Introduction
Malignant melanoma is a serious disease arising from
melanocytes which threatens human health in China and worldwide
(1,2). The incidence and mortality rate of
malignant melanoma continues to increase at a higher rate than that
of any other type of malignancy (3,4).
Although melanoma is curable if detected at an early localized
stage, metastatic malignant melanoma has already become a
therapeutic challenge (5).
Hundreds of patients with advanced stage III or IV melanoma,
particularly those with metastatic disease, have participated in
studies of immunological therapy having failed on chemotherapy
(6). Thus, ways to prevent and
treat malignant melanoma using immunological methods are urgently
required. Vaccination has been used for centuries, causing
mortality due to infectious disease in humans to profoundly
decrease, but a number of serious global diseases with no effective
vaccines remain, including acquired immunodeficiency syndrome,
influenza, malaria and cancer. It is harder to produce effective
immune responses when using vaccines as a treatment for cancer,
compared with when using preventive cancer vaccines (7,8). The
cancer vaccine for cervical tumors is the first vaccine to prevent
human cancer. With the success of the cervical cancer vaccine, an
increasing number of researchers have been working to identify
novel and effective cancer vaccines. Thus, the aim of the present
study is to identify novel vaccines that elicit stronger and more
directed antitumor immune responses.
Cancer vaccine antigens include purified or
recombinant proteins or peptides. They are frequently poorly
immunogenic and require an effective adjuvant to help elicit
protective immune responses based on antibodies or activated T
cells (9–11). Polyinosinic-cytidylic acid (poly
I:C) is a synthetic double-stranded RNA that has been used as an
adjuvant (12). Poly I:C can act
with distinct types of pathogen recognition receptors, which bind
to toll-like receptor 3 (TLR3) or activate cytosolic RNA helicases,
including retinoic acid-inducible gene 1 (RIG-I) and melanoma
differentiation-associated gene 5 (MDA5) (13,14).
Therefore, activation of TLR3 and MDA5 could trigger effective
inflammatory responses. Rapid innate immunity would be elicited and
the magnitude and durability of type-1 T helper (Th1) cell immunity
and CD8+ T cell immunity would be optimized compared
with either pathway alone (15–18).
Therefore, poly I:C was selected as the adjuvant for cancer
vaccines in the present study.
The use of animal models is important in the study
of malignant melanoma (19). The
metastatic B16 mouse melanoma cell line originates from C57BL/6
mice and has a high metastatic frequency that easily mimics
clinical metastatic melanoma (20). Thus, C57BL/6 mice bearing B16
melanoma were used as a mouse model for malignant melanoma in the
present study. B16 melanoma lysates were used as the antigen
combined with effective Th1 response-related poly I:C as an
adjuvant in the cancer vaccine, which could effectively elicit the
innate and adaptive immune responses. The objective of this study
was to explore the role and identify the effectiveness of in
vivo vaccination with B16 cell lysates on tumors in the mouse
model. The study may aid the development of a vaccine for malignant
melanoma and provide novel therapeutic ideas for this currently
untreatable disease.
Materials and methods
Cell line
The B16 melanoma cell line was maintained in our
laboratory at the Chinese PLA General Hospital (Beijing, China) and
cultured in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen
Life Technologies, Carlsbad, CA, USA) with 10% fetal bovine serum
(FBS; Thermo Trace Ltd, Melbourne, Australia) at 37°C in an
atmosphere of 95% air and 5% CO2.
Mice
Male 6–8-week-old C57BL/6 mice were purchased from
Vital River Biotechnology Co., Ltd. (Beijing, China). Animals were
maintained in micro-isolator cages in specific pathogen-free
conditions. They were handled under aseptic conditions following a
protocol approved by the Institutional Animal Care and Use
Committee of the Chinese PLA General Hospital (Beijing, China). All
studies were approved by the Animal Study Committee of the Chinese
PLA General Hospital.
Animal grouping and immunization
The mice were randomly divided into three groups.
Each group contained more than six mice. Animals were injected
intraperitoneally twice on days 1 and 15, with 50 μg B16 cell
lysate antigen or 50 μg B16 cell lysate plus 50 μg poly I:C, 1.5%
Al(OH)3 or with PBS. After the final immunization, the
C57BL/6 mice were inoculated intraperitoneally with
1×105 melanoma cells suspended in 100 μl PBS.
