Introduction
Lung cancer is the leading cause of
cancer-associated mortality worldwide and non-small cell lung
cancer (NSCLC) represents ~80–85% of all lung cancers (1). Unfortunately, the majority of
patients are diagnosed at an advanced stage, when drug therapy is
the main treatment option. Although systemic chemotherapy improves
the lung cancer survival rate, treatment outcomes for advanced
NSCLC remain unsatisfactory.
Epidermal growth factor receptor (EGFR) belongs to
the ErbB family of plasma membrane receptor tyrosine kinases and is
unduly expressed in numerous cancer types, including 40–80% of
NSCLCs (2,3). Patients whose tumors harbor
somatic-activating mutations within the EGFR gene greatly benefit
from treatment with EGFR-tyrosine kinase inhibitors (EGFR-TKIs)
(4). The recent development of
anticancer drugs that target EGFR has improved the survival rates
and triggered a paradigm shift for the treatment of NSCLC patients
(5,6).
However, all patients with EGFR mutant tumors will
inevitably develop progressive disease following 10–14 months of
EGFR-TKI treatment, a concept referred to clinically as acquired
resistance (7). Currently, novel
treatment strategies are urgently required to overcome acquired
resistance to EGFR-TKIs. The combination of continued EGFR-TKIs
with concurrent chemotherapy has been proposed as a strategy to
overcome acquired resistance (8).
A recent retrospective analysis demonstrated improved response
rates (RR) when EGFR-TKIs were continued with chemotherapy,
following evident disease progression on the TKIs alone (9). Additionally, several strategies
combining chemotherapy with continued EGFR-TKIs at the time of
progression have been put forward.
Preclinical and clinical studies suggested that the
synergy of targeted agents with conventional cytotoxic agents may
not only have lower toxicity but also be an improved, more
effective cancer treatment option (10). Gefitinib, erlotinib, docetaxel and
pemetrexed have been widely used for second-line treatment of
advanced NSCLC. Gefitinib and pemetrexed have distinctly improved
tolerability and lower toxicity compared with docetaxel, and their
use has promptly increased as a result (11,12).
Gefitinib is a tyrosine kinase inhibitor (TKI) that
inhibits the EGFR through competitive inhibition of the ATP-binding
site in the tyrosine kinase domain (13). It has been approved for its
clinical benefit in the treatment of advanced NSCLC patients who
have previously received platinum-based chemotherapy (14). It inhibits the activation of ERK
and AKT and induces growth arrest of tumor cells (15). Gefitinib causes cell growth arrest
in G0/G1 phase and induces apoptosis in a variety of tumor cell
types (16,17). EGFR-TKIs have demonstrated
promising synergistic effects in combination with various cytotoxic
drugs against various human tumor types in vitro and in
vivo (18–20).
Pemetrexed is a multitargeted antifolate cytotoxic
agent which potently inhibits multiple key enzymes, including
thymidylate synthase (TS), dihydrofolate reductase (DHFR) and
glycinamide ribonucleotide formaldehyde transferase (GARFT)
(21). Pemetrexed arrests cells
mainly in the S phase of the cell cycle and induces apoptosis in
various solid tumor types, including NSCLC (22). Phase III clinical trials
demonstrated that pemetrexed has equal efficacy and milder toxicity
and improved safety profiles compared with previous standard
cytotoxic drugs in first- and second-line treatment (23,24).
According to the above information, it was
hypothesized that the combination of an EGFR-TKI and a
chemotherapeutic agent may represent a more effective therapeutic
strategy for NSCLC patients who are resistant to EGFR-TKI therapy
alone. In the present study, the effects of gefitinib in
combination with pemetrexed were examined in gefitinib-sensitive
and gefitinib-acquired resistant NSCLC cell lines in
vitro.
Materials and methods
Drugs
Gefitinib (IRESSA) was obtained from AstraZeneca
(London, England, UK) and was dissolved in dimethylsulfoxide (DMSO;
Sigma, St. Louis, MO, USA) to a stock concentration of 10 mmol/l.
Pemetrexed (Alimta; Eli Lilly, Indianapolis, IN, USA) was obtained
commercially from the pharmacy at The Third Affiliated Hospital of
Anhui Medical University (Hefei, China) and was dissolved in 0.9%
NaCl to a final concentration of 10 mmol/l. The two drugs were
stored at −20°C in tightly sealed sterile tubes and diluted to the
desired concentrations in culture medium prior to use. The final
concentration of DMSO in Dulbecco’s modified Eagle’s medium (DMEM;
Hyclone, Logan, UT, USA) was kept at <0.1% and equal amounts of
the solvent were added to the control cells.
Cell culture
The EGFR-TKI-sensitive human NSCLC cell line PC9 and
the gefitinib-acquired-resistant cell line PC9/GR were provided by
Guangdong Lung Cancer Institute (Guangdong, China) and were
cultured in DMEM supplemented with 10% heat-inactivated fetal
bovine serum (Hyclone), penicillin (100 U/ml), streptomycin (100
μg/ml) and l-glutamine (2 mM) in a humidified atmosphere with 5%
CO2 at 37°C and then harvested with trypsin-EDTA when
the cells reached exponential growth.
