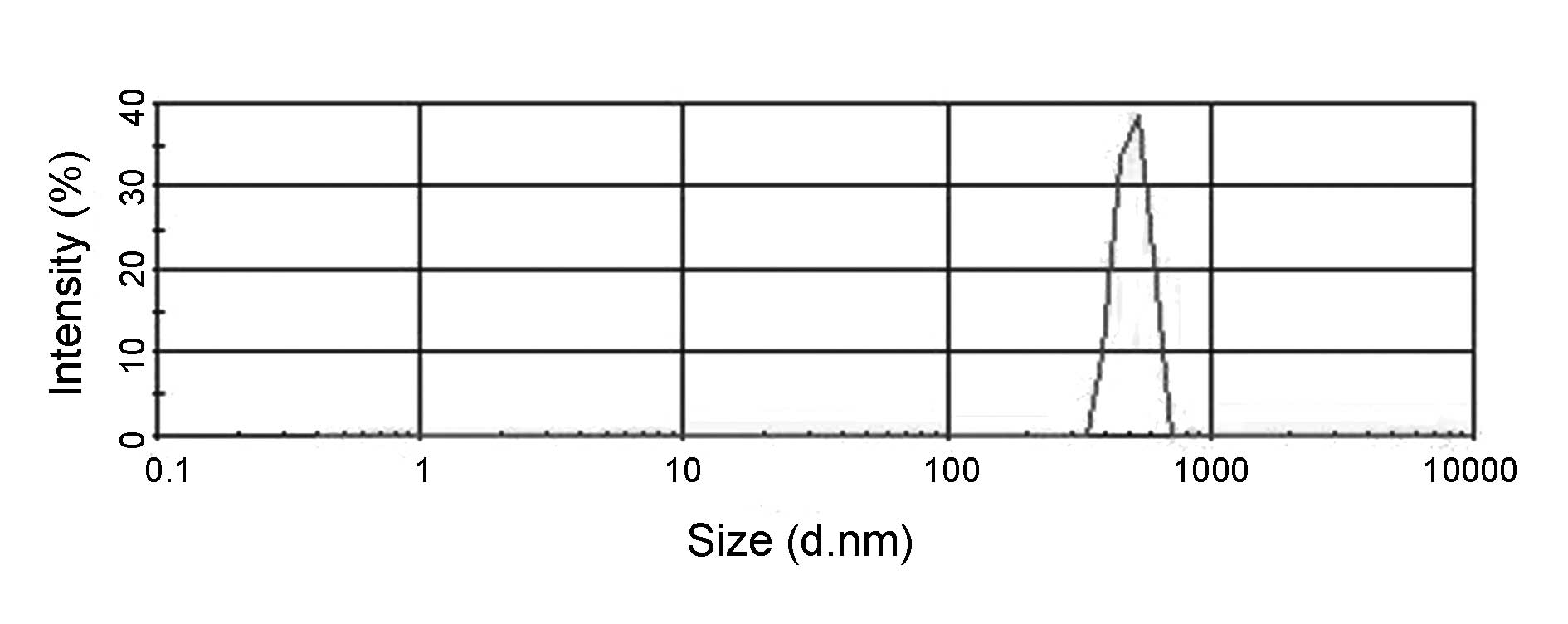
microbubble was round following sterilization. As shown in Figs. 2 and 3, the particle size ranged from 295–468
nm with a mean of 360 nm for N-N-Mbs, or 369–618 nm with a mean of
508 nm for LHRH-N-Mbs. The surface potential in the two groups was
the same (−14.6 mV). As shown in Fig.
4, following being kept at room temperature (20–25°C) for 14
days, LHRH-N-Mbs appeared round, evenly distributed and showed no
signs of aggregation. No significant difference was identified in
particle size nor potential compared with those of freshly prepared
LHRH-N-Mbs (P>0.05). Following being kept at room temperature
for 17 days, however, the shape of the microbubbles altered and
they became uneven.