Tumor cell lysate
B16 cells were collected and washed three times with
phosphate-buffered saline (PBS) buffer. Eight snap freeze-thaw
cycles between liquid N2 and 37°C were conducted. The
cells were centrifuged at 500 × g to obtain the lysate, which was
then filtered with a 70-mm Falcon filter (BD Biosciences,
Erembodegen, Belgium). Coomassie blue staining method was used to
measure the amount of protein and was performed according to the
manufacturer’s instructions (Benda Biotechnology, Co., Shanghai,
China). The lysate was separated and kept frozen in liquid
N2 until required.
Splenocyte proliferation assay
Cell proliferation levels were determined using the
MTT assay (21). One week after
the final immunization, single-cell suspensions from the mice in
each group were prepared under sterile conditions. Red blood cells
(RBCs) were lysed using lysis buffer containing 0.75%
NH4Cl in Tris-buffer. Cell concentrations were adjusted
to 3×106 cells/ml in DMEM supplemented with 10% FBS.
Samples (100 μl) of the suspensions were dispensed into 96-well
round-bottom culture plates (Costar, Tewksbury, MA, USA) and
incubated with 10 μg/ml B16 melanoma cell lysate for 48 h at 37°C
in a 5% CO2 humid incubator.
Quantitative PCR analysis of mRNA
expression
Splenocytes from the immunized mice were cultured in
six-well plates for 24 h at 37°C in the presence of 5%
CO2, with or without 10 μg/ml B16 cell lysate. Total RNA
was extracted with an RNApure kit (Bioteke, Beijing, China) and
retrotranscribed with murine leukemia virus (MLV) reverse
transcriptase (RT) (Invitrogen Life Technologies). PCR
amplifications were performed using a 7500 Real-Time PCR system
(Applied Biosystems, Foster City, CA, USA) and each sample was
tested in triplicate. Thermal cycling conditions were 40 cycles of
12 sec at 95°C and 1 min at 60°C using SYBR-Green (Invitrogen Life
Technologies). β-actin was used as the internal reference gene. The
primers used were as follows: Interferon-γ (IFN-γ),
5′-CAGCAACAGCAAGGCGAAA-3′ and 5′-CTGGACCTGTGGGTTGTTGAC-3′; β-actin,
5′-AGAGGGAAATCGTGCGTGAC-3′ and 5′-CAATAGTGATGACCTGGCCGT-3′.
Fluorescence-activated cell sorter (FACS)
analysis
Seven days after the final immunization, single cell
suspensions were performed for spleen T-cell subtype analysis. RBCs
from 50 μl heparin-treated orbital blood were lysed with RBC lysis
buffer (eBioscience, San Diego, CA, USA). Lymphocytes were stained
with 100 μl PBS plus 1% bovine serum albumin and 0.1%
NaN3 together with 5 μl fluorescein
isothiocyanate-conjugated anti-CD3 monoclonal antibody (mAb)
followed by simultaneous staining with 5 μl phycoerythrin anti-CD4
or anti-CD8 mAb, and then incubated for 20 min at 4°C. Flow
cytometry was performed using CellQuest software and a FACScan flow
cytometer (Becton Dickinson, San Jose, CA, USA), with FlowJo
software (Tree Star Inc., Ashland, OR, USA) used for data
analysis.
Statistical analysis
Survival curves of the animals treated with
different protocols were plotted according to the Kaplan-Meier
method. Statistical significance in different treatment groups was
compared using the log-rank test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Antigen-specific splenocytes proliferate
in mice immunized with B16 cell lysates plus poly I:C
In order to detect whether the splenocytes of the
mice immunized with B16 cell lysates plus poly I:C had an elevated
antigen-specific proliferation rate, the cell numbers were measured
by MTT assay. Ten days after the final immunization, spleens from
mice in each group were removed and the rate of splenocyte
proliferation was measured. After in vitro stimulation with
B16 lysates for 24 h, the splenocytes from the mice immunized with
B16 lysates in combination with poly I:C were significantly more
numerous than those in either control group (P<0.01; Fig. 1). The cells treated with
Concanavalin A (ConA) were used as positive controls and untreated
cells were used as negative controls.