Growth inhibition assay
The antiproliferative effects of gefitinib and
pemetrexed as single agents on cells were evaluated by an MTT
assay. Exponentially growing cells were seeded in 96-well plastic
plates at a density of 3,500 and 4,000 cells/well for PC9 and
PC9/GR, respectively. Cells were then cultured overnight and
treated with various concentrations of gefitinib and pemetrexed for
72 h. Following exposure to each drug for 72 h in 96-well plates,
20 μl MTT solution was added to each well and cells were incubated
for 4 h at 37°C. The colored formazan product was then dissolved by
adding 150 μl DMSO. The 96-well plates were placed on a shaker for
10 min at room temperature to thoroughly dissolve the formazan
product. Then the optical density (OD) of each well was measured at
490 nm on an ELISA plate reader (Bio-Rad Laboratories, Inc.
Winooski, VT, USA). The percentage of cell growth inhibition
resulting from each drug was calculated as: [(OD490control
cells − OD490treated cells)/OD490control
cells] × 100%. The IC50-value was the
concentration resulting in 50% cell growth inhibition by a 72 h
exposure to the drug(s) compared with the untreated control cells.
Six replicate wells were used for each drug concentration and the
testing was conducted independently at least three times.
Drug combination studies
The antiproliferative effects of the interaction
between gefitinib and pemetrexed were evaluated by measuring the
combination index (CI), a quantitative representation of
pharmacological interactions between two drugs. The combination
drug doses for constant ratios of the IC50-values were
calculated from the previous growth inhibition assay. Thus, the
CI-value was calculated using 0.125, 0.25, 0.5, 1, 2 and 4 times
the IC50 of gefitinib and pemetrexed combination doses.
The CI-values of interactions between gefitinib and pemetrexed were
analyzed according to the Chou and Talaly method using the CompuSyn
software (ComboSyn, Inc., Paramus, NJ, USA). CI <1, CI =1 and CI
>1 indicate a synergistic, additive and antagonistic effect,
respectively (25).
Cell cycle analysis by flow
cytometry
Cells (1×106/well) were plated in
six-well dishes and treated with gefitinib and pemetrexed as single
agents and in combination at the concentration of the
IC50-value for 72 h as described above. At the end of
each exposure, the adhered cells were harvested by trypsinization,
washed twice with phosphate-buffered saline (PBS) and fixed with
75% cold ethanol at 4°C overnight. Following removal of the
ethanol, the cells were washed twice in PBS and then resuspended in
1 ml of propidium iodide (PI)/Triton X-100 staining solution [PBS
containing 0.1% Triton X-100 (Sigma), 200 μg/ml RNAse A (Sigma) and
50 μg/ml PI (Sigma)] in the dark for 30 min. The cell cycle was
assessed by flow cytometry and the percentage of cells in G1, S and
G2/M phases of the cell cycle were calculated using ModFit LT™
software, version 4.0 (Verity Software House, Topsham, ME,
USA).
Flow cytometric analysis of
apoptosis
Cells in the exponential growth phase were plated in
six-well plates, allowed to attach overnight and treated with
gefitinib and pemetrexed alone or in combination using the
concentration of the IC50-values for 72 h. Following 72
h of treatment, adherent and floating cells were collected, washed
twice with pre-cooled (4°C) PBS and resuspended in 400 μl binding
buffer. Cells were first incubated with 5 μl Annexin V-fluorescein
isothiocyanate (FITC) at room temperature in the dark for 15 min
and then with 10 μl PI (40 μg/ml) at room temperature in the dark
for 5 min. Cell suspensions were transferred to flow cytometry test
tubes and detected by flow cytometry. Cells with no drug treatment
were used as a control. Data were analyzed by CellQuest software
(Becton Dickinson, San Jose, CA, USA)
Western blot analysis
Cells were seeded at a density of 6×105
in 100 mm2 dishes for 24 h prior to treatment. Following
72 h of incubation with single or double drug combinations, cells
were washed with ice-cold PBS solution and scraped in lysis buffer.
The lysates were centrifuged at 13,380 × g for 30 min at 4°C and
then the supernatant was collected. Equivalent amounts of proteins
were analyzed by SDS-PAGE and transferred to PVDF membranes.
Appropriate primary antibodies to p-AKT, AKT, phosphorylated
extracellular-signal-regulated kinase (p-ERK), ERK, B-cell lymphoma
2 (Bcl-2) and β-actin purchased from Cell Signaling Technology,
Inc. (Beverly, MA, USA) were used. Proteins were visualized with a
horseradish peroxidase-coupled secondary antibody from Cell
Signaling Technology, Inc. Positive bands were detected using
enhanced chemiluminescence reagents (Millipore, Billerica, MA,
USA). β-actin was used as a loading control. The band densities
were scanned for densitometric analysis using ImageJ 1.43 software
(NIH Image, Bethesda, MD, USA).
Statistical analysis
All data were assayed in three independent
experiments. The results were presented as the mean ± standard
deviation. Student’s t-test and one-way analysis of variance were
used to assess the statistical significance between values and a
level of P<0.05 was considered to indicate a statistically
significant difference.
