Introduction
Lung cancer is the most common cause of
cancer-related mortality worldwide. With >1,000,000 new cases
per year, lung cancer represents the most frequent lethal neoplasm
in males, while its incidence increases progressively in females
(1). Lung cancer is a
heterogeneous disease clinically, histologically, biologically and
molecularly (2). The two main
types of lung cancer, non-small cell lung cancer (NSCLC;
representing 80–85% of cases) and small cell lung cancer
(representing 15–20% of cases) are identified based on
histological, clinical and immunohistochemical characteristics, and
also differ molecularly with numerous genetic alterations
exhibiting subtype specificity (3). Despite considerable efforts, only
5–10% of these patients survive 5 years following diagnosis, and
surgery accounts for the majority of these long-term survivors
(4). Therefore, tumor angiogenesis
and tumor metastasis to multiple organs is a critical problem for
patients with lung cancer. The prevention and treatment of tumor
angiogenesis and tumor metastasis are clinically important
(5).
It is well appreciated that heat shock proteins
(Hsps) are activated in the mammalian heart in response to numerous
physiological or pathological stresses and, consequently, provide
cardioprotection (6,7). HspB6, also referred to as Hsp20,
belongs to a small Hsp family (15–30 kDa), which includes ≥10
members (HspB1-B10) (8,9). While HspB6 can be detected in various
tissues, it is most highly expressed in muscle cells (8–10).
Previously, Wang et al demonstrated that HspB6-engineered
mesenchymal stem cells augmented the secretion of growth factors,
including vascular endothelial growth factor (VEGF), basic
fibroblast growth factor (bFGF) and intercellular adhesion molecule
1 (ICAM-1), and promoted myocardial angiogenesis (11). Numerous studies indicate that
certain Hsps (Hsp90, Hsp70, Hsp60 and aB-crystallin) are detectable
outside a variety of cell types, including neuronal cells,
monocytes, macrophages, endothelial cells and tumor cells of
epithelial origin (12–16). Notably, HspB6 is detectable in the
blood and is considered to inhibit platelet aggregation (17). Furthermore, the authors identified
what they hypothesized to be a novel function for the extracellular
HspB6 in hearts, which was as a mediator of angiogenesis through
directly interacting with VEGF receptor 2 (VEGFR-2) (11).
HspB6 is important in endothelial proliferation and
tumor growth of several types of cancer (18). However, its effects on lung cancer
and the biological function of HspB6 remain unclear (19,20).
To further address the roles of HspB6 in lung cancer, we
transplanted Lewis lung carcinoma (LLC) cells into BALB/c mice to
examine the roles of HspB6 in the processes of tumorigenesis and
metastasis in vivo. Tumor size, lymph node weight and
angiogenic factors in the tumor growth phase following implantation
were detected and compared. In the present study, we provide
definitive evidence of the importance of HspB6 in LLC implanted
tumor growth and metastasis.
Materials and methods
Reagents and antibodies
Mouse recombinant protein HspB6 was purchased from
BioChem Technology, Inc. (Shanghai, China). Alexa Fluor®
488 Annexin V/Dead Cell Apoptosis kit (cat no. V13245) and
PE-labeled swine anti-rat IgG polyclonal antibodies were purchased
from Invitrogen Life Technologies (Carlsbad, CA, USA). Rat
anti-mouse CD31 (MEC13.3) monoclonal antibodies were purchased from
BD Pharmingen (San Diego, CA, USA). Goat anti-mouse VEGF (sc-1836)
polyclonal antibodies, goat anti-mouse bFGF (C-18) polyclonal
antibodies and goat anti-mouse ICAM-1 (sc-1511) polyclonal
antibodies were purchased from Santa Cruz Biotechnology, Inc.
(Santa Cruz, CA, USA).
Cell line and mice
LLC cells, a murine NSCLC cell line, was purchased
from Shanghai Biotechnology (Shanghai, China). Cells were
maintained in DMEM (Sigma-Aldrich, St. Louis, MO, USA) supplemented
with 10% FCS. BALB/c mice weighing 20–25 g were kindly supplied by
the Immunology Laboratory of Soochow University (Suzhou, China) and
were kept in our animal facility under specific pathogen-free
conditions. All animal experiments were approved by the Guideline
for the Care and Use of Laboratory Animals of the Chinese Medical
Academy and the Soochow University Animal Care Committee (Suzhou,
China). Animals were kept in groups of five and fed regular
laboratory chow and water ad libitum. A 12-h day and night
cycle was maintained.