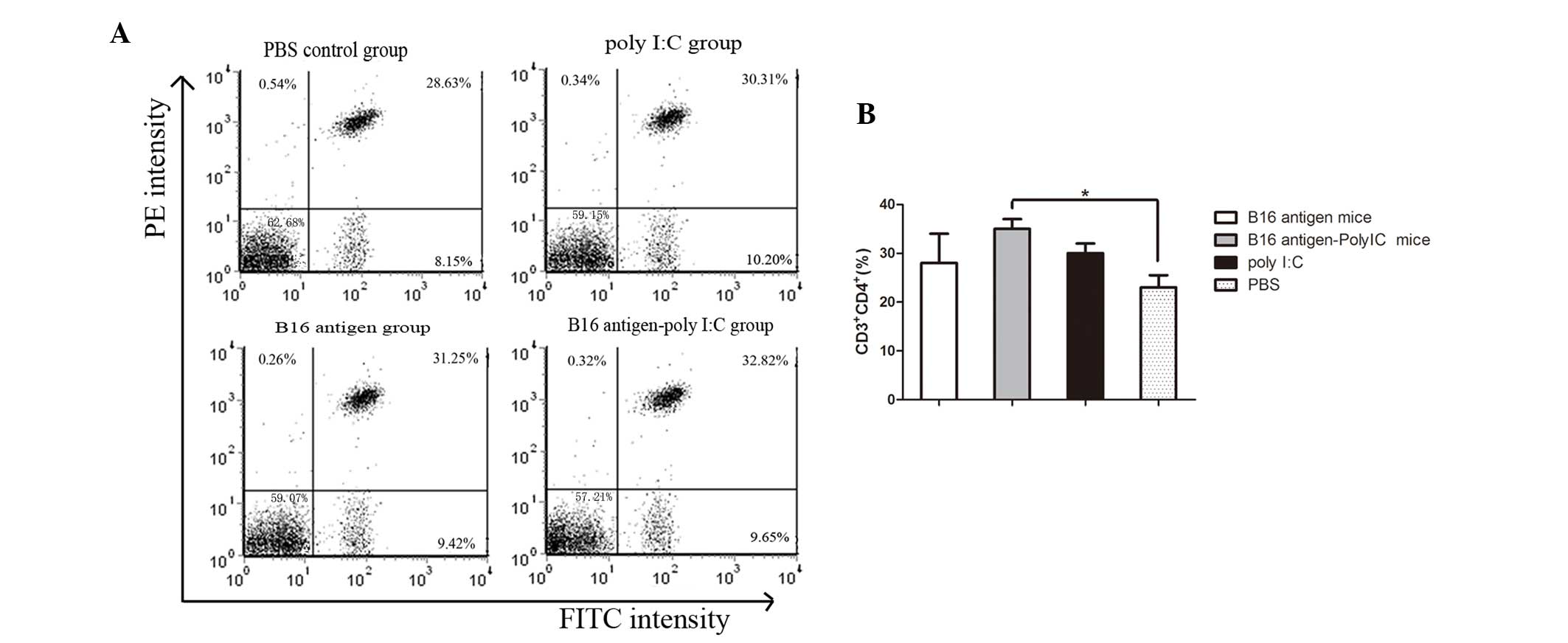
Number of CD4+CD3+
T lymphocytes and CD8+CD3+ T lymphocytes
increases in mice immunized with B16 cell lysate plus poly I:C
T-cell subsets were also analyzed. The percentages
of T helper cells (CD4+CD3+ T lymphocytes)
and cytotoxic T cells (CD8+CD3+ T
lymphocytes) were determined by flow cytometry. As shown in
Fig. 2, the mice immunized with
B16 cell lysate plus poly I:C contained a higher percentage of
CD3+CD4+ T lymphocytes in their peripheral
blood than that of the mice injected with PBS. In addition, the
frequency of CD8+CD3+ T lymphocytes in the
peripheral blood appeared to increase in the mice immunized by B16
cell lysates plus poly I:C compared with that of the mice injected
with PBS (Fig. 3).
IFN-γ expression determined by qPCR and
cytokine secretion measured by ELISA
C57BL/6 mice were immunized twice with B16 cell
lysates in combination with poly I:C, the antigen alone, or PBS.
Ten days after the final immunization, spleens were removed and
splenocytes of single cell suspension were prepared. In order to
compare the cell-mediated immune responses among the three groups,
splenocytes from the immunized mice were in vitro-stimulated
with B16 antigen for 24 h and IFN-γ mRNA levels were analyzed using
qRT-PCR (amplification curve shown in Fig. 4A). The mean relative IFN-γ mRNA
expression in the mice immunized with B16 cell lysate plus poly I:C
was significantly higher than that in the mice immunized with the
B16 cell lysate only (P<0.01; Fig.
4B). IFN-γ protein levels were also examined by ELISA. As
expected, the mean IFN-γ production in the mice immunized with B16
cell lysate was higher than that in the antigen-immunized group
following in vitro stimulation with B16 cell lysates
(Fig. 4C).