Results
Dose-dependent antiproliferative effects
of gefitinib and pemetrexed in human NSCLC cell lines
The antiproliferative activity of gefitinib and
pemetrexed as single agents on PC9 and PC9/GR cells were evaluated
using an MTT assay. The cells were exposed to the varying
concentrations of gefitinib (1.25–80 nmol/l for PC9 and 0.25–16
μmol/l for PC9/GR cells) or pemetrexed (0.05–3.2 μmol/l for both
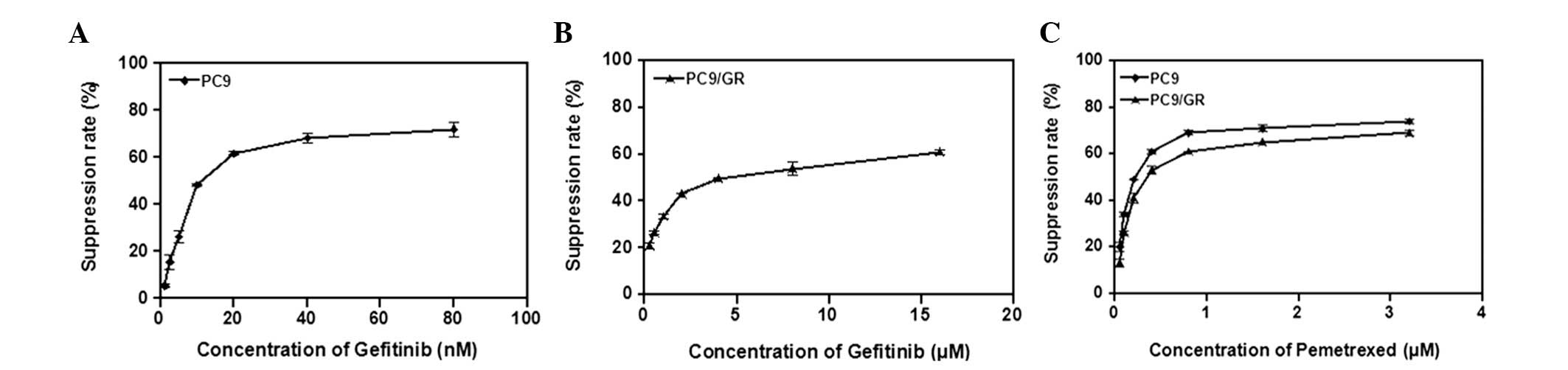
cell lines) for 72 h. Fig. 1
illustrates the growth-inhibitory effect of gefitinib on the parent
PC9 cell line and its resistant subline, PC9/GR. The
IC50-value of gefitinib in PC9 cells was 0.016 μmol/l,
as compared with 4.94 μmol/l in PC9/GR cells (306-fold resistance).
Dose-dependent growth inhibition by pemetrexed was observed in the
two NSCLC cell lines, where PC9/GR cells exhibited no cross
resistance to pemetrexed, with IC50-values of <1
μmol/l in all cases (Table I).
This concentration is markedly lower than the mean peak plasma
concentration of pemetrexed achievable in patients (i.e., 120–230
μmol/l), indicating a unexpectedly high sensitivity of NSCLC cells
to this agent in vitro (26,27).
 | Table IIC50-values of gefitinib
and pemetrexed were determined by the MTT assay. |
Table I
IC50-values of gefitinib
and pemetrexed were determined by the MTT assay.
|
IC50 | PC9 (nM) | PC9/GR (μM) |
|---|
| Pemetrexed | 292.66±26.01 | 0.51±0.023 |
| Gefitinib | 16.05±1.85 | 4.94±0.440 |
Effects of combined gefitinib and
pemetrexed treatment in human EGFR-TKI-sensitive and
EGFR-TKI-resistant cells
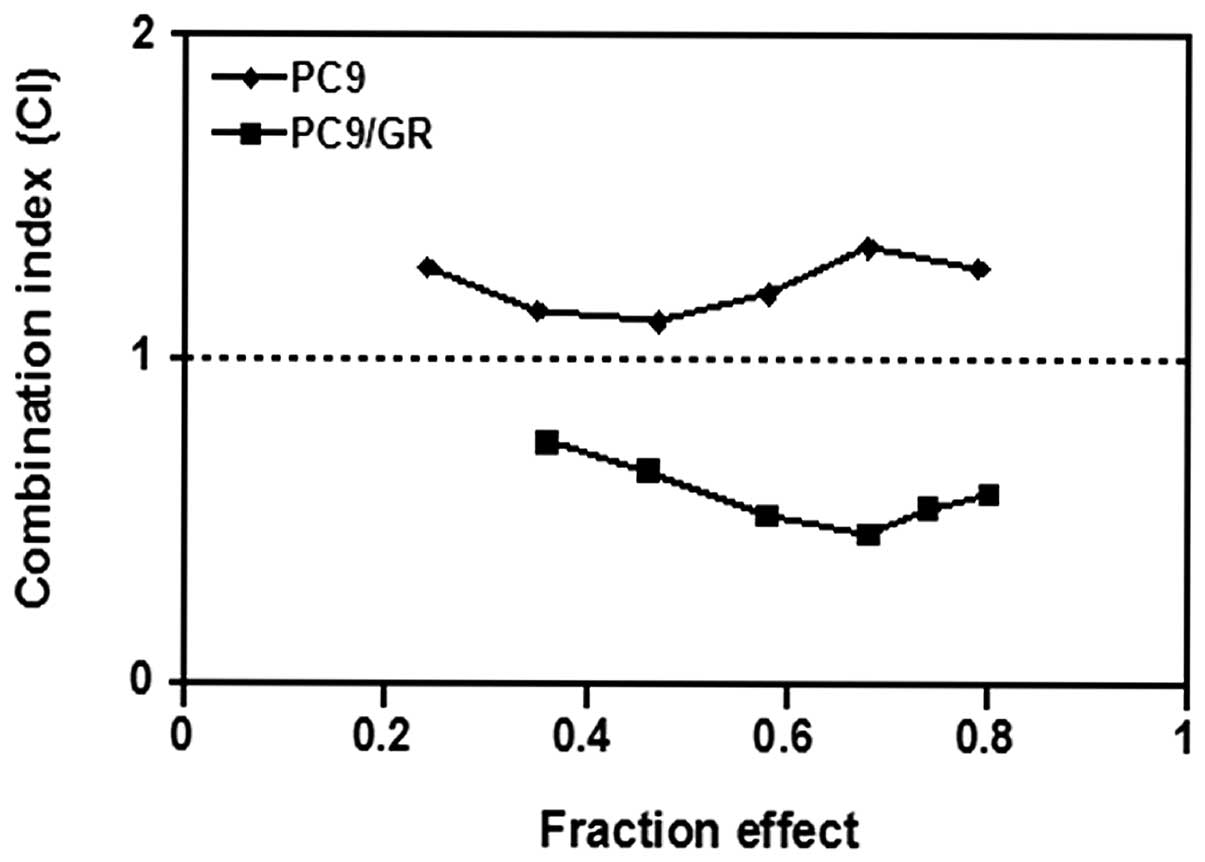
The interaction between gefitinib and pemetrexed on
PC9 and PC9/GR cell lines was evaluated. They were exposed to
various concentrations of gefitinib and pemetrexed concomitantly
for 72 h. As illustrated in Fig.
2, the PC9/GR cells, when treated with gefitinib and pemetrexed
concurrently, CI-values were all <1, with mean CI-values of
0.59, suggesting a synergistic interaction between gefitinib and
