Introduction
Neuroblastoma is the most common extracranial solid
tumor of childhood and exhibits various clinical manifestations and
diverse prognoses (1,2). Certain tumor cells undergo
spontaneous regression while others exhibit continuous progression.
The prognosis of neuroblastoma is associated with the age of onset,
the extent of disease and the biological characteristics of the
tumor. MYCN gene amplification is an independent high risk factor
for neuroblastoma. MYCN regulates various genes through multiple
biochemical signaling pathways and thereby promotes the growth of
malignant tumors (3). Previous
studies have shown that the expression levels of the MYCN gene were
associated with a variety of abnormal micro (mi)RNA regulation
patterns (4–7). miRNAs are a class of endogenous
non-coding RNAs ~22 nt in length, which may inhibit the translation
of specific target mRNAs and induce its degradation, thereby
affecting cell proliferation, differentiation, apoptosis and other
biological processes (8). Various
miRNA molecules have been associated with neuroblastoma; one of the
MYCN gene regulating factors in neuroblastoma is the miRNA17–92
cluster (9–11).
The tyrosine kinase (Trk) receptor family includes
TrkA, TrkB and TrkC. Previous studies have shown that the Trk
family is associated with the prognosis of neuroblastoma (12). Neuroblastoma with TrkA expression
results in an improved prognosis, as TrkA combines with its ligand,
nerve growth factor (NGF), to promote spontaneous regression or
differentiation of the tumor. However, neuroblastoma with TrkB
expression results in a poor prognosis due to amplification of the
MYCN gene. TrkB ligands from neuroblastoma, via autocrine or
paracrine survival pathways, may enhance the viability, drug
resistance and angiogenesis of TrkB-expressing tumors (13). To the best of our knowledge, no
studies have confirmed the association between the miRNA17–92
cluster and the TrK family. In the present study, the miRNA17–92
cluster regulated by MYCN gene was hypothesized to be associated
with the TrK family and thus affect the prognosis of neuroblastoma.
One of the miRNA members of miRNA17–92 cluster, miRNA-92a, was
selected to investigate its effect on human neuroblastoma cells and
the underlying mechanisms.
Materials and methods
Cell culture
Primary BE(2)-M17 human neuroblastoma cell lines
with an amplified MYCN gene were purchased from the cell stores of
the Chinese Academy of Medical Sciences (Beijing, China), and
cultured in Dulbecco’s modified Eagle’s medium (DMEM; Hyclone
Laboratories, Inc., Logan, UT, USA) supplemented with 10% fetal
bovine serum (FBS; Gibco Life Technologies, Grand Island, NY, USA)
and 1% penicillin/streptomycin (Hyclone Laboratories, Inc.) at 37°C
in a 5% CO2 atmosphere.
Gene transfection
After 24 h of culture, the cells were starved in
DMEM without 10% FBS and 100 units penicillin/streptomycin, and
divided into four groups. The appropriate quantity of the following
reagents was added to each of the four groups respectively:
miRNA-92a mimics negative control (NC) sense,
5′-UUCUCCGAACGUGUCACGUTT-3′; miRNA-92a mimics NC antisense,
5′-ACGUGA CACGUUCGGAGAATT-3′; miRNA-92a mimics sense,
5′-UAUUGCACUUGUCCCGGCC UGU-3′; miRNA-92a mimics antisense,
5′-AGGCCGGGACAA GUGCAAUAUU-3′; miRNA-92a inhibitor NC sense,
5′-CAGUACUUUUGUGUA GUACAA-3′; and miRNA-92a inhibitors antisense,
5′-ACAGGCCGGGACAAGUGCA AUA-3′ (all purchased from Gene Pharma,
Shanghai, China). Lipofectamine 2000 (Invitrogen Life Technologies,
Carlsbad, CA, USA) was added to the culture media of each of above
groups and oligonucleotides were adjusted to a final concentration
of 160 nM. All cells were incubated in DMEM containing 10% FBS at
37°C in a humidified incubator with 5% CO2. The
miRNA-92a mimics and inhibitor were fluorescently labeled with
fluorescein (Sigma-Aldrich, St. Louis, MO, USA) for verification of
transfection.
Total RNA extraction
Total RNA from cultured cell lines was extracted at
0, 24, 48 and 72 h post-transfection following the manufacturer’s
instructions of the RNAiso Plus reagent (Takara Bio Inc., Otsu,
Japan). The concentration and quality of the RNA were determined
with GeneQuan Pro (Biochrom Ltd., Cambridge, UK), with the
OD260nm/OD280nm between 1.8 and 2.2. The RNA was stored at
−80°C.