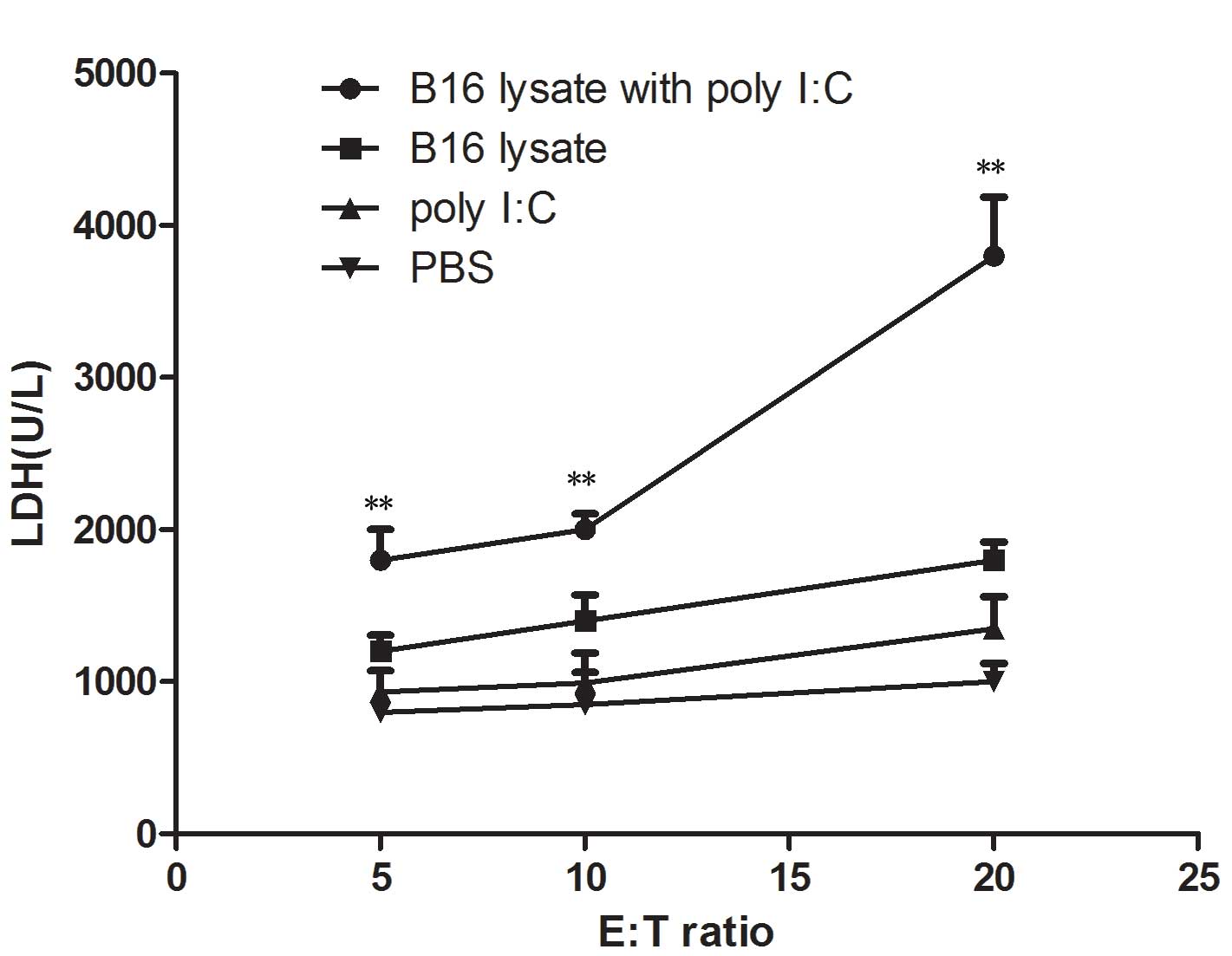
Cytotoxic T lymphocyte (CTL)
activity
To demonstrate the cytotoxic activity of splenocytes
from the immunized mice for B16 cells, the release of cytosolic
lactate dehydrogenase (LDH) into the culture medium by a damaged
B16 melanoma cell line was tested. Ten days after the final
immunization, damage to the membranes of B16 melanoma cells was
evaluated in a 24 h cytotoxicity assay by measuring LDH release.