pemetrexed in cells with acquired EGFR-TKI resistance. However,
similar results were not identified in PC9 cells. In
EGFR-TKI-sensitive PC9 cells, concurrent administration resulted in
antagonistic effects (CI >1), with mean CI-values of 1.24
(Fig. 2).
Effects of pemetrexed and gefitinib on
the cell cycle
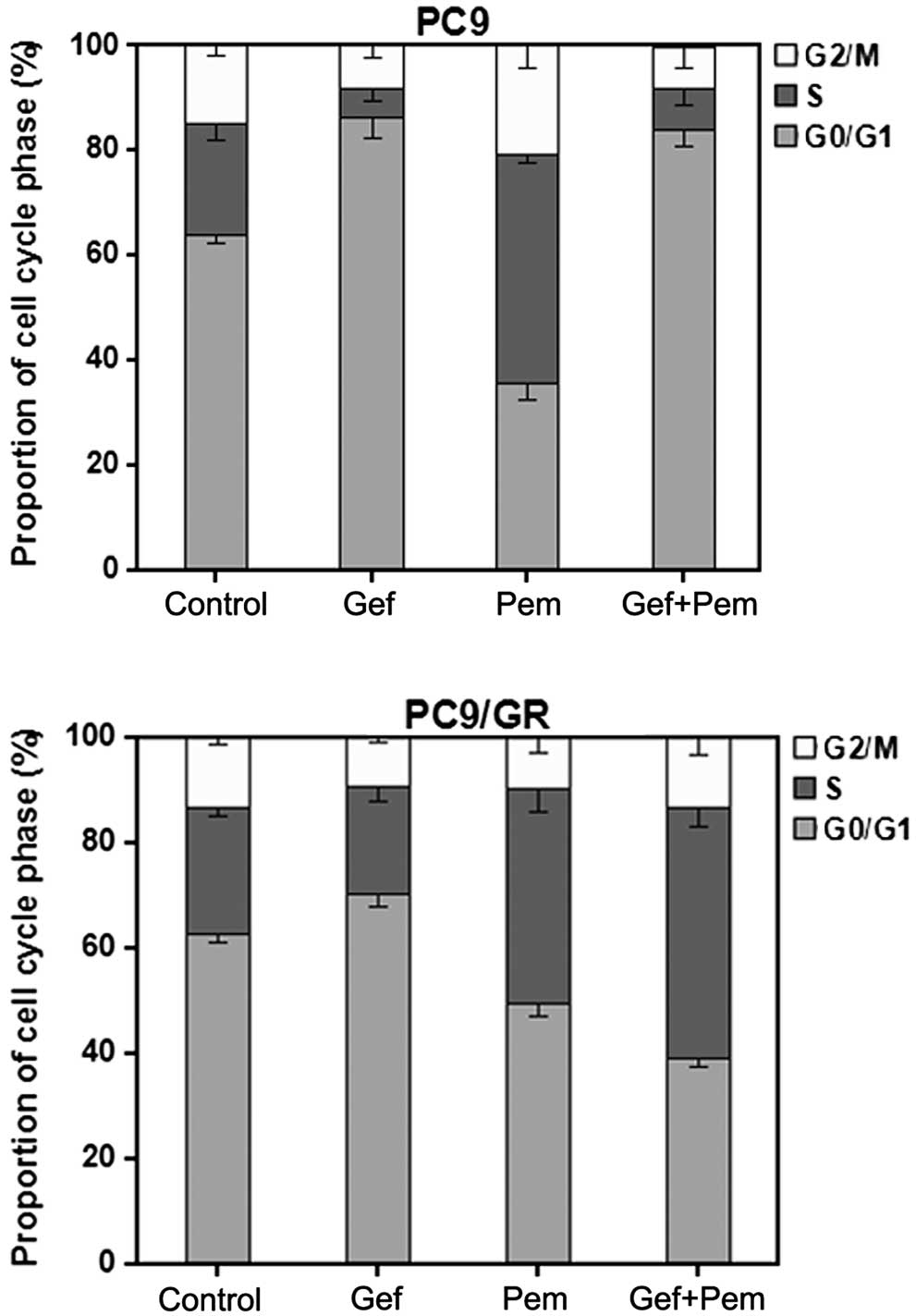
The cell cycle distribution was analyzed in cells
exposed to pemetrexed or gefitinib alone or concurrently for 72 h
by flow cytometry. The cell cycle effects of different exposures as
compared with the unexposed control cells are demonstrated in
Fig. 3. As illustrated in Fig. 3, in the EGFR-TKI-sensitive PC9
cells, when treated with gefitinib alone, there was a significant
cell cycle arrest at the G0/G1-phase (P<0.01), while in
EGFR-TKI-resistant PC9/GR cells, when administered with gefitinib
alone, there was no evident additional G0/G1-phase arrest.
Pemetrexed alone induced S-phase arrest in ~41% of PC9/GR cells
(P<0.01) and resulted in S-phase arrest in ~44% of PC9 cells
(P<0.01). Concurrent exposure of PC9 cells to pemetrexed and
gefitinib resulted in a similar cell cycle arrest pattern (mainly
G1-phase arrest) to that observed with gefitinib administered alone
and a corresponding reduction of arrest in S-phase (P<0.05),
which prevented the S-phase arrest effect of pemetrexed. By
contrast, when PC9/GR cells were exposed to both drugs, alterations
in the cell cycle phase distribution and overlapping effects of
pemetrexed and gefitinib were observed, which may amplify the
cytotoxicity of pemetrexed and gefitinib.
Effects of pemetrexed or gefitinib alone
or in combination on cell apoptosis
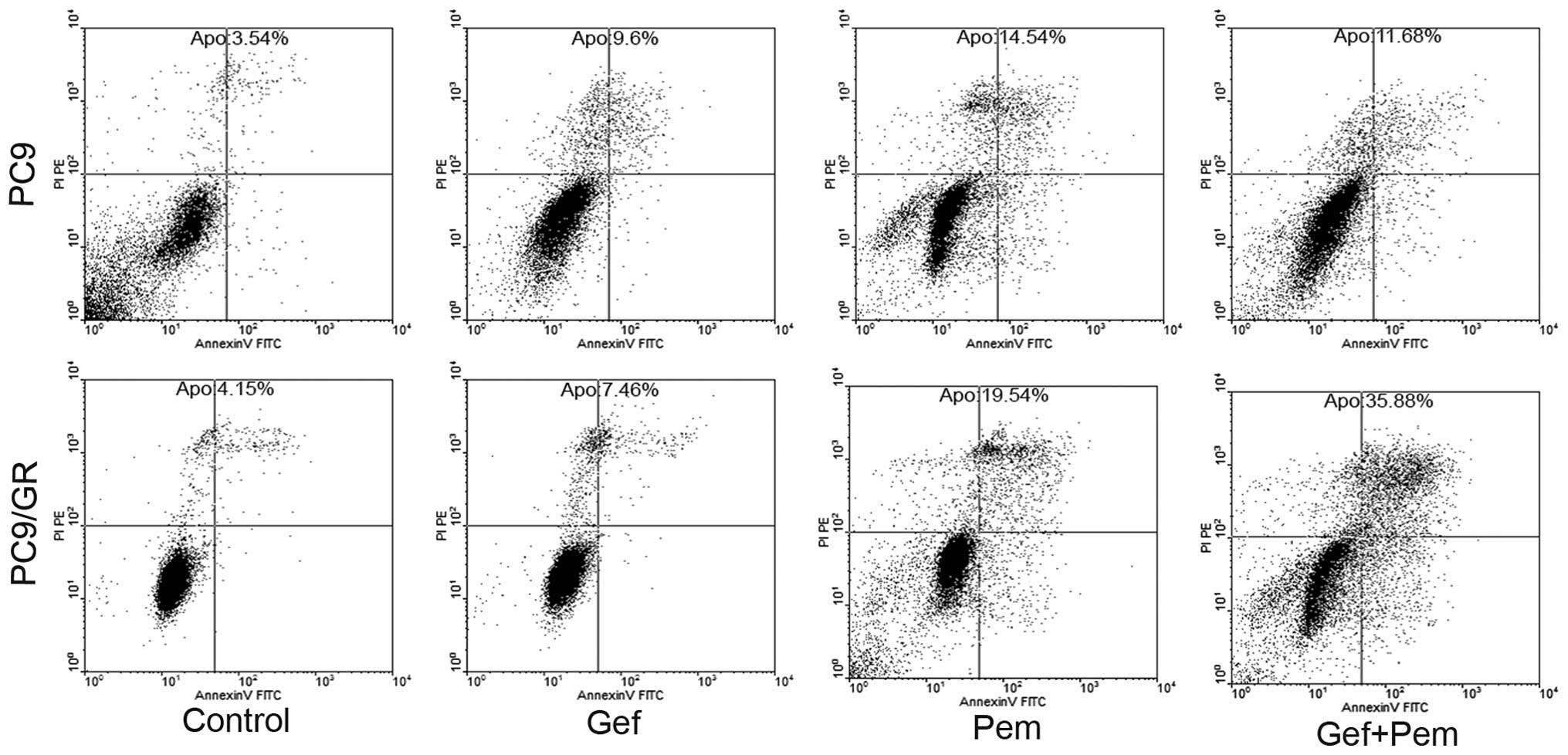
To examine whether the observed growth inhibition
was due to enhanced apoptosis, the rates of apoptosis of each
single or combined drug treatment in EGFR-TKI-sensitive and
EGFR-TKI-resistant cell lines were evaluated using the