Surface LHRH binding rate of LHRH-N-Mbs
N-N-Mbs bind to biotinylated LHRH antibodies via their
biotin-avidin connection
Following LHRH-N-Mbs being washed four times, bright
fluorescence was observed using fluorescence microscopy. The
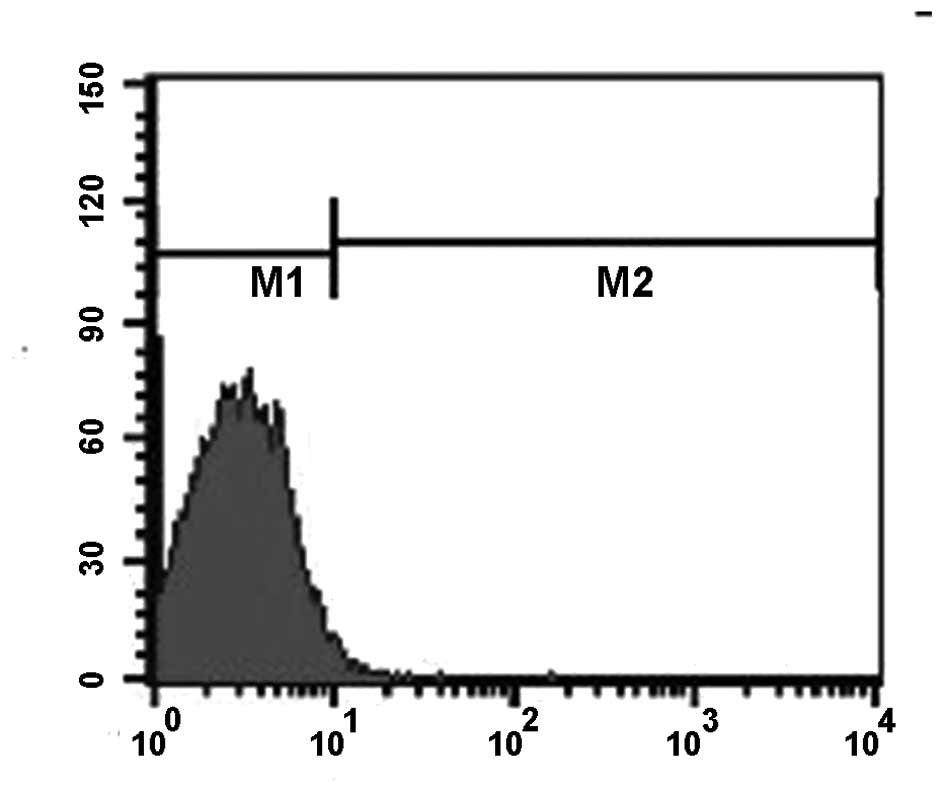
fluorescence diminished following further washing. Flow cytometry
data demonstrated that the binding rate between LHRH-N-Mbs and the
secondary antibody was 75.6% (Fig.
5). The binding rate between the secondary antibody and N-N-Mbs
containing avidin was 0.83% (Fig.
6), which was significantly different from that between
LHRH-N-Mbs and the secondary antibody (P<0.05). Little to no
fluorescence was detected for the N-N-Mbs incubated with the
secondary antibody by fluorescence microscopy.
In vitro targeting experiment
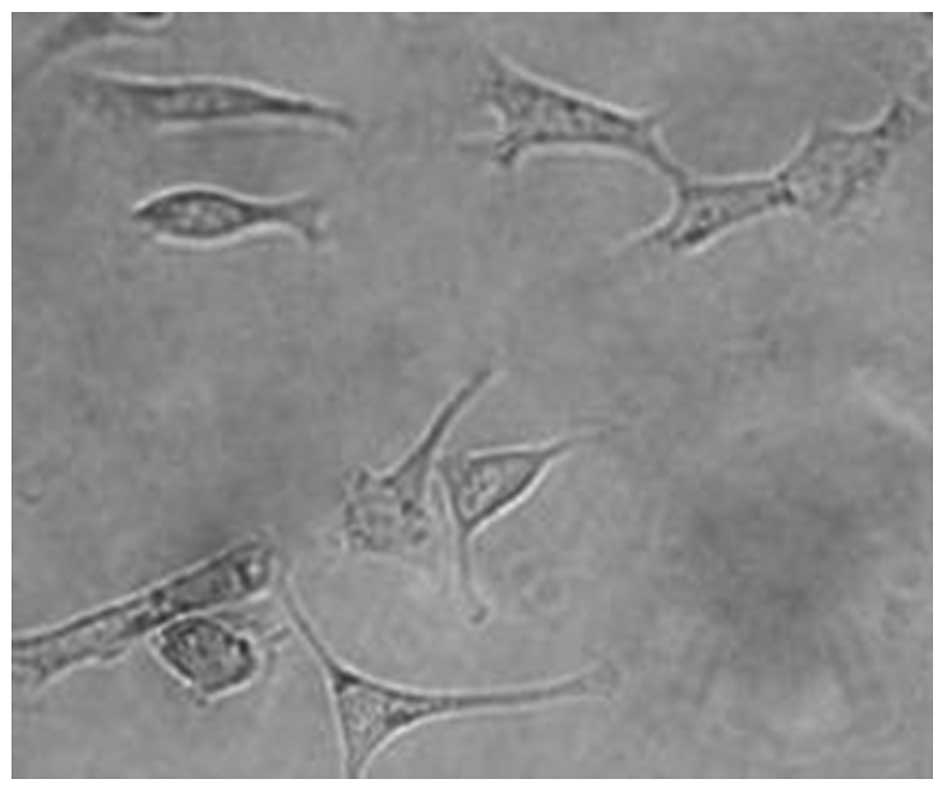
Following cells being incubated with LHRH-N-Mbs,
LHRH-N-Mb was adherent to the surrounding OVCAR-3 cells and formed
a rosette-like structure as observed under the light microscope
(Fig. 7).