LDH release assays were performed with splenocytes as effector
cells and B16 melanoma cells as target cells. The
effector-to-target cell ratios were 5:1, 10:1 and 20:1. As shown in
Fig. 5, the CTL response was
significantly higher in the mice immunized with B16 lysate plus
poly I:C than that in those immunized with B16 lysate alone
(P<0.01) or PBS (P<0.01). B16 melanoma cells and splenocytes
from the immunized mice did not release any LDH when measured at 24
h. These were cultured alone in DMEM medium and were used as
negative controls.
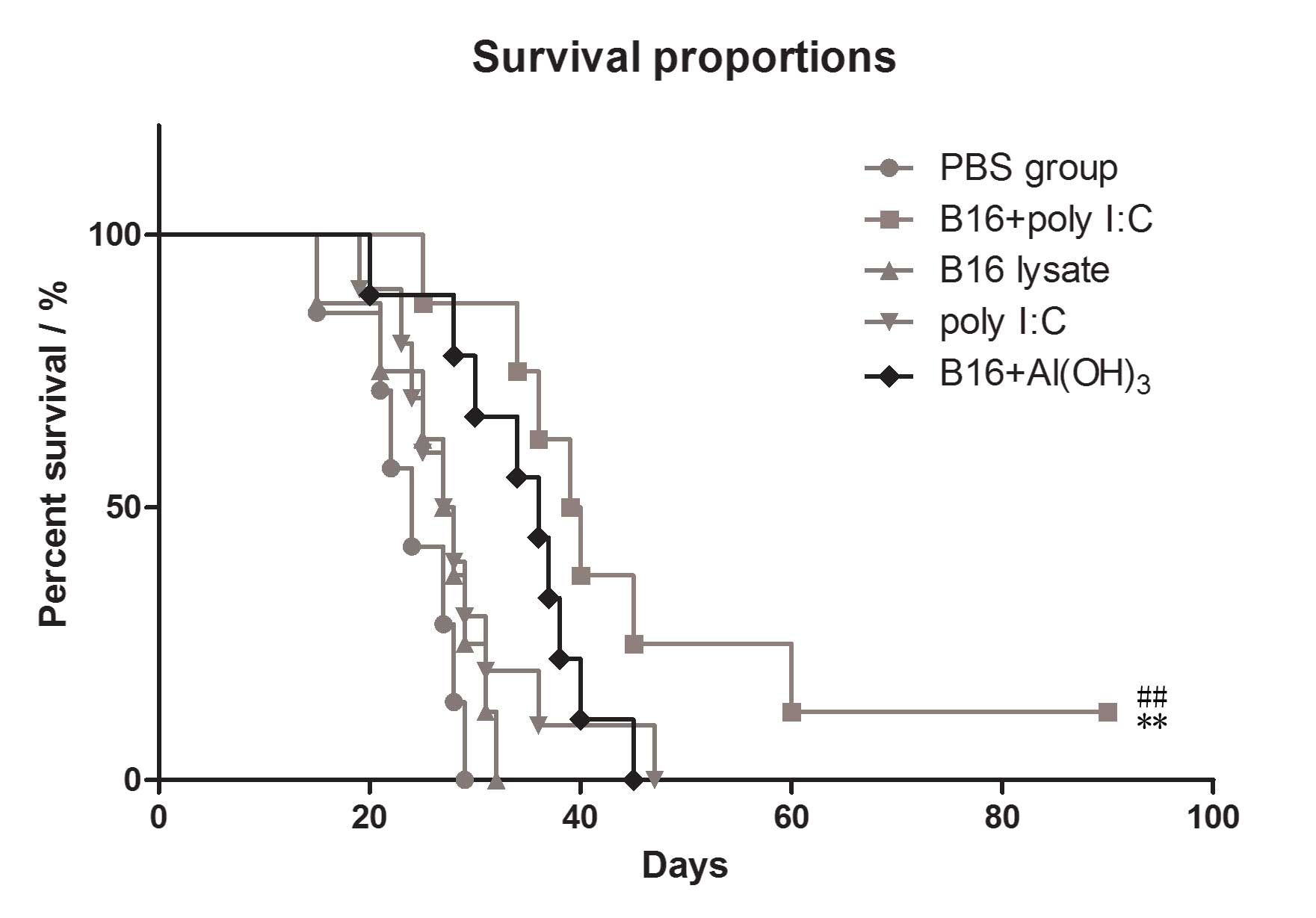
Improved antitumor effects in B16 lysate
plus poly I:C vaccinated mice compared with those in mice immunized
with B16 lysate or PBS only
To assess in vivo antitumor responses in the
immunized mice, the survival rates were evaluated in the immunized
groups. Following the final immunization, all the mice received an
intraperitoneal challenge of 1×105 B16 melanoma cells.