concentration of the IC50-value for each drug with
subsequent analysis using an Annexin V/PI assay. As demonstrated in
Fig. 4, the rates of apoptosis
induced by pemetrexed in PC9 and PC9/GR after 72 h were 14.54 and
19.54%, respectively. Treatment with gefitinib alone for 72 h
resulted in the apoptotic rates of 9.6 and 7.64% in PC9 and PC9/GR
cells, respectively. However, exposure to pemetrexed combined with
gefitinib resulted in a decrease in the induction of apoptosis in
11.68% of PC9 cells. By contrast, the rates of apoptosis induced by
concurrent exposure in PC9/GR cells after 72 h were significantly
increased to 35.88%. These data indicated that concurrent exposure
to pemetrexed and gefitinib resulted in a negative interaction in
gefitinib-sensitive cells and a synergistic interaction in
gefitinib-resistant NSCLC cells.
Gefitinib or pemetrexed alone or in
combination modify expression levels of EGFR signaling-associated
proteins
ERK and AKT are important downstream targets of the
EGFR pathway. To further elucidate the potential mechanisms
involved in regulating the interaction between pemetrexed and
gefitinib, western blot analysis was used to evaluate the effects
of single or combined drugs on the EGFR downstream signaling
pathway, the levels of phosphorylated ERK and phosphorylated AKT in
gefitinib-sensitive and gefitinib-resistant cells. As illustrated
in Fig. 5, gefitinib inhibited the
activation of the EGFR downstream signaling mediators ERK and AKT
effectively in gefitinib-sensitive PC9 cells, whereas the
inhibitory effects of gefitinib on these signaling pathways in
gefitinib-resistant PC9/GR cells were significantly less than those
in parent PC9 cells. These results suggested that downregulation of
activated AKT and ERK is correlated with cellular sensitivity to
gefitinib. Following 72 h of exposure to pemetrexed at
concentrations of the IC50-values, the levels of p-ERK
and p-AKT were upregulated in PC9/GR cells as compared with