Quantitative polymerase chain reaction
(qPCR) for miRNA and TrkA mRNA expression
qPCR analysis for miRNA-92a was performed in
triplicate with the One Step Primescript® miRNA cDNA
Synthesis kit (Takara Bio Inc.) and SYBR® Premix Ex
TaqTMII (Perfect Real Time; Takara Bio Inc.) according to the
manufacturer’s instructions. U6 small nuclear RNAs served as
internal controls. The mixture was incubated for 30 sec at 95°C for
1 cycle, followed by 5 sec at 95°C and 30 sec at 60°C for 40 cycles
using the DNA Engine Opticon2 (MJ Research Inc. Waltham, MA). The
fold-change in miRNA-92a expression levels was calculated using ΔCT
and 2−ΔΔCT. The following primers were used
respectively: miRNA-92a sense, 5′-TATTGCACTTGTCCCGGCCTG-3′; the
miRNA-92a antisense strand was constructed using general primers;
U6 sense, 5′-TCGCTTCGGCAGCACATA-3′; U6 antisense,
5′-TTGCGTGTCATCCTTGCG-3′ (Sunbiotech Co. Ltd., Beijing, China).
TrkA mRNA expression levels were measured at 24 and
48 h post-transfection by qPCR, which was performed in triplicate
using the same method as for miRNA-92a. β-actin served as the
internal control. The following primers were used respectively:
TrkA mRNA sense, 5′-TATTGCACTTGTCC CGGCCTG-3′; TrkA mRNA antisense,
5′-ACAAGGAGCAG CGTAGAAAGGA-3′; β-actin sense, 5′-TGACGTGGACATC
CGCAAAG-3′; β-actin antisense, 5′-CTGGAAGGTGGA CAGCGAGG-3′
(Sunbiotech Co. Ltd.).
Cell Counting Kit-8 (CCK-8) assay
The transfected neuroblastoma cells were seeded into
96-well plates (5.0×103 cells/well) containing 100 μl
DMEM medium supplemented with 10% FBS. Cell viability was detected
by CCK-8 assay (Dojin Laboratories, Kumamoto, Japan) at 0, 24, 48
and 72 h post-transfection. The absorbance at 450 nm (A450) of each
well was read on a Model 550 spectrophotometer (Bio-Rad, Hercules,
CA, USA).
Transwell migration assay
Cellular migration was measured using a modification
of the method as reported previously (14), using 24-well Transwell cell culture
chambers filtered with multiporous polycarbonate membranes (Corning
Inc., NY, USA). The BE(2)-M17 cells were cultured for 6 h in
serum-free DMEM medium without antibiotics following transfection.
Each group of cells was digested to form a cell suspension and
adjusted to a density of either 2×105 cells per ml
(miRNA-92a mimics and miRNA-92a mimics NC groups) or
3×105 cells per ml (miRNA-92a inhibitor and miRNA-92a
inhibitor NC groups). A volume of 100 μl cell suspension cultured
in 500 μl DMEM medium with 10% FBS was extracted and plated onto a
24-well Transwell plate. The plates were placed in a humidified 5%
CO2 incubator for 36 h at 37°C. The upper surface of the
membrane was wiped with cotton swabs to remove non-migrated cells,
and the remaining cells were fixed in 95% ethanol (Beijing Chemical
Works, Beijing, China) for 10 min and then stained with 0.1%
crystal violet (Sigma-Aldrich) for 15 min. Digital images of cells
were obtained using the DMI4000B DFC500 inverted microscope (Leica
Microsystems AG, Wetzlar, Germany; magnification, ×200). The number
of cells in each image was counted by Scion Image software (Scion
Corporation, Torrance, CA, USA). Each treatment in the migration
assay was performed in triplicate.
Flow cytometry assay for TrkA protein
expression
A total of ~1.0×106 cells were fixed in
phosphate-buffered saline (PBS) with 4% formaldehyde for 10 min at
37°C and subsequently incubated with the primary rabbit polyclonal
anti-human TrkA antibody (Abcam Plc, Cambridge, UK) at 50 μg/ml for
1 h. Following three washes with PBS, the cells were incubated with
2 μg/ml anti-rabbit IgG for 1 h (Cell Signaling Technology Inc.,
Boston, MA, USA). The cells were resuspended in PBS and analyzed
with the CytomicsTM FC500 flow cytometer (Beckman Coulter Inc.
Fullerton, CA, USA).