The results revealed that subcutaneous immunization of C57BL/6 mice
with B16 cell lysate plus poly I:C conferred improved protection
against B16 melanoma cells than did immunization with B16 cell
lysate or PBS alone. The survival rate of the B16 plus poly I:C
group was significantly higher than that of the B16 lysate group
and PBS group (P=0.029 vs. B16 lysate group, P=0.003 vs. PBS
group), as shown in Fig. 6. Also
the antitumor effects in the mice immunized by B16 antigen plus
poly I:C were significantly greater than those in mice immunized by
B16 antigen plus Al2(OH)3, which is used as
the positive adjuvant in market.
Discussion
Cancer vaccines have been studied for several
decades and are intended either to prevent the development of
cancer or to treat existing cancers (22–24).
However, advances in this field have been slower than those in
other forms of immunotherapy (24–26).
In order to overcome the poor immunogenicity of tumors,
administration of tumor antigens with an effective adjuvant is
theoretically a good strategy. The adjuvant could be a molecule
that is able to activate dendritic cells (DCs) and induce potent
antitumor T-cell immune responses (27). Ligands of toll-like receptors
(TLRs) are the best candidates to activate DCs and can lead to DC
maturation. Thus, with the aim of inducing potent antitumor T-cell
responses, poly I:C, the ligand of TLR3, was selected as the
adjuvant of the tumor antigen to strongly activate DCs and
facilitate T-cell priming in the present study (14,26,28,29).
The results of the study clearly demonstrate that poly I:C was an
effective adjuvant for B16 cell lysates and successfully induced
effective antitumor immune responses.
It is reported that antitumor activity requires the
participation of CD3+CD4+ and
CD3+CD8+ T lymphocytes (30,31).
Th1 cells exhibit a critical role in cellular immunity by releasing
cytokines that activate CD8+ T cells. Thus, activation
of CD4 T helper cells is an important step for the priming of
memory CTL responses. CD8+ T cells are the main effector
cells with CTL activity, however the main cells producing cytokines
are CD4+ Th1 cells, including interleukin-2, IFN-γ and
tumor necrosis factor-α. In the present study, the antigen-specific
Th1 responses and CTL response after the final immunization in
different groups of mice were measured. Supporting the idea that
the induction of IFN-γ suggests polarization towards the Th1
response, the group of mice immunized with B16 cell lysate in
combination with poly I:C produced increased levels of IFN-γ and
specific CTL activity when stimulated in vitro with B16
melanoma cell lysates. Increased levels of IFN-γ and CTL activity
contributed to the observed antitumor effect in the mice immunized
with B16 cell lysate plus poly I:C. In addition, this was
consistent with the potent antigen-specific antitumor immunity
previously observed in the murine B16 melanoma challenge model
(32–34). The survival rate of the mice
immunized with B16 cell lysate in combination with poly I:C was
significantly higher than that of the group immunized with B16
antigen or PBS only. It was found in previous studies that
immunization with B16 plus poly I:C was able to fully protect mice
in prophylactic vaccination experiments, not only in the short-term
but also in the long-term (35–37).
Although the B16 melanoma cell lysate was used as a
cancer antigen to assess the antitumor effects of the cancer
vaccine in the current study, it is reasonable to hypothesize that
poly I:C could confer adjuvant properties when used in combination
with a variety of viral antigenic peptides or tumor-specific
antigens. In addition, the findings of this study imply that the
adjuvant poly I:C may be useful for eliciting immune responses or
breaking immune tolerance in cases of spontaneous tumors as well as
in cases of infections caused by the hepatitis B virus, the human
papillomavirus, and the human immunodeficiency virus.
In conclusion, in vivo experiments with the
mouse model in the present study demonstrated that the mice that
received B16 cell lysate plus poly I:C exhibited enhanced antitumor
prophylactic and therapeutic efficacy, which was associated with
increased IFN-γ production and induction of cytotoxic T lymphocyte
activity. It is hypothesized that this strategy could be useful for
the treatment of malignant tumors and metastasis.
References
|
1
|
Lee C, Collichio F, Ollila D and Moschos
S: Historical review of melanoma treatment and outcomes. Clin
Dermatol. 31:141–147. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen LL, Jaimes N, Barker CA, Busam KJ and
Marghoob AA: Desmoplastic melanoma: a review. J Am Acad Dermatol.