Cell culture and tumor implantation
model
LLC cells were grown in RPMI-1640 culture medium
(Invitrogen Life Technologies, Grand Island, NY, USA) containing
10% FBS, 2 mM L-glutamine, 2 mM sodium pyruvate, 20 mM HEPES, 1%
non-essential amino acids, 100 μg/ml streptomycin and 100 U/ml
penicillin. Cells were maintained at 37°C with 5% CO2.
Cells were progressively passed to larger plates and allowed to
reach ~90% confluence. Cells were harvested by trypsinization,
washed with HBSS buffer (Invitrogen Life Technologies) three times
and resuspended at a density of 1.0×107 cells/ml in
serum-free HBSS buffer. LLC cells (1×106) in 100 μl HBSS
buffer were injected subcutaneously into 8-week-old male BALB/c
mice. Tumor growth was assessed on days 6, 8, 10, 14, 16 and 18
after LLC cells injection by the measurement of two bisecting
diameters in each tumor using calipers. The size of the tumor was
determined by direct measurement of the tumor dimensions. The
volume was calculated according to the equation: V =
(LxW2) × 0.5, where V=volume, L=length and W=width
(21). On day 14 following tumor
implantation, the mice were anesthetized and sacrificed by
dislocation of the cervical spine, and the tumor tissues were
dissected and weighed.
Macroscopic assessments of cervical lymph
node metastasis
The mice were sacrificed on day 14 following the
implantation of LLC cells, and cervical tissues surrounding tumor
masses were excised en bloc. The nearby cervical lymph nodes
were removed and their weights were measured immediately.
Quantitative polymerase chain reaction
(qPCR)
Total RNA was extracted from LLC cells using an
RNeasy Mini kit (Qiagen, Tokyo, Japan). The resultant RNA
preparations were further treated with ribonuclease-free
deoxyribonuclease (DNase) I (Invitrogen Life Technologies Inc.,
Gaithersburg, MD, USA) to remove genomic DNA. Total RNA (2 μg) was
reverse-transcribed at 42°C for 1 h in 20 μl of reaction mixture
containing mouse Moloney leukemia virus reverse transcriptase and
hexanucleotide random primers (Qiagen). The PCR solution contained
2 μl of cDNA, the specific primer set (0.2 μM final concentration)
and 12.5 μl of SYBR Premix Ex Taq™ (SYBR Premix Ex Taq Perfect
Real-Time PCR kit; Takara, Bio, Inc., Shiga, Japan) in a final
volume of 25 μl. qPCR was performed using an iCycler iQ Multicolor
Real-Time PCR Detection system (170–8740; Bio-Rad, Hercules, CA,
USA). The PCR parameters were as follows: Initial denaturation at
95°C for 1 min, followed by 40 cycles of 95°C for 5 sec and 60°C
for 30 sec. The relative gene expression levels were calculated
using the 2−ΔΔCt method, where Ct represents the
threshold cycle, and GAPDH was used as a reference gene. The primer
sequences are shown in Table
I.
 | Table ISpecific sets of primers of qPCR. |
Table I
Specific sets of primers of qPCR.
| Gene | Primer sequences |
|---|
| VEGF | F:
5′-CGCCGCAGGAGACAAACCGAT-3′
R: 5′-ACCCGTCCATGAGCTCGGCT-3′ |
| bFGF | F:
5′-AAGAGCGACCCACACGTCAAAC-3′
R: 5′-GTAACACACTTAGAAGCCAGCAGCC-3′ |
| ICAM-1 | F:
5′-CCGGTCCTGACCCTGAGCCA-3′
R: 5′-ATTGGACCTGCGGGGTGGGT-3′ |
| GAPDH | F:
5′-ACCACAGTCCATGCCATCAC-3′
R: 5′-TCCACCACCCTGTTGCTGTA-3′ |
Western blot analysis
Cell lysates from dissected tumor tissues 14 days
following injection were prepared. Briefly, protein samples were
dissolved in Laemmli buffer (Bio-Rad), boiled for 5–10 min and
centrifuged for 2 min at 10,000 × g to remove insoluble materials.