untreated cells. By contrast, in the PC9 cells, exposure to
pemetrexed did not result in increased p-AKT and p-ERK levels.
In addition, it was identified that when the PC9/GR
cells were exposed to a combination of pemetrexed and gefitinib for
72 h, the levels of p-AKT and p-ERK were decreased compared with
the levels observed in the control or single-agent treatment,
whereas, in the PC9 cells, the combination of the two drugs
increased the levels of p-AKT and p-ERK. However, there was no
significant variation in the total ERK and AKT expression levels
compared with the control (Fig.
5). These results suggested that although gefitinib alone did
not inhibit p-AKT and p-ERK1/2 expression, as was expected in the
gefitinib-resistant cells, gefitinib blocked the pemetrexed-induced
activation of phosphorylated ERK and phosphorylated AKT when
exposed concurrently.
Effects of gefitinib and pemetrexed
treatment on Bcl-2 levels
To further elucidate the mechanisms of cell death
induced by treatment with pemetrexed and gefitinib, the expression
of Bcl-2 was detected by western blot analysis. As illustrated in
Fig. 6, it was identified that the
basal levels of Bcl-2 were higher in PC9/GR cells than those in PC9
cells and the inhibitory effect of gefitinib on Bcl-2 in PC9/GR
cells was noticeably lower than that in PC9 cells. When the two
cell lines were exposed to the concurrent treatment, the levels of
Bcl-2 were effectively downregulated in gefitinib-resistant cells,
while in gefitinib-sensitive PC9 cells, upregulation of Bcl-2
expression levels was observed as compared with the single-agent
treatment.
Discussion
Currently, platinum-based chemotherapy is considered
to be the mainstay of first-line treatment for advanced NSCLC
(28). Despite the optimization of
chemotherapeutic strategies, there have been no second-line
combination chemotherapy regimens with a proven a survival benefit,
due to excessive toxicities and the rapidly declining clinical
condition of patients (29,30).