Statistical analysis
All data were analyzed by SPSS software (version 13;
SPSS, Inc., Chicago, IL, USA) and the significance of the
difference between groups was determined by Student’s t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effect of miRNA-92a transfection and its
changes in expression
In order to analyze the effect of miRNA-92a
transfection and its expression, the solution of miRNA-92a mimics,
miRNA-92a mimics NC, miRNA-92a inhibitors and miRNA-92a inhibitor
NC was replaced 6 h later following the transfection. Tumor cells
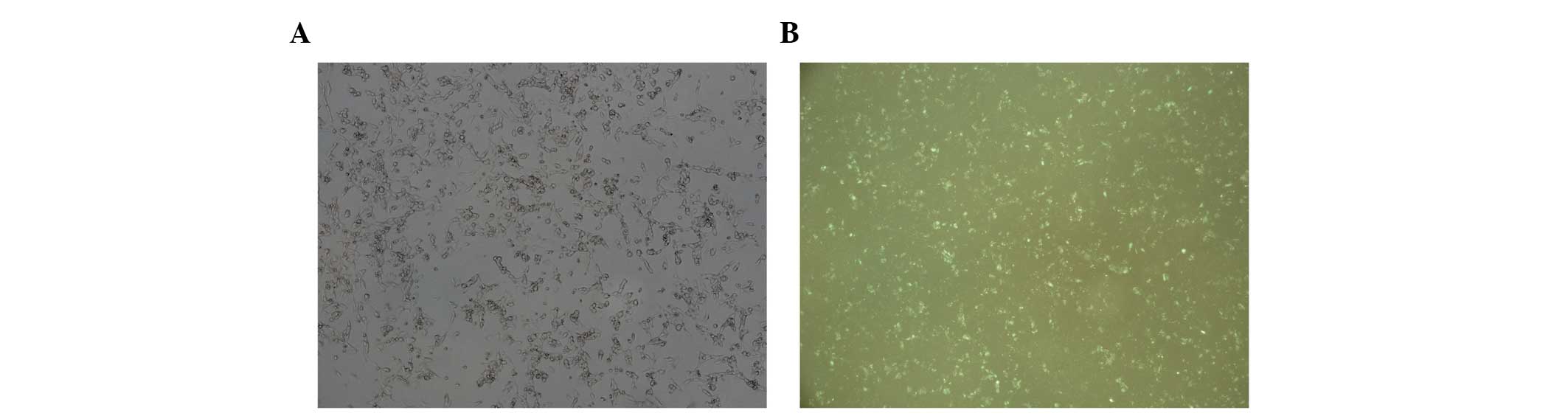
were focused under the fluorescence microscope at a magnification
of ×100. The cells were observed under natural light and under
fluorescence in the same field of vision (Fig. 1). Granular fluorescence was
observed in a large number of the tumor cells which indicated miRNA
transfection of the cells. To further verify transfection, total
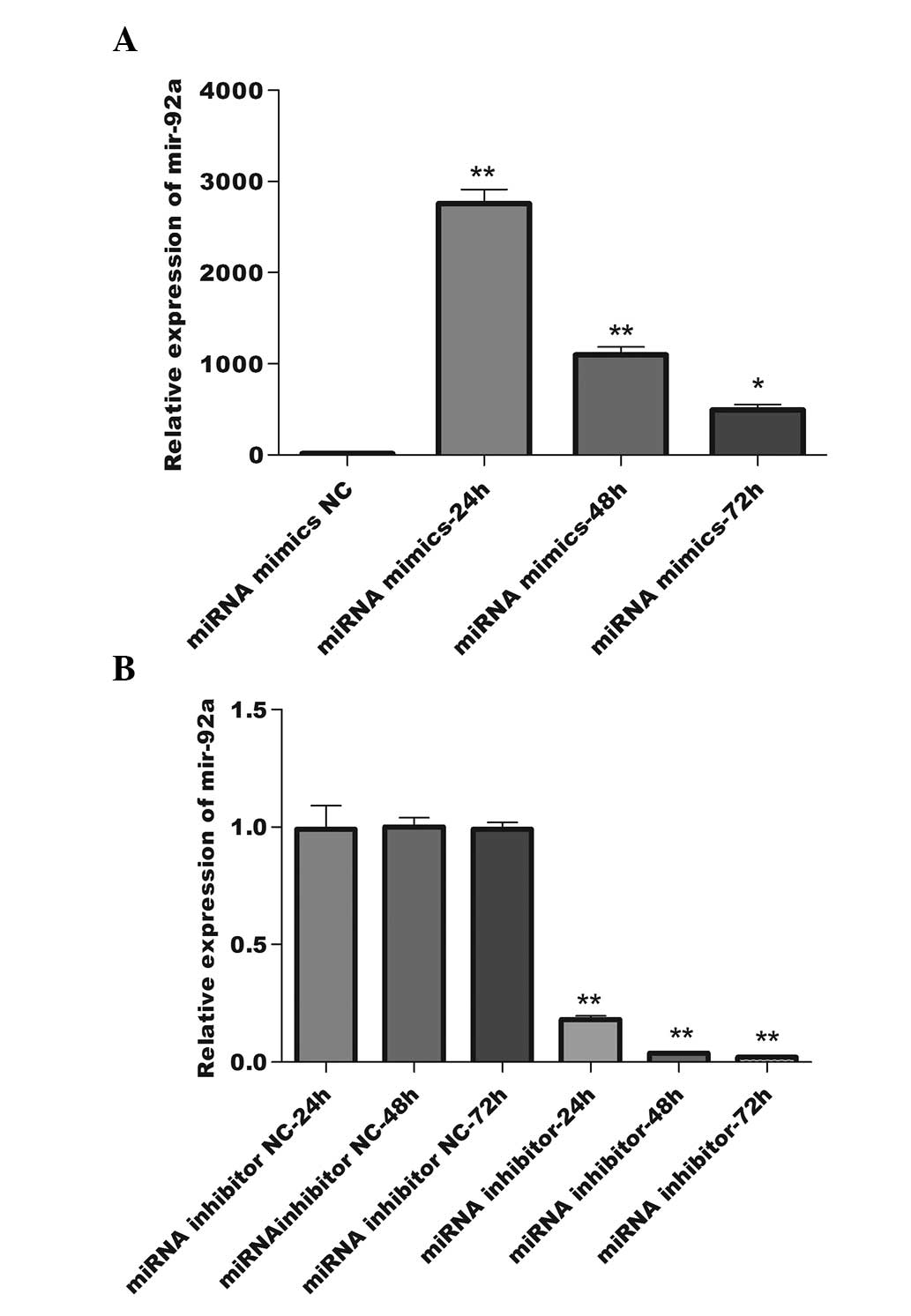
RNA at 24, 48 and 72 h after transfection was isolated and qPCR was
employed to detect the relative expression levels of miRNA-92a at
three time points. The maximum miRNA-92a expression level following
miRNA-92a mimics transfection was detected at 24 h after
transfection, then gradually decreased from this level (Fig. 2A). The miRNA-92a expression levels
were reduced in comparison with the NC group 24 h after miRNA-92a
inhibitor transfection and the inhibition reached a peak at 48 h,
which lasted until 72 h post-transfection (Fig. 2B). These results demonstrate that
the interference effect was significantly enhanced 24 h
post-transfection with miRNA-92a mimics (P<0.01) and 48 h
post-transfection with miRNA-92a inhibitors (P<0.01).