68:825–833. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vourc’h-Jourdain M, Martin L and Barbarot
S; aRED. Large congenital melanocytic nevi: therapeutic management
and melanoma risk: a systematic review. J Am Acad Dermatol.
68:493–498. 2013.PubMed/NCBI
|
|
4
|
Ma C and Armstrong AW: Severe adverse
events from the treatment of advanced melanoma: a systematic review
of severe side effects associated with ipilimumab, vemurafenib,
interferon alfa-2b, dacarbazine and interleukin-2. J Dermatolog
Treat. 25:401–408. 2014. View Article : Google Scholar
|
|
5
|
Singh S, Nagpal SJ, Murad MH, et al:
Inflammatory bowel disease is associated with an increased risk of
melanoma: a systematic review and meta-analysis. Clin Gastroenterol
Hepatol. 12:210–218. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gogas H, Polyzos A and Kirkwood J:
Immunotherapy for advanced melanoma: fulfilling the promise. Cancer
Treat Rev. 39:879–885. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Engell-Noerregaard L, Hansen TH, Andersen
MH, Thor Straten P and Svane IM: Review of clinical studies on
dendritic cell-based vaccination of patients with malignant
melanoma: assessment of correlation between clinical response and
vaccine parameters. Cancer Immunol Immunother. 58:1–14. 2009.
View Article : Google Scholar
|
|
8
|
Zhang S, Wang Q and Miao B: Review:
dendritic cell-based vaccine in the treatment of patients with
advanced melanoma. Cancer Biother Radiopharm. 22:501–507. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Harris RC, Chianese-Bullock KA, Petroni
GR, et al: The vaccine-site microenvironment induced by injection
of incomplete Freund’s adjuvant, with or without melanoma peptides.
J Immunother. 35:78–88. 2012.PubMed/NCBI
|
|
10
|
Cho DY, Yang WK, Lee HC, et al: Adjuvant
immunotherapy with whole-cell lysate dendritic cells vaccine for
glioblastoma multiforme: a phase II clinical trial. World
Neurosurg. 77:736–744. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang ZY, Xing Y, Liu B, et al: Protective
antitumor immunity induced by tumor cell lysates conjugated with
diphtheria toxin and adjuvant epitope in mouse breast tumor models.
Chin J Cancer. 31:295–305. 2012. View Article : Google Scholar
|
|
12
|
Cui Z and Qiu F: Synthetic double-stranded
RNA poly(I:C) as a potent peptide vaccine adjuvant: therapeutic
activity against human cervical cancer in a rodent model. Cancer
Immunol Immunother. 55:1267–1279. 2006. View Article : Google Scholar
|
|
13
|
Kato A, Truong-Tran AQ, Scott AL,
Matsumoto K and Schleimer RP: Airway epithelial cells produce B
cell-activating factor of TNF family by an IFN-beta-dependent
mechanism. J Immunol. 177:7164–7172. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Inao T, Harashima N, Monma H, et al:
Antitumor effects of cytoplasmic delivery of an innate adjuvant
receptor ligand, poly(I:C), on human breast cancer. Breast Cancer
Res Treat. 134:89–100. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rajan JV, Warren SE, Miao EA and Aderem A:
Activation of the NLRP3 inflammasome by intracellular poly I:C.
FEBS Lett. 584:4627–4632. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Trumpfheller C, Caskey M, Nchinda G, et
al: The microbial mimic poly IC induces durable and protective
CD4+ T cell immunity together with a dendritic cell
targeted vaccine. Proc Natl Acad Sci USA. 105:2574–2579. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wörnle M, Sauter M, Kastenmüller K, et al:
Novel role of toll-like receptor 3, RIG-I and MDA5 in poly (I:C)
RNA-induced mesothelial inflammation. Mol Cell Biochem.
322:193–206. 2009.
|
|
18
|
Longhi MP, Trumpfheller C, Idoyaga J, et
al: Dendritic cells require a systemic type I interferon response
to mature and induce CD4+ Th1 immunity with poly IC as
adjuvant. J Exp Med. 206:1589–1602. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bose A, Lowe DB, Rao A and Storkus WJ:
Combined vaccine+axitinib therapy yields superior antitumor
efficacy in a murine melanoma model. Melanoma Res. 22:236–243.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kayaga J, Souberbielle BE, Sheikh N, et
al: Anti-tumour activity against B16-F10 melanoma with a GM-CSF
secreting allogeneic tumour cell vaccine. Gene Ther. 6:1475–1481.