Recently, targeted anticancer drugs, including the EGFR-TKIs
gefitinib and erlotinib, have been approved for the treatment of
lung cancer (31). However,
although targeted therapies have been successfully developed, only
a small subset of NSCLC patients with EGFR mutant tumors benefit
from the EGFR-TKIs, and patients who initially respond to the
treatment often ultimately develop acquired resistance to these
targeted therapies (32).
Therefore, the development of new treatment strategies for NSCLC to
overcome acquired resistance to EGFR-TKIs is urgently required.
A key issue in the novel treatment modalities
against advanced NSCLC is the integration of EGFR-TKIs with
chemotherapy. Preclinical studies have revealed that
coadministration with EGFR-TKIs enhanced the effect of different
cytotoxic agents against various tumor models, including NSCLC
cells (33,34). However, there have been few studies
that used combinations of chemotherapy and targeted anticancer
agents in the treatment of NSCLCs with acquired EGFR-TKI
resistance.
PC9 is a lung adenocarcinoma cell line, which has a
deletion mutation within the kinase domain of EGFR and is highly
sensitive to EGFR-TKIs. These characteristics are similar to those
of NSCLC with clinical responsiveness to gefitinib. The PC9/GR
subline is specifically resistant to gefitinib, which is similar to
that of patients with acquired resistance to EGFR-TKIs. In the
present study, the antiproliferative effects of gefitinib, as a
single agent and in combination with pemetrexed in the
EGFR-TKI-sensitive PC9 cell line and its resistant subline,
PC-9/GR, was investigated.
It was identified that gefitinib and pememtrexed
exhibited dose-dependent growth inhibition when used as single
agents in the two lung cancer cells, but in PC9/GR cells,
significant antiproliferative effects of gefitinib were observed
only at higher concentrations. The IC50-values of
gefitinib to PC9/GR cells were higher than those to PC9 cells.
These results evidently demonstrated the significant in
vitro resistance of PC9/GR cells to gefitinib. Synergism was
observed when gefitinib was combined with pemetrexed in the PC9/GR
cells, whereas antagonistic interactions were noted in the PC9
cells.
Previous studies have demonstrated controversial
results regarding the concurrent antiproliferative effects in
EGFR-TKI-sensitive cell lines (34,35).
The discrepancies in the antiproliferative effects of combined
administration may be in part due to the different heritage
characteristics of the cell lines and different drug exposure
conditions. Therefore, patients may benefit from the concomitant
administration of pemetrexed and gefitinib, particularly in those
with acquired resistance to EGFR-TKIs. In addition, the results of
the present study also demonstrated consistent results in the rate
of apoptosis.
The mechanisms of these synergistic and antagonistic
effects may be explained by differences in the changes induced in
the cell cycle. In the present study, pemetrexed arrested the cell
cycle at the S-phase in the cell lines, whereas gefitinib caused
significant G0/G1 phase accumulation only in the PC9 cell line.
When exposed to a combination of pemetrexed and gefitinib, the
proportion of cells in S phase increased compared with the control
in the PC9/GR cells. Due to the fact that gefitinib was not able to
induce additional G1-phase arrest in PC9/GR cells with acquired
resistance to gefitinib, continuous daily administration of
gefitinib is expected to have a minimal negative effect on the
cytotoxicity of pemetrexed. However, in PC9 cells, concurrent
exposure resulted in a mainly G0/G1-phase arrest, which is similar
to gefitinib administered alone. The mechanisms underlying the
observed antagonistic effects of concurrent administration of
EGFR-TKIs and chemotherapy are relevant to pharmacodynamic
separation, since G0/G1-phase arrest induced by EGFR-TKIs in NSCLC
possibly interferes with the cell cycle-specific cytotoxicity of
chemotherapy, resulting in decreased cytotoxicity (36).
By contrast, part of this growth inhibition may be
explained by apoptosis. Bcl-2 is an anti-apoptotic member of the
Bcl-2 family, which is a key apoptosis regulator that is
downregulated in numerous cell types in response to treatment
leading to activation of apoptosis (37,38).
The present study demonstrated that combination treatment of
gefitinib and pemetrexed resulted in high levels of Bcl-2
activation in gefitinib-sensitive PC9 cells, whereas downregulation
of the anti-apoptotic protein Bcl-2 was observed in PC9/GR cells.
These results indicated that downregulation of the antiapoptotic
protein Bcl-2 may be involved in combination-induced cell
death.
To elucidate the mechanism underlying the different
antiproliferative effects of gefitinib combined with pemetrexed,
drug-induced growth signaling pathway expression levels following
pemetrexed exposure were evaluated. These results demonstrated that
pemetrexed increased the levels of p-ERK and p-AKT in the
gefitinib-resistant PC9/GR cells. By contrast, in the PC9 cells,
pemetrexed exposure did not result in increased p-AKT and p-ERK
levels. Following this, a possible association between sensitivity
to gefitinib and inhibition of EGFR downstream signaling molecules
p-AKT and p-ERK was evaluated. Previous studies have demonstrated
that inhibition of PI3K/AKT activity correlates with the
sensitivity to EGFR-TKIs in NSCLC cells (39,40).