Protein (30 μg per lane) was separated by SDS/PAGE (12%; Bio-Rad)
and transferred onto Immobilon-P membranes (Millipore, Bedford, MA,
USA). The inhibited membranes were probed overnight (4°C) with goat
anti-mouse VEGF, bFGF or ICAM-1 antibodies (Santa Cruz
Biotechnology, Inc.; sc-1836, 1:100), respectively, and rabbit
anti-mouse GAPDH (Santa Cruz Biotechnology, Inc.; N-21, 1:200).
Subsequently, the membranes were incubated with secondary
horseradish peroxidase-conjugated antibody and immunoreactive bands
were visualized using ECL reagent (Pierce Biotechnology, Inc.,
Rockford, IL, USA). Immunoreactive bands corresponding to target
proteins were quantified by Image J analysis (http://rsb.info.nih.gov/ij/download.html) and
normalized to those of GAPDH. Blots are representative of at least
three experiments.
Flow cytometric analysis of CD31-positive
and apoptotic cells
Tumor tissues dissected from BALB/c mice were minced
with scissors and were homogenized using a homogenizer in RPMI-1640
medium. Cell suspensions were then passed over a nylon filter with
a 100-μm pore size. The resultant cells were further stained with
rat anti-mouse CD31 mAb followed by staining with PE-conjugated
swine anti-rat IgG mAb. Fluorescence intensities were determined
using a FACSCalibur (Becton-Dickinson, Franklin Lakes, NJ, USA),
together with the samples stained with non-immunized swine IgG as
an isotype control. In the apoptosis assay, cell suspensions were
washed in cold phosphate-buffered saline (PBS) in the absence of an
inducing agent. The resultant cells were re-centrifuged and the
supernatant was discarded. Cell pellets were resuspended in 100
μl/test annexin-binding buffer. Alexa Fluor® 488 annexin
V (5 μl; component A) and 1 μl 100 μg/ml of PI working solution
were added to each 100-μl cell suspension followed by incubation at
room temperature for 15 min. Following the incubation period, 400
μl of 1X annexin-binding buffer was added, gently mixed and kept on
ice. As soon as possible, the stained cells were analyzed by a flow
cytometry calibur.
Wound scratch assays
In order to explore the effects of HspB6 on the
migration of LLC cells, wound scratch assays were performed. The
assay was simple and inexpensive and the experimental conditions
can be easily modified for different purposes. In brief, cells were
seeded into six-well plates at a density that, following 24 h of
growth, reached 70–80% confluence as a monolayer. The monolayer was
gently, slowly and perpendicularly scratched with a new 1-ml
pipette tip across the center of the well. The resulting gap
distance was therefore equal to the outer diameter of the end of
the tip. Following this, the wells were gently washed twice with
medium to remove the detached cells. Subsequently, the wells were
replenished with fresh medium. Cells were treated with 1 μg/ml of
human recombinant HspB6 in experimental wells and PBS in control
wells. Cells were grown for an additional 48 h and images of the
cells were captured on a microscope (BX43; Olympus Imaging,
Shinjuku, Tokyo, Japan) at 0, 12, 24 and 48 h. The gap distance was
quantitatively evaluated using Image J software. Each experimental
group was repeated multiple times.
Determination of the rate of cell
proliferation
The rate of proliferation was determined using Cell
Counting Kit 8 (CCK8; Dojindo Laboratories, Kumamoto, Japan). Cells
(5×103 cells per well) were incubated in 96-well plates
and maintained in complete medium 24 h following HspB6 stimulation.
After 48, 96 and 120 h, 10 μl of sterile CCK8 dye was added to the
cells and incubated for 4 h at 37°C. Spectrometric absorbance at a
wavelength of 450 nm was measured on an enzyme immunoassay analyzer
(ELx-800; Molecular Devices, Sunnyvale, CA, USA) following 4 h of
incubation. Experiments were performed at least three times, with
six replicate measurements, and data are presented as the mean
optical density ± standard deviation.