Blocking experiment
Following OVCAR-3 cells being pre-incubated with
LHRH antibody, no binding between LHRH-N-Mbs and OVCAR-3 cells was
observed and no rosette formation was detected (Fig. 8).
Discussion
Patients with ovarian cancer (80%) are diagnosed at
the intermediate or late stages of the malignancy (3,4),
which is one of the key factors contributing to the high mortality
rates of ovarian cancer. Targeted ultrasound molecular imaging
technology (12–14) makes early, qualitative and location
diagnosis of ovarian cancer possible. In the present study, the
preparation and characterization of a nano-microbubble contrast
targeting agent, which is the key component of ultrasound molecular
imaging, was investigated.
In the present study, N-N-Mbs and LHRH-N-Mbs for
targeting ovarian cancer cells were generated using
lyophilization/sonication and the biotin-avidin bridge method
(24,25). The particle size ranged from
295–468 nm with a mean of 360 nm for N-N-Mbs, or 369–618 nm with a
mean of 508 nm for LHRH-N-Mbs. It was hypothesized that the
biotinylated LHRH antibody may occupy a certain space, which would
lead to a larger size of LHRH-N-Mbs. The in vitro targeting
experiments suggested that OVCAR-3 cells highly expressing LHRH
receptors efficiently bind to LHRH-N-Mbs and form a rosette-like
structure. Importantly, following OVCAR-3 cells being pre-incubated
with biotinylated LHRH antibodies, which blocked the interaction of
receptors with its ligands, no binding of LHRH-N-Mbs to OVCAR-3
cells was observed and no rosette formation was detected,
suggesting that LHRH-N-Mbs specifically bind to OVCAR-3 cells
through the interaction between the biotinylated LHRH antibody and
the LHRH receptor.
Whilst the size of LHRH-N-Mbs is slightly larger
than that of N-N-MBS, they remain within the nanometer scale and
are smaller than the vascular endothelial gap (16,26).
Following penetration of the nano-scale contrast agent into the
blood vessels and reaching its target site, it binds to ovarian
cancer cells through the LHRH receptor-antibody interaction, which
leads to an extended residing time in the targeted tissue and
organs. As low echo-reflecting nano-particles selectively
accumulate at the target area with long residual time,
nano-particles are able to produce a significantly enhanced signal
at the target area and show an extremely low background noise,
making LHRH-N-Mbs an ideal contrast agent at the molecular level.
Adaptation of this molecular imaging technology would markedly
improve the accuracy and resolution of early stage ovarian cancer
diagnosis. Furthermore, the physicochemical properties of
LHRH-N-Mbs did not significantly change even following being stored
at room temperature for half a month, suggesting it is stable. This
feature further suggests the use of this contrast agent in future
in vivo experiments.
In terms of in vitro experiments, the use of
LHRH-N-Mbs has less interference factors, differing from the
complications of in vivo studies. The first challenge of
in vivo experiments is the low concentration of the contrast
agent due to the dilution in the blood. In addition, the contrast
agent faces shear stress (27,28)
in the circulatory system, phagocytic immune response and other
harsh conditions (18,29). Therefore, the efficient and
specific targeting of LHRH-N-Mbs in an in vivo setting, to a
large extent, relies on the amount of LHRH antibodies in the
LHRH-N-Mbs and the conjugative strength between the antibody and
the microbubble.