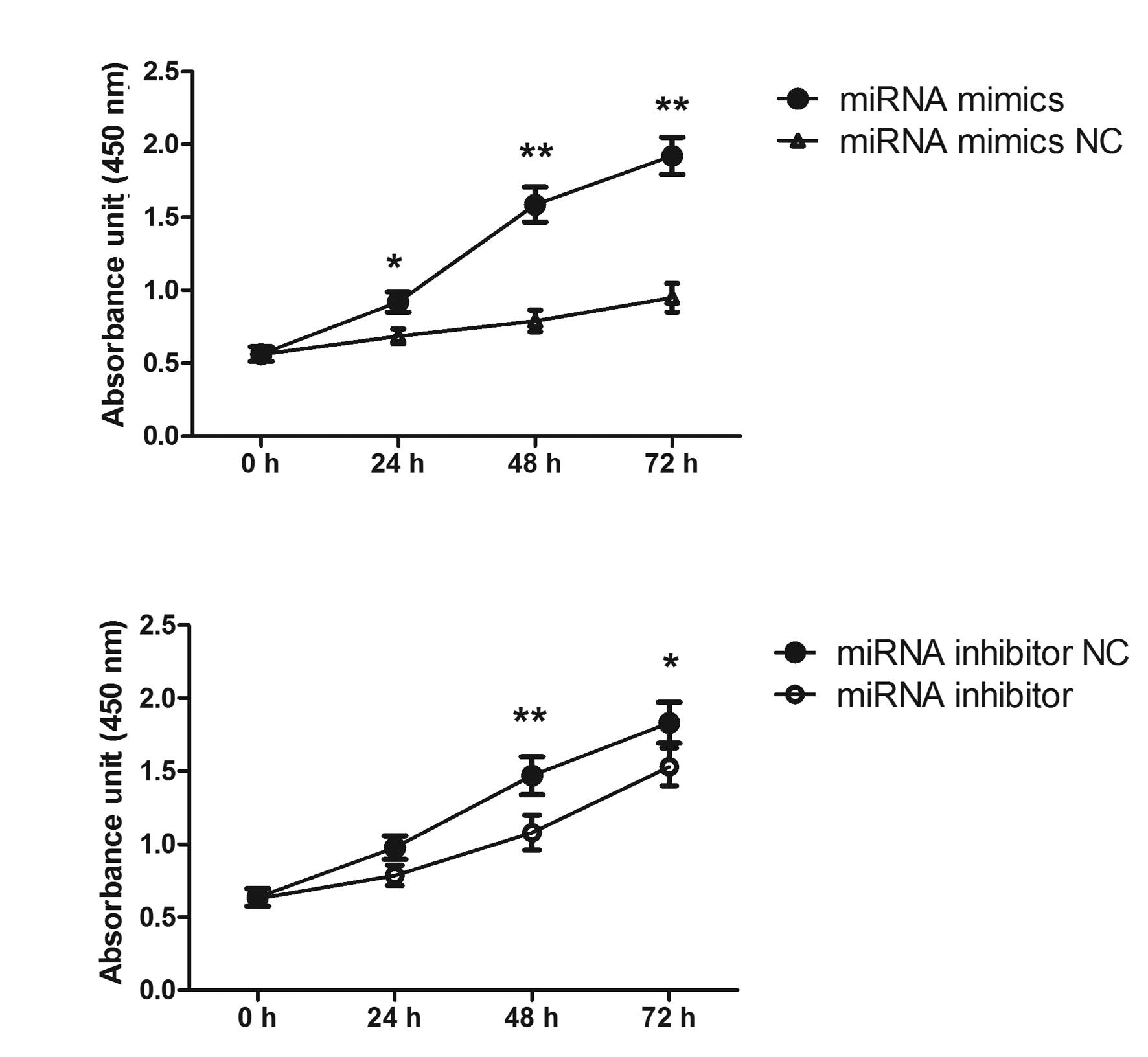
miRNA-92a promotes the proliferation of
BE(2)-M17 human neuroblastoma cells
In order to investigate the functional role of
miRNA-92a in neuroblastoma cells, the effect of miRNA-92a mimics
and miRNA-92a inhibitors on the proliferation of BE(2)-M17 human
neuroblastoma cell line was examined. The cells were transfected
with either miRNA or NC for 24, 48 and 72 h. CCK-8 assay and direct
cell count revealed that overexpression of miRNA-92a mimics
significantly increased the proliferation of BE(2)-M17 human
neuroblastoma cells and overexpression of miRNA-92a inhibitors
significantly inhibited the proliferation of neuroblastoma cells
(Fig. 3).