Statistical analysis
The means and standard error of the mean were
calculated for all parameters determined in the study. Data were
analyzed statistically using one-way analysis of variance or
two-tailed Student’s t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Tumor growth and angiogenesis is enhanced
in HspB6-injected groups
To investigate the effects of HspB6 in tumor growth,
we firstly grafted LLC cells subcutaneously into HspB6-injected
group and control group mice, and evaluated the development of
solid tumors surrounding the injection sites. The tumors in the
HspB6-injected groups were significantly larger in size than those
in the control groups after day 8. On day 14 following
implantation, the mean tumor mass in HspB6-injected groups was
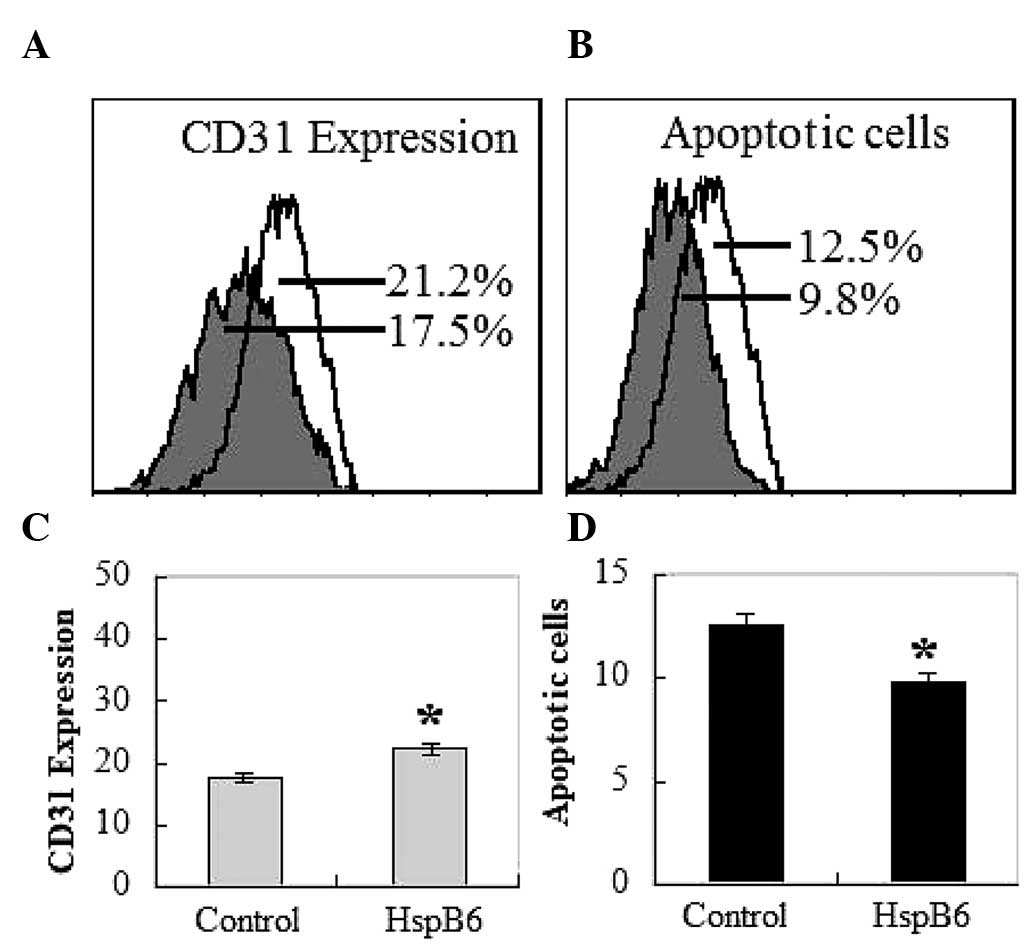
increased to almost double of that in the control mice (Fig. 1A and B). Flow cytometry examination
of the xenografts revealed that the vascularization of tumor
tissues was markedly increased by intraperitoneal injection of
HspB6 (Fig. 2A and C). CD31
expression of vascular endothelial cells was ~25% higher in tumors
grafted in the HspB6-injected groups than those grafted in the
control groups (Fig. 2C).