The interaction between biotin and avidin is highly
specific and the high affinity binding is not affected by the
dilution of the reagent, which minimizes the nonspecific effect in
the practical application. The binding of biotinylated antibody and
avidin is quick, specific and has no effect on the antibody
activity. The antibody and avidin complex is also stable as it
cannot be washed off during the incubation and washing steps nor in
an organic solvent (24,25,30,31).
In the present study, LHRH-N-Mb was prepared by
linking a biotinylated LHRH antibody to N-N-Mbs containing avidin.
Despite repeated washing, the flow cytometry data demonstrated that
the binding rate between the secondary antibody and LHRH-N-Mb was
75.6%, while the binding rate between N-N-Mb containing avidin and
the secondary antibody was only 0.83%. As the same amount of avidin
was added to LHRH-N-Mbs and N-N-Mbs, this ruled out the possibility
that more binding between the secondary antibody and the
microbubble was due to more avidin. These data convincingly suggest
that biotinylated LHRH antibody, instead of avidin, binds to the
secondary antibody. This strong interaction provides an important
basis for future in vivo applications.
Numerous problems of targeting nano-microbubbles
remain, including the complexity of the preparation (32), the stability in the blood and the
induced immune response (33),
which require further investigation. However, with improvements in
technology, ultrasound molecular imaging targeting technology, a
non-invasive detection method for early stage cancer diagnosis,
possesses great application potential.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (no. 81060217) and the Science and
Technology Program Foundation of Jiangxi Province (no.
2010BSA15000).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar
|
|
2
|
Lech A, Daneva T, Pashova S, et al:
Ovarian cancer as a genetic disease. Front Biosci (Landmark Ed).
18:543–563. 2013. View
Article : Google Scholar
|
|
3
|
Bast RC Jr, Skates S, Lokshin A and Moore
RG: Differential diagnosis of a pelvic mass: improved algorithms
and novel biomarkers. Int J Gynecol Cancer. 22(Suppl 1): S5–S8.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Leung F, Dimitromanolakis A, Kobayashi H,
Diamandis EP and Kulasingam V: Folate-receptor 1 (FOLR1) protein is
elevated in the serum of ovarian cancer patients. Clin Biochem.
46:1462–1468. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li J, Chen H, Mariani A, et al: HE4
(WFDC2) Promotes Tumor Growth in Endometrial Cancer Cell Lines. Int
J Mol Sci. 14:6026–6043. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ferraro S, Braga F, Lanzoni M, Boracchi P,
Biganzoli EM and Panteghini M: Serum human epididymis protein 4 vs
carbohydrate antigen 125 for ovarian cancer diagnosis: a systematic
review. J Clin Pathol. 66:273–281. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Azzam AZ, Hashad DI and Kamel NA:
Evaluation of HE4 as an extrabiomarker to CA125 to improve
detection of ovarian carcinoma: is it time for a step forward? Arch
Gynecol Obstet. 288:167–172. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lenhard M, Stieber P, Hertlein L, et al:
The diagnostic accuracy of two human epididymis protein 4 (HE4)
testing systems in combination with CA125 in the differential
diagnosis of ovarian masses. Clin Chem Lab Med. 49:2081–2088. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hamed EO, Ahmed H, Sedeek OB, Mohammed AM,
Abd-Alla AA and Abdel Ghaffar HM: Significance of HE4 estimation in
comparison with CA125 in diagnosis of ovarian cancer and assessment
of treatment response. Diagn Pathol. 8:112013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Varga D, Deniz M, Schwentner L and
Wiesmüller L: Ovarian cancer: in search of better marker systems
based on DNA repair defects. Int J Mol Sci. 14:640–673. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Arits AH, Stoot JE, Botterweck AA, Roumen
FJ and Voogd AC: Preoperative serum CA125 levels do not predict
suboptimal cytoreductive surgery in epithelial ovarian cancer. Int
J Gynecol Cancer. 18:621–628. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Deshpande N, Ren Y, Foygel K, Rosenberg J
and Willmann JK: Tumor angiogenic marker expression levels during
tumor growth: longitudinal assessment with molecularly targeted
microbubbles and US imaging. Radiology. 258:804–811. 2011.
View Article : Google Scholar
|
|
13
|
Willmann JK, Kimura RH, Deshpande N, Lutz
AM, Cochran JR and Gambhir SS: Targeted contrast-enhanced
ultrasound imaging of tumor angiogenesis with contrast microbubbles
conjugated to integrin-binding knottin peptides. J Nucl Med.