Effect of miRNA-92a on BE(2)-M17 human
neuroblastoma cell migration
To reveal whether miRNA-92a was involved in the
regulation of migration of neuroblastoma cells, a Transwell
migration assay was conducted. The number of cells across the
membrane in the miRNA mimics transfected group (32.4±3.7) was
increased by 37.3% compared with that in the control group
(23.6±2.1), which was significantly different (P<0.05) (Fig. 4A and 4B). The number of cells
across the membrane in the group of cells transfected with the
miRNA-92a inhibitors (21.3±2.3) was decreased by 40.8% compared
with that in the control group (36.0±7.5) which was also
significantly different (P<0.05, Fig. 4C and D). These data indicate that
transfection with miRNA mimics was capable of enhancing the
migration of cells, while transfection with miRNA inhibitors
reduced the migration of cells.
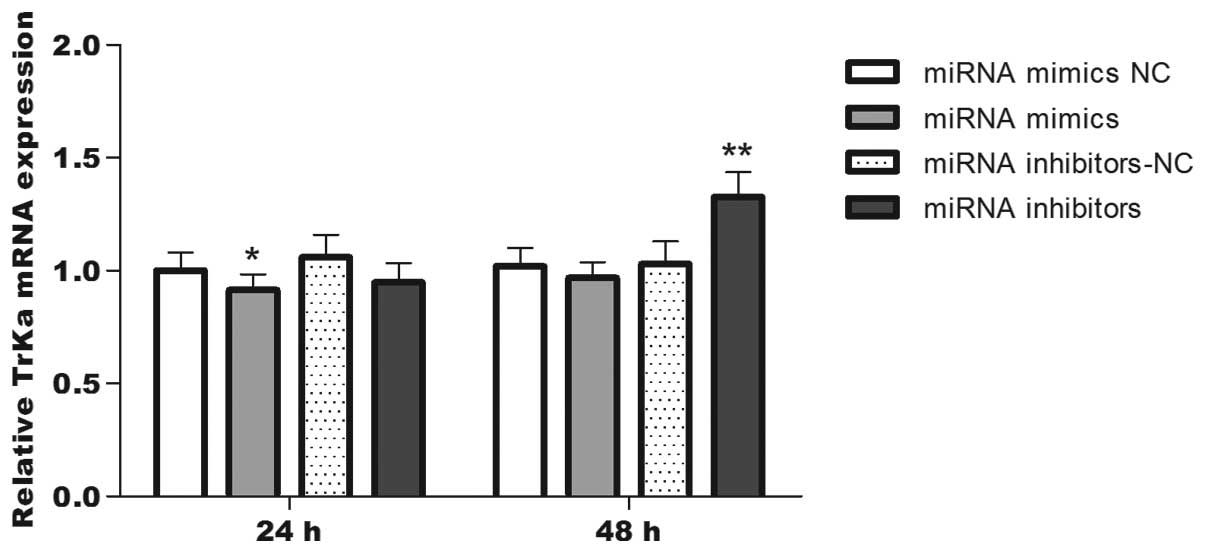
TrkA expression levels are inversely
correlated with the miRNA-92a expression levels
In view of the established role of miRNA-92a as an
effector for indirect MYCN-induced downregulation of protein-coding
genes, it was hypothesized that this miRNA may regulate TrkA. To
investigate this possibility, TrkA mRNA expression following
conditional up- or downregulation of miRNA-92a was verified in
BE(2)-M17 human neuroblastoma cell lines. Total RNA was extracted
at 24 and 48 h post-transfection and qPCR was used to detect TrkA
mRNA expression at these two time points; the TrkA protein
expression levels were detected by flow cytometry. The data from
the qPCR and flow cytometry measurements revealed that the TrkA
mRNA expression levels (Fig. 5)
and the average fluorescence value (Table I) at 24 h post-transfection were
significantly reduced in the miRNA-92a mimics transfection group
compared with the control group, although no statistical difference
was detected at 48 h. However, when the miRNA-92a inhibitor
transfection group was compared with its control, the TrkA mRNA
expression levels and the average fluorescence value at 48 h
post-transfection were significantly reduced, although no
statistical difference was identified at 24 h. These results
demonstrated that the expression levels of TrkA protein in the
cells transfected with miRNA-92a mimics were significantly reduced
(5.8% decrease; P<0.01) at 24 h post-transfection compared with
the cells in the control group, while the expression levels of TrkA
protein in the cells transfected with miRNA-92a inhibitor were
significantly elevated (18.2% increase; P<0.01) at 48 h
post-transfection compared with the cells in the control group.