Tumor metastasis is enhanced in the
HspB6-injected groups
To examine the effects of HspB6 in tumor metastasis,
cervical lymph nodes surrounding implanted tumor masses were
dissected and weighed. We revealed that the weights of the cervical
lymph nodes were markedly increased in HspB6-injected groups
implanted with LLC cells in comparison with those of the nodes in
the control mice. (Fig. 3A). The
subcutaneous implantation of LLC cells in HspB6-injected groups was
demonstrated to result in enhanced metastatic growth in the
cervical lymph nodes combined with promoting the tumor volume at
the implanted site.
HspB6 reduces cell apoptosis
To rule out the possibility that the inhibition of
cell growth was due to cytotoxic effects, an apoptosis assay was
performed. Cells positive for Annexin V-FITC and negative for PI
are in the early stage of apoptosis, as shown in the Q4 quadrant,
while cells positive for Annexin V-FITC and PI are in the late
stages of apoptosis or necrosis, as shown in the Q2 quadrant
(22). Thus, the degree of
apoptosis correlates with the amount of positive Annexin V-FITC
cells. As depicted in Fig. 2B and
2D, there is little binding of Annexin V-FITC on tumor cells
implanted in the HspB6 groups compared with the tumor cells
implanted in the control mice. The binding for the control mice
group ranges from 9.7±2.5 to 12.8±3.1% on day 14. While the binding
for the HspB6 group decreased from 6.9±2.7 to 9.5±2.6% on day 14.
Combined with these observations, it is clear that the effect of
HspB6 on LLC tumors is primarily mediated by reducing apoptotic
cell death.
Angiogenic factor gene and protein
expression is increased in tumor masses implanted in HspB6
mice
The increased tumor angiogenesis caused large tumor
foci in the xenografts of HspB6 groups, however not in the control
groups. We also identified that the expression levels of VEGF, bFGF
and ICAM-1 protein and mRNA in tumor tissues were markedly higher
in HspB6 groups than in the control mice (Fig. 4A and B). The increasing result was
confirmed by quantification of the relative abundance of VEGF, bFGF
and ICAM-1 to GAPDH using a densitometer (Fig. 4A and C). These results suggest that
HspB6 is important in tumor-associated VEGF, bFGF and ICAM-1
production and the accompanying angiogenesis.
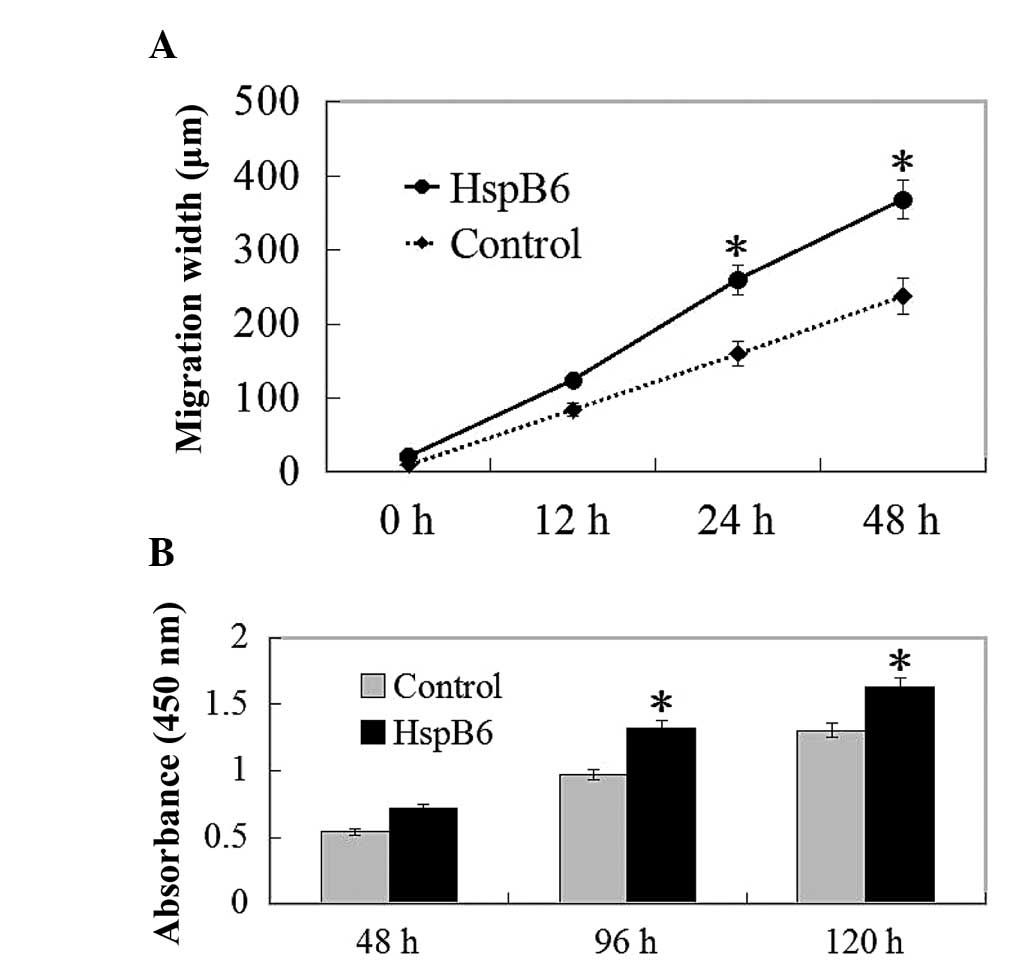
Migration restoration is induced
following wound scratching with HspB6 stimulation
In order to further delineate the effects of HspB6
on the biological function of LLCs, we next examined the roles of
HspB6 on the migration of LLC cells. LLCs efficiently seal linear
scratch wounds following 48 h of culture, and this migratory
process was clearly induced by HspB6 supplemented into the culture
medium. Cell migration into the wounded area was markedly increased
after 24 h in the HspB6 stimulation group (Fig. 5A). These data indicated that an
increase in the migration of LLCs following HspB6 stimulation was
responsible for the attenuated wound healing response.
Effects on the proliferation of LLCs