51:433–440. 2010. View Article : Google Scholar
|
|
14
|
Barua A, Bitterman P, Bahr JM, et al:
Detection of tumor-associated neoangiogenesis by Doppler
ultrasonography during early-stage ovarian cancer in laying hens: a
preclinical model of human spontaneous ovarian cancer. J Ultrasound
Med. 29:173–182. 2010.
|
|
15
|
Xing Z, Wang J, Ke H, et al: The
fabrication of novel nanobubble ultrasound contrast agent for
potential tumor imaging. Nanotechnology. 21:1456072010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rapoport NY, Nam KH, Gao Z and Kennedy A:
Application of Ultrasound for Targeted Nanotherapy of Malignant
Tumors. Acoust Phys. 55:594–601. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Krupka TM, Solorio L, Wilson RE, Wu H,
Azar N and Exner AA: Formulation and characterization of echogenic
lipid-Pluronic nanobubbles. Mol Pharm. 7:49–59. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Brigger I, Dubernet C and Couvreur P:
Nanoparticles in cancer therapy and diagnosis. Adv Drug Deliv Rev.
54:631–651. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Anderson CR, Hu X, Zhang H, et al:
Ultrasound molecular imaging of tumor angiogenesis with an integrin
targeted microbubble contrast agent. Invest Radiol. 46:215–224.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xing W, Zhigang W, Bing H, et al:
Targeting an ultrasound contrast agent to folate receptors on
ovarian cancer cells: feasibility research for ultrasonic molecular
imaging of tumor cells. J Ultrasound Med. 29:609–614.
2010.PubMed/NCBI
|
|
21
|
Völker P, Gründker C, Schmidt O, Schulz KD
and Emons G: Expression of receptors for luteinizing
hormone-releasing hormone in human ovarian and endometrial cancers:
frequency, autoregulation, and correlation with direct
antiproliferative activity of luteinizing hormone-releasing hormone
analogues. Am J Obstet Gynecol. 186:171–179. 2002.
|
|
22
|
Engel JB, Schally AV, Buchholz S, Seitz S,
Emons G and Ortmann O: Targeted chemotherapy of endometrial,
ovarian and breast cancers with cytotoxic analogs of luteinizing
hormone-releasing hormone (LHRH). Arch Gynecol Obstet. 286:437–442.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yin T, Wang P, Zheng R, et al: Nanobubbles
for enhanced ultrasound imaging of tumors. Int J Nanomedicine.
7:895–904. 2012.PubMed/NCBI
|
|
24
|
Reches M and Gazit E: Biological and
chemical decoration of peptide nanostructures via biotin-avidin
interactions. J Nanosci Nanotechnol. 7:2239–2245. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bayer EA and Wilchek M: Avidin-biotin
technology: preparation of avidin conjugates. Methods Mol Biol.
10:143–148. 1992.PubMed/NCBI
|
|
26
|
Oeffinger BE and Wheatley MA: Development
and characterization of a nano-scale contrast agent. Ultrasonics.
42:343–347. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Saxer T, Zumbuehl A and Müller B: The use
of shear stress for targeted drug delivery. Cardiovasc Res.
99:328–333. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tan J, Shah S, Thomas A, Ou-Yang HD and
Liu Y: The influence of size, shape and vessel geometry on
nanoparticle distribution. Microfluid Nanofluidics. 14:77–87. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Diaz-López R, Tsapis N, Santin M, et al:
The performance of PEGylated nanocapsules of perfluorooctyl bromide
as an ultrasound contrast agent. Biomaterials. 31:1723–1731.
2010.PubMed/NCBI
|
|
30
|
Morag E, Bayer EA and Wilchek M:
Reversibility of biotin-binding by selective modification of
tyrosine in avidin. Biochem J. 316:193–199. 1996.PubMed/NCBI
|
|
31
|
Wang W, Liu GJ, Xie XY, et al: Development
and evaluation of lipid microbubbles targeted to
alpha(v)beta(3)-integrin via biotin-avidin bridge. J Microencapsul.
29:177–184. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Klibanov AL: Ligand-carrying gas-filled
microbubbles: ultrasound contrast agents for targeted molecular
imaging. Bioconjug Chem. 16:9–17. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Klibanov AL: Ultrasound molecular imaging
with targeted microbubble contrast agents. J Nucl Cardiol.
14:876–884. 2007. View Article : Google Scholar : PubMed/NCBI
|