Overall, the miRNA-92a inhibitor exhibited a greater effect on TrkA
protein than the miRNA-92a mimics.
 | Table IAverage fluorescence intensity of Trk
protein by fluorescence-activated cell sorting. |
Table I
Average fluorescence intensity of Trk
protein by fluorescence-activated cell sorting.
| Group | miRNA-92a mimics
NC | miRNA-92a mimics | miRNA-92a inhibitors
NC | miRNA-92a
inhibitors |
|---|
| Average fluorescence
intensity at 24 h | 31.26±0.26 | 29.45±0.36a | 28.05±0.43 | 26.72±0.99 |
| Average fluorescence
intensity at 48 h | 25.12±0.76 | 24.25±0.28 | 22.42±0.86 | 26.51±0.36a |
Discussion
miRNAs are large groups of gene regulatory molecules
which influence a number of gene encoding proteins. There is
abundant evidence revealing >1,400 types of miRNA important in
the pathogenesis of human diseases. One study has shown that miRNA
inhibits the dedifferentiation and plasticity of cells in the
process of tumor formation through the regulation of protein
expression and the cell differentiation process. Furthermore, miRNA
affects the cyclical adjustment of tumor cells, the integrity of
the genomes, the stress response, apoptosis and metastasis
(15).
The miRNA genes are located in introns or non-coding
regions of the chromosome and are first transcribed as primary
transcripts of 500–3,000 nt termed pri-miRNA. These molecules are
then cut to ~70-bp miRNA precursors and then to ~22-bp mature miRNA
assembled into RNA-induced silencing complexes to perform RNA
interference gene silencing. When the miRNA molecule and the 3′UTR
nucleotide of the target mRNA are complementary, the complex may
inhibit the translation of the mRNA to protein and may induce the
degradation of target mRNA (8,16).
In the present study, double-stranded miRNA mimics,
which have greater stability than single stranded ones, were
selected to increase miRNA-92a expression levels. The miRNA-92a
expression levels significantly increased (P<0.01) with miRNA
mimics compared to an NC, achieving a peak 24 h after transfection
and then gradually decreasing at 48 and 72 h after transfection.
The miRNA-92a expression levels were found to progressively
decrease compared with the NC following transfection with miRNA
inhibitors. The effective regulation of miRNA-92a expression by
transfection indicated that the research was reliable.
Studies have revealed that miRNA may affect a
multitude of target genes and that one target gene may be regulated
by multiple miRNA molecules (17–19).
The miR-17–92 cluster has been considered to be the most effective
carcinogenic miRNA, and hundreds of target genes of this cluster
have been reported (20–23). One study observed a clear reduction
in the expression levels of endogenous p21 mRNA and protein in
SK-N-AS 17-5p cluster cells, as well as in SK-N-AS cells
transiently transfected with miR-17-5p, although not in those
transfected with miRNA-92 (21).
Another study demonstrated that miRNA-17–92 is a potent inhibitor
of transforming growth factor (TGF)-β signaling. By functioning
upstream and downstream of pSMAD2, miRNA-17–92 activation triggers
downregulation of multiple key effectors along the TGF-β signaling
cascade as well as direct inhibition of the TGF-β-responsive gene,
but miRNA-92a did not affect the luciferase signals of SMAD2
(9). This suggests that every
member of the miRNA-17–92 cluster exhibits its own characteristics,
and the signaling pathway and mechanism of miRNA-92a may be
different from the miRNA-17–92 cluster, although this requires
further investigation.
Neuroblastoma cells have been observed to undergo
differentiation and dissipation when TrkA is highly expressed,
while cells with low TrkA expression levels exhibit the opposite
behavior and become more invasive (3). In the present study, the
proliferation and migration of neuroblastoma cells were found to be
enhanced when TrkA expression was reduced, while the proliferation
and migration capacity decreased when the TrkA expression was
increased in the neuroblastoma cells.
Numerous studies have shown that high expression of
TrkA in neuroblastoma tissue is associated with improved clinical
characteristics in patients and lower MYCN gene amplification,
while patients in the advanced stages of neuroblastoma usually
exhibit lower expression levels of TrkA and the tumors cannot be
treated with NGF to inhibit differentiation (24–26).
The NGF/TrkA signaling pathway is important in promoting
neuroblastoma differentiation and natural regression (13,27,28).