To investigate the biological result of HspB6, we
also examined the proliferation of LLCs. With the stimulation of
HspB6, the proliferation of LLCs was determined by the MTT assay.
HspB6 promotes cell proliferation, as determined by the MTT assay,
up to 24 h following stimulation with HspB6 (Fig. 5B).
Discussion
HspB6 is recognized to act intracellularly as a
molecular chaperone and confers protection against various
hazardous conditions (3,23). Zhang et al (18) revealed that extracellular HspB6
physically bound to the VEGF receptor and thereby activated the
downstream signaling pathway (Akt and ERK). As a result, myocardial
angiogenesis was enhanced in HspB6-overexpressing hearts.
Overexpression of HspB6 in the heart not only resists
stress-triggered cardiomyocyte death via the intrinsic
anti-apoptotic pathways, but also causes increased secretion of
HspB6 outside cardiomyocytes, which may function in autocrine or
paracine signaling, enhancing the survival of cardiomyocytes and
non-cardiomyocytes. Furthermore, the circulating HspB6 has been
demonstrated to bind to platelets and inhibit their aggregation
(20,24), which may be beneficial for the
treatment of ischemic heart disease. This suggests that HspB6 may
serve as a novel cardiokine and benefits hearts at multiple levels
and that the HspB6 protein may have an advantage over VEGF in
promoting myocardial angiogenesis. In the present study, we
identified a novel role for HspB6 in facilitating LLC tumor growth
by measuring the tumor mass volume in vivo. This indicated
that implanted tumor growth of LLCs was tightly correlated with the
HspB6 protein, suggesting a possible novel biological function of
the HspB6 protein in tumors. This provides a possible therapeutic
approach of targeting HspB6 to treat lung cancer.
Lung cancer is notorious for its high probability of
lymphatic metastasis even in its early stage. A previous study
reported that nodal micrometastases were identified in up to 36% of
the resected lungs from the patients with peripheral NSCLC and the
presence of metastases to the lymph nodes has been demonstrated to
immensely reduce the survival rates (25–27).
Consequently, the development of a strategy to suppress lymphatic
metastasis appears to be critical in the treatment of lung cancer
patients. Among the numerous factors associated with the metastatic
process, innate immunity of antitumor metastasis is the first
defensive response (28). We
hypothesized that HspB6 affects LLC xenografted tumors by inducing
tumor cell growth and metastasis. The results of the present study
demonstrated that cervical lymph nodes surrounding tumor masses in
the HspB6 groups were markedly higher in weight than those in the
control mice (Fig. 3). Notably,
tumor volumes implanted in HspB6 groups were larger in size
compared with those in the control mice (Fig. 1). These findings confirm that HspB6
is crucial in initiating the metastatic process of implanted LLC
tumor cells in vivo and concomitantly suggesting that they
also induce the proliferation of tumor cells.