1999. View Article : Google Scholar
|
|
21
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival: application to proliferation and
cytotoxicity assays. J Immunol Methods. 65:55–63. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kirkwood JM, Moschos S and Wang W:
Strategies for the development of more effective adjuvant therapy
of melanoma: current and future explorations of antibodies,
cytokines, vaccines, and combinations. Clin Cancer Res.
12:2331s–2336s. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kirkwood JM, Strawderman MH, Ernstoff MS,
Smith TJ, Borden EC and Blum RH: Interferon alfa-2b adjuvant
therapy of high-risk resected cutaneous melanoma: the Eastern
Cooperative Oncology Group Trial EST 1684. J Clin Oncol. 14:7–17.
1996.PubMed/NCBI
|
|
24
|
Terando A, Sabel MS and Sondak VK:
Melanoma: adjuvant therapy and other treatment options. Curr Treat
Options Oncol. 4:187–199. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Krishnan L, Deschatelets L, Stark FC,
Gurnani K and Sprott GD: Archaeosome adjuvant overcomes tolerance
to tumor-associated melanoma antigens inducing protective CD8 T
cell responses. Clin Dev Immunol. 2010:5784322010. View Article : Google Scholar
|
|
26
|
Mechl Z and Kopecný J: Current results
with surgery and adjuvant chemotherapy in malignant melanoma. Arch
Geschwulstforsch. 56:367–371. 1986.(In German).
|
|
27
|
Olivier A, Sainz-Perez A, Dong H,
Sparwasser T, Majlessi L and Leclerc C: The adjuvant effect of TLR
agonists on CD4(+) effector T cells is under the indirect control
of regulatory T cells. Eur J Immunol. 41:2303–2313. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang YK, Zheng Z, Cheng CX, Wang LY, Li
YR and Qiu F: The antitumor effect of the toll-like receptor 3
ligand polyinosinic-cytidylic acid as an adjuvant. Cancer Immunol
Immunother. 62:237–244. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Martínez-Gil L, Goff PH, Hai R,
García-Sastre A, Shaw ML and Palese P: A Sendai virus-derived RNA
agonist of RIG-I as a virus vaccine adjuvant. J Virol.
87:1290–1300. 2013.PubMed/NCBI
|
|
30
|
Wang S, Du W, Zhang H, et al: Biological
characteristics and antitumor activity of CIK cells activated by
recombinant human fibronectin for human lung cancer cell lines in
vitro. Zhongguo Fei Ai Za Zhi. 13:277–281. 2010.PubMed/NCBI
|
|
31
|
Starska K, Głowacka E, Kulig A,
Lewy-Trenda I, Bryś M and Lewkowicz P: The role of tumor cells in
the modification of T lymphocytes activity - the expression of the
early CD69+, CD71+ and the late
CD25+, CD26+, HLA/DR+ activation
markers on T CD4+ and CD8+ cells in squamous
cell laryngeal carcinoma. Part I Folia Histochem Cytobiol.
49:579–592. 2011.PubMed/NCBI
|
|
32
|
Török L: Adjuvant interferon treatment of
melanoma. Magy Onkol. 47:105–107. 2003.(In Hungarian).
|
|
33
|
Mitchell MS, Kan-Mitchell J, Kempf RA,
Harel W, Shau HY and Lind S: Active specific immunotherapy for
melanoma: phase I trial of allogeneic lysates and a novel adjuvant.
Cancer Res. 48:5883–5893. 1988.PubMed/NCBI
|
|
34
|
Jasani B, Navabi H and Adams M: Ampligen:
a potential toll-like 3 receptor adjuvant for immunotherapy of
cancer. Vaccine. 27:3401–3404. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
May M, Kendel F, Hoschke B, et al:
Adjuvant autologous tumour cell vaccination in patients with renal
cell carcinoma. Overall survival analysis with a follow-up period
in excess of more than 10 years. Urologe A. 48:1075–1083. 2009.(In
German).
|
|
36
|
May M, Brookman-May S, Hoschke B, et al:
Ten-year survival analysis for renal carcinoma patients treated
with an autologous tumour lysate vaccine in an adjuvant setting.
Cancer Immunol Immunother. 59:687–695. 2010.
|
|
37
|
Markowicz S, Nowecki ZI, Rutkowski P, et
al: Adjuvant vaccination with melanoma antigen-pulsed dendritic
cells in stage III melanoma patients. Med Oncol. 29:2966–2977.
2012. View Article : Google Scholar : PubMed/NCBI
|