High expression of TrkA protein in neuroblastoma cells is
associated with differentiation and regression (28–30),
while lower TrkA expression is associated with increased
invasiveness. One study suggested that activated Ras in the
NGF/TrkA signaling pathway stimulates nuclear translocation of p53
and induces growth arrest by induction of p21WAF1 in PC12 cells
(31). Activation of TrkA induces
the phosphorylation and activation of SHC, phoshoinositide 3-kinase
and phospholipase Cγ1, which are the primary effectors of Trk
activity in NGF-treated PC12 cells (32). Ras/MAPK and AKT are activated
downstream of these signaling pathways. Ras sequentially activates
a series of kinases, including RAF1, mitogen-activated protein
kinase kinase, mitogen-activated protein kinase (MAPK) and
ribosomal S6 kinase (RSK) (13).
MAPK and RSK translocate to the nucleus to initiate the activation
of transcription factors that regulate NGF-inducible genes, leading
to survival and neuronal differentiation. Other signaling proteins
important for normal biological responses to ligand binding include
SH2B/APS, fibroblast growth factor receptor substrate 2 and AKT
(32). Further studies may be
conducted to investigate whether miRNA-92a regulates the Ras/MAPK
signaling pathway.
In the present study, transfection with miRNA-92a
inhibitors was found to exert a more marked impact on TrkA protein
expression levels than transfection with miRNA-92a mimics. Since
the BE(2)-M17 neuroblastoma cell line exhibits MYCN gene
amplification, the cell line was hypothesized to have high
expression levels of miRNA-92a. Therefore, reducing the expression
of miRNA-92a is more efficient than increasing the expression.
Furthermore, the expression levels of the target protein were found
to be reduced at 24 h after transfection with the miRNA-92a mimics,
although these were restored to the levels of the NC after 48 h.
This may be considered to be an intracellular adjustment mechanism
to correct target protein expression back to normal levels.
Although an association between the expression
levels of miRNA-92a and TrkA has been observed, the mechanism and
signaling pathway of TrkA expression regulated by miRNA-92a remains
unclear. The present study has limitations in that there is not a
great quantity of data and the findings have not been replicated in
other neuroblastoma cell lines. If a particular solution were found
to inhibit or restore the TrkA signaling pathway when the study is
repeated, the results may be more convincing.
In conclusion, the present study demonstrated that
the biological behavior of neuroblastoma cells was markedly altered
when the expression levels of miRNA-92a were elevated or reduced.
The proliferation and migration capacity of neuroblastoma cells
exhibited a positive correlation with the expression levels of
miRNA-92a in tumor cells, and a negative correlation with TrkA
protein expression levels. miRNA-92a may affect neuroblastoma cell
proliferation and migration capacity by regulating TrkA protein
expression levels. This may provide an experimental basis for the
treatment of neuroblastoma with miRNA-92a.
Acknowledgements
This study was supported by the China National 863
Program (grant no. 2012AA020804).
Abbreviations:
|
DMEM
|
Dulbecco’s modified Eagle’s medium
|
|
miRNA
|
microRNA
|
|
NGF
|
nerve growth factor
|
|
PBS
|
phosphate-buffered saline
|
|
qPCR
|
quantitative polymerase chain
reaction
|
|
Trk
|
tyrosine kinase
|
References
|
1
|
Maris JM, Mosse YP, Bradfield JP, et al:
Chromosome 6p22 locus associated with clinically aggressive
neuroblastoma. N Engl J Med. 358:2585–2593. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schwab M, Westermann F, Hero B and
Berthold F: Neuroblastoma: biology and molecular and chromosomal
pathology. Lancet Oncol. 4:472–480. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Alaminos M, Mora J, Cheung NK, et al:
Genome-wide analysis of gene expression associated with MYCN in
human neuroblastoma. Cancer Res. 63:4538–4546. 2003.PubMed/NCBI
|
|
4
|
Mestdagh P, Fredlund E, Pattyn F, et al:
MYCN/c-MYC-induced microRNAs repress coding gene networks
associated with poor outcome in MYCN/c-MYC-activated tumors.
Oncogene. 29:1394–1404. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
O’Donnell KA, Wentzel EA, Zeller KI, Dang
CV and Mendell JT: c-Myc-regulated microRNAs modulate E2F1
expression. Nature. 435:839–843. 2005.PubMed/NCBI
|
|
6
|
Ma L, Young J, Prabhala H, et al: miR-9, a
MYC/MYCN-activated microRNA, regulates E-cadherin and cancer
metastasis. Nat Cell Biol. 12:247–256. 2010.PubMed/NCBI
|
|
7
|
Welch C, Chen Y and Stallings RL:
MicroRNA-34a functions as a potential tumor suppressor by inducing
apoptosis in neuroblastoma cells. Oncogene. 26:5017–5022. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bartel DP: MicroRNAs: genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mestdagh P, Boström AK, Impens F, et al:
The miR-17–92 microRNA cluster regulates multiple components of the
TGF-beta pathway in neuroblastoma. Mol Cell. 40:762–773. 2010.
|
|
10
|
Lovén J, Zinin N, Wahlström T, et al:
MYCN-regulated microRNAs repress estrogen receptor-alpha (ESR1)
expression and neuronal differentiation in human neuroblastoma.