We also revealed that the mRNA and protein
expression of potent angiogenic factors, VEGF, bFGF and ICAM-1,
were significantly more enhanced in LLC-implanted tumor tissues in
HspB6 groups compared with the control mice. In 1971, Folkman, who
became known as the ‘father of tumor angiogenesis’, first
emphasized the importance of tumor vascularity for tumor growth
(29). He described how, if a
tumor could be stopped from growing its own blood supply, it would
wither and die. Various in vitro and in vivo studies
have since uncovered the role of VEGF as a central player in
physiological and pathological angiogenesis (30). Concomitantly, we revealed that LLC
implanted tumors exhibited increased angiogenesis in the HspB6
groups more than that in the control mice using anti-mouse CD31 mAb
by FACS. These results suggested that HspB6 may be critical in
tumor growth by upregulating the expression of VEGF, bFGF and
ICAM-1 angiogenic factors and thus increasing tumor angiogenesis.
Further exploration regarding the mechanisms by which HspB6
regulates the expression of VEGF, bFGF and ICAM-1 is required.
However, the biological function of HspB6 and its regulation of
tumor angiogenesis is an interesting topic for clinical
discussion.
We also detected the effects of HspB6 on LLC cell
migration and cell proliferation in vitro by wound
scratching and CCK-8 assays, respectively, to observe the direct
effects of HspB6 on tumor cells. The results demonstrated that LLC
migration and proliferation were induced following HspB6
stimulation. This may provide evidence that HspB6 is critical in
tumor growth by multiple pathways, including the promotion of the
expression of angiogenic factors, tumor cell migration and tumor
cell proliferation. Antagonist-like anti-HspB6 neutralizing
antibody could be used to inhibit lung tumor growth.
In summary, we demonstrated that HspB6 is critically
involved in LLC implanted tumor growth, tumor metastasis and tumor
angiogenesis. The results demonstrated that HspB6 induced tumor
growth, tumor metastasis and tumor angiogenesis. Furthermore, the
expression of VEGF, bFGF and ICAM-1, the most important of the
proangiogenic factors, was enhanced more than in the control mice
groups. Furthermore, less apoptotic cells were produced than in the
control mice groups. All these findings suggest that HspB6 could
enhance cancer growth by the synthesis pathway. Thus, our results
provide new information regarding the mechanisms by which HspB6
induces its effects on tumor growth.
Acknowledgements
This study was supported by the Suzhou Natural
Science Foundation (no. W2012FZ085).
References
|
1
|
Stanley K and Stjernswärd J: Lung cancer -
a worldwide health problem. Chest. 96:1S–5S. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Larsen JE and Minna JD: Molecular biology
of lung cancer: clinical implications. Clin Chest Med. 32:703–740.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nakagawa K, Sasaki Y, Kato S, et al:
22-Oxa-1alpha, 25-dihydroxyvitamin D3 inhibits metastasis and
angiogenesis in lung cancer. Carcinogenesis. 26:1044–1054. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thatcher N: New perspectives in lung
cancer. 4. Haematopoietic growth factors and lung cancer treatment.
Thorax. 47:119–126. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Weynants P, Marchandise FX and Sibille Y:
Pulmonary perspective: immunology in diagnosis and treatment of
lung cancer. Eur Respir J. 10:1703–1719. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Latchman DS: Heat shock proteins and
cardiac protection. Cardiovasc Res. 51:637–646. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Willis MS and Patterson C: Hold me tight:
Role of the heat shock protein family of chaperones in cardiac
disease. Circulation. 122:1740–1751. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fan GC, Chu G and Kranias EG: Hsp20 and
its cardioprotection. Trends Cardiovasc Med. 15:138–141. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fan GC and Kranias EG: Small heat shock
protein 20 (Hsp20) in cardiac hypertrophy and failure. J Mol Cell
Cardiol. 51:574–577. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Edwards HV, Cameron RT and Baillie GS: The
emerging role of HSP20 as a multifunctional protective agent. Cell
Signal. 23:1447–1454. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang X, Zhao T, Huang W, et al:
Hsp20-engineered mesenchymal stem cells are resistant to oxidative
stress via enhanced activation of Akt and increased secretion of
growth factors. Stem Cells. 27:3021–3031. 2009.PubMed/NCBI
|
|
12
|
Schmitt E, Gehrmann M, Brunet M, et al:
Intracellular and extracellular functions of heat shock proteins:
repercussions in cancer therapy. J Leukoc Biol. 81:15–27. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Calderwood SK, Mambula SS, Gray PJ Jr, et
al: Extracellular heat shock proteins in cell signaling. FEBS Lett.
581:3689–3694. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhan R, Leng X, Liu X, et al: Heat shock
protein 70 is secreted from endothelial cells by a non-classical
pathway involving exosomes. Biochem Biophys Res Commun.
387:229–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Song X and Luo Y: The regulatory mechanism
of Hsp90alpha secretion from endothelial cells and its role in
angiogenesis during wound healing. Biochem Biophys Res Commun.
398:111–117. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hecker JG and McGarvey M: Heat shock
proteins as biomarkers for the rapid detection of brain and spinal
cord ischemia: a review and comparison to other methods of
detection in thoracic aneurysm repair. Cell Stress Chaperones.
16:119–131. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Niwa M, Kozawa O, Matsuno H, et al: Small
molecular weight heat shock-related protein, HSP20, exhibits an
anti-platelet activity by inhibiting receptor-mediated calcium
influx. Life Sci. 66:PL7–PL12. 2000.PubMed/NCBI
|
|
18
|
Zhang X, Wang X, Zhu H, et al: Hsp20
functions as a novel cardiokine in promoting angiogenesis via
activation of VEGFR2. PLoS One. 7:e327652012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Noda T, Kumada T, Takai S, et al:
Expression levels of heat shock protein 20 decrease in parallel
with tumor progression in patients with hepatocellular carcinoma.
Oncol Rep. 17:1309–1314. 2007.PubMed/NCBI
|
|
20
|
Myung JK, Afjehi-Sadat L,
Felizardo-Cabatic M, et al: Expressional patterns of chaperones in
ten human tumor cell lines. Proteome Sci. 2:82004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Srivastava S, Lundqvist A and Childs RW:
Natural killer cell immunotherapy for cancer: a new hope.
Cytotherapy. 10:775–783. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Levy EM, Roberti MP and Mordoh J: Natural
killer cells in human cancer: from biological functions to clinical
applications. J Biomed Biotechnol. 2011:6761982011.PubMed/NCBI
|
|
23
|
Hwang YJ, Kim J, Park DS and Hwang KA:
Study on the Immunomodulation Effect of Isodon japonicus Extract
via Splenocyte Function and NK Anti-Tumor Activity. Int J Mol Sci.
13:4880–4888. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kozawa O, Matsuno H, Niwa M, et al: HSP20,
low-molecular-weight heat shock-related protein, acts
extracellularly as a regulator of platelet functions: a novel
defense mechanism. Life Sci. 72:113–124. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ichinose Y, Yano T, Asoh H, et al:
Prognostic factors obtained by a pathologic examination in
completely resected non-small-cell lung cancer. An analysis in each
pathologic stage. J Thorac Cardiovasc Surg. 110:601–605. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
van Velzen E, Snijder RJ, Brutel de la
Rivière A, et al: Type of lymph node involvement influences
survival rates in T1N1M0 non-small cell lung carcinoma. Lymph node
involvement by direct extension compared with lobar and hilar node
metastases. Chest. 110:1469–1473. 1996.PubMed/NCBI
|
|
27
|
Mountain CF and Dresler CM: Regional lymph
node classification for lung cancer staging. Chest. 111:1718–1723.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Costantini C and Cassatella MA: The
defensive alliance between neutrophils and NK cells as a novel arm
of innate immunity. J Leukoc Biol. 89:221–233. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Folkman J: Tumor angiogenesis: therapeutic
implications. N Engl J Med. 285:1182–1186. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Senger DR, Galli SJ, Dvorak AM, et al:
Tumor cells secrete a vascular permeability factor that promotes
accumulation of ascites fluid. Science. 219:983–985. 1983.
View Article : Google Scholar
|