Proc Natl Acad Sci USA. 107:1553–1558. 2010.PubMed/NCBI
|
|
11
|
Nara K, Kusafuka T, Yoneda A, Oue T,
Sangkhathat S and Fukuzawa M: Silencing of MYCN by RNA interference
induces growth inhibition, apoptotic activity and cell
differentiation in a neuroblastoma cell line with MYCN
amplification. Int J Oncol. 30:1189–1196. 2007.PubMed/NCBI
|
|
12
|
Kogner P, Barbany G, Dominici C, Castello
MA, Raschellá G and Persson H: Coexpression of messenger RNA for
TRK protooncogene and low affinity nerve growth factor receptor in
neuroblastoma with favorable prognosis. Cancer Res. 53:2044–2050.
1993.PubMed/NCBI
|
|
13
|
Brodeur GM, Minturn JE, Ho R, et al: Trk
receptor expression and inhibition in neuroblastomas. Clin Cancer
Res. 15:3244–3250. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Valster A, Tran NL, Nakada M, Berens ME,
Chan AY and Symons M: Cell migration and invasion assays. Methods.
37:208–215. 2005. View Article : Google Scholar
|
|
15
|
Negrini M, Nicoloso MS and Calin GA:
MicroRNAs and cancer - new paradigms in molecular oncology. Curr
Opin Cell Biol. 21:470–479. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Woo CW, Tan F, Cassano H, Lee J, Lee KC
and Thiele CJ: Use of RNA interference to elucidate the effect of
MYCN on cell cycle in neuroblastoma. Pediatr Blood Cancer.
50:208–212. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Krek A, Grün D, Poy MN, et al:
Combinatorial microRNA target predictions. Nat Genet. 37:495–500.
2005. View
Article : Google Scholar
|
|
18
|
Bang-Berthelsen CH, Pedersen L, Fløyel T,
Hagedorn PH, Gylvin T and Pociot F: Independent component and
pathway-based analysis of miRNA-regulated gene expression in a
model of type 1 diabetes. BMC Genomics. 12:972011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xie SY, Li YJ, Wang PY, Jiao F, Zhang S
and Zhang WJ: miRNA-regulated expression of oncogenes and tumor
suppressor genes in the cisplatin-inhibited growth of K562 cells.
Oncol Rep. 23:1693–1700. 2010.PubMed/NCBI
|
|
20
|
Olive V, Jiang I and He L: mir-17–92, a
cluster of miRNAs in the midst of the cancer network. Int J Biochem
Cell Biol. 42:1348–1354. 2010.
|
|
21
|
Fontana L, Fiori ME, Albini S, et al:
Antagomir-17–5p abolishes the growth of therapy-resistant
neuroblastoma through p21 and BIM. PLoS One. 3:e22362008.
|
|
22
|
Fontana L, Pelosi E, Greco P, et al:
MicroRNAs 17–5p-20a-106a control monocytopoiesis through AML1
targeting and M-CSF receptor upregulation. Nat Cell Biol.
9:775–787. 2007.
|
|
23
|
Haug BH, Henriksen JR, Buechner J, et al:
MYCN-regulated miRNA-92 inhibits secretion of the tumor suppressor
DICKKOPF-3 (DKK3) in neuroblastoma. Carcinogenesis. 32:1005–1012.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Westermark UK, Wilhelm M, Frenzel A and
Henriksson MA: The MYCN oncogene and differentiation in
neuroblastoma. Semin Cancer Biol. 21:256–266. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Peterson S and Bogenmann E: The RET and
TRKA pathways collaborate to regulate neuroblastoma
differentiation. Oncogene. 23:213–225. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nakagawara A: Molecular basis of
spontaneous regression of neuroblastoma: role of neurotrophic
signals and genetic abnormalities. Hum Cell. 11:115–124.
1998.PubMed/NCBI
|
|
27
|
Nakagawara A, Arima M, Azar CG, Scavarda
NJ and Brodeur GM: Inverse relationship between trk expression and
N-myc amplification in human neuroblastomas. Cancer Res.
52:1364–1368. 1992.PubMed/NCBI
|
|
28
|
Kogner P, Barbany G, Dominici C, Castello
MA, Raschellá G and Persson H: Coexpression of messenger RNA for
TRK protooncogene and low affinity nerve growth factor receptor in
neuroblastoma with favorable prognosis. Cancer Res. 53:2044–2050.
1993.PubMed/NCBI
|
|
29
|
Nakagawara A: Trk receptor tyrosine
kinases: a bridge between cancer and neural development. Cancer
Lett. 169:107–114. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nakagawara A: The NGF story and
neuroblastoma. Med Pediatr Oncol. 31:113–115. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hughes AL, Gollapudi L, Sladek TL and Neet
KE: Mediation of nerve growth factor-driven cell cycle arrest in
PC12 cells by p53. Simultaneous differentiation and proliferation
subsequent to p53 functional inactivation. J Biol Chem.
275:37829–37837. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kaplan DR and Miller FD: Signal
transduction by the neurotrophin receptors. Curr Opin Cell Biol.
9:213–221. 1997. View Article : Google Scholar : PubMed/NCBI
|