Consistent with this, the present study identified that in the PC9
cell line, which is highly sensitive to gefitinib, there was a
strong inhibition of p-AKT and p-ERK following treatment with
gefitinib. However, there was no inhibition of p-AKT and p-ERK
following treatment with gefitinib in the gefitinib-resistant
PC9/GR cell line. These data suggested that in the
gefitinib-sensitive NSCLC cell lines, the AKT and ERK pathways are
more dependent on signals transduced by EGFR than is the case in
the gefitinib-resistant NSCLC cell lines.
When gefitinib was combined with pemetrexed, a
significant decrease in p-AKT and p-ERK was observed in the PC9/GR
cells, as compared with the control. However, the levels of p-AKT
and p-ERK increased when gefitinib and pemetrexed were applied
together to the PC9 cells. The results of the present study
suggested that only NSCLC tumors that respond to pemetrexed by
upregulating p-AKT and p-ERK will respond to the addition of
EGFR-targeted TKIs. Furthermore, a number of previous studies have
demonstrated similar results: Only cell lines that responded with
increased EGFR activation following chemotherapy were
synergistically growth inhibited following the addition of
gefitinib to chemotherapy (33,41).
These effects on p-AKT and p-ERK in NSCLC cells may explain the
synergistic or antagonist growth inhibitory effects observed in the
two cell lines treated with gefitinib and pemetrexed.
In conclusion, the present study demonstrated the
effect of in vitro single-agent gefitinib and concurrent
growth inhibitory effects of gefitinib and pemetrexed in the
EGFR-TKI-sensitive and the EGFR-TKI-resistant NSCLC cell lines. It
was identified that gefitinib combined with pemetrexed generated
synergistic effects in cells with acquired gefitinib resistance and
antagonistic effects in gefitinib-sensitive cells. The present
study suggested that EGFR-TKIs combined with pemetrexed may be
beneficial to NSCLC patients with acquired EGFR-TKI resistance. The
ongoing IMPRESS (NCT01544179) randomized phase III trial, which
evaluates continued administration of gefitinib with the addition
of chemotherapy, compared with chemotherapy alone in EGFR
mutation-positive patients who have progressed on first-line
gefitinib, will indicate whether this preclinical observation is
clinically relevant or of therapeutic value.
Acknowledgements
This study was supported by a grant from the Anhui
Provincial Natural Science Research Program of Higher Education
Institutions Foundation of China (no. KJ2012A157) and by The
Central Laboratory of The Third Affiliated Hospital of Anhui
Medical University. The authors are grateful to Dr Xuchao Zhang for
providing the PC9 and PC9/GR cell lines.
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
2
|
Salomon DS, Brandt R, Ciardiello F and
Normanno N: Epidermal growth factor-related peptides and their
receptors in human malignancies. Crit Rev Oncol Hematol.
19:183–232. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ennis BW, Lippman ME and Dickson RB: The
EGF receptor system as a target for antitumor therapy. Cancer
Invest. 9:553–562. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lynch TJ, Bell DW, Sordella R, et al:
Activating mutations in the epidermal growth factor receptor
underlying responsiveness of non-small-cell lung cancer to
gefitinib. N Engl J Med. 350:2129–2139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Maemondo M, Inoue A, Kobayashi K, et al:
Gefitinib or chemotherapy for non-small-cell-lung cancer with
mutated EGFR. N Engl J Med. 362:2380–2388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Masago K, Fujita S, Togashi Y, et al:
Clinicopathologic factors affecting the progression free survival
of patients with advanced non-small-cell lung cancer after
gefitinib therapy. Clin Lung Cancer. 12:56–61. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jackman D, Pao W, Riely GJ, et al:
Clinical definition of acquired resistance to epidermal growth
factor receptor tyrosine kinase inhibitors in non-small-cell lung
cancer. J Clin Oncol. 28:357–360. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lovly CM and Horn L: Strategies for
overcoming EGFR resistance in the treatment of advanced-stage
NSCLC. Curr Treat Options Oncol. 13:516–526. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Goldberg SB, Oxnard GR, Digumarthy S, et
al: Chemotherapy with erlotinib or chemotherapy alone in advanced
non-small cell lung cancer with acquired resistance to EGFR
tyrosine kinase inhibitors. Oncologist. 18:1214–1220. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Haddad R, Allen A, Wirth L, Tishler R and
Posner M: Integrating novel agents into the curative treatment of
head and neck cancer. Expert Rev Anticancer Ther. 6:157–159. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hanna N, Shepherd FA, Fossella FV, et al:
Randomized phase III trial of pemetrexed versus docetaxel in
patients with non-small cell lung cancer previously treated with
chemotherapy. J Clin Oncol. 22:1589–1597. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fukuoka M, Yano S, Giaccone G, et al:
Multi-institutional randomized phase II trial of gefitinib for
previously treated patients with advanced non-small-cell lung
cancer (The IDEAL 1 Trial) [corrected]. J Clin Oncol. 21:2237–2246.
2003.
|
|
13
|
Grunwald V and Hidalgo M: Developing
inhibitors of the epidermal growth factor receptor for cancer
treatment. J Natl Cancer Inst. 95:851–867. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim ES, Hirsh V, Mok T, et al: Gefitinib
versus docetaxel in previously treated non-small-cell lung cancer
(INTEREST): a randomised phase III trial. Lancet. 372:1809–1818.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Steins MB, Reinmuth N, Bischoff H,
Kindermann M and Thomas M: Targeting the epidermal growth factor
receptor in non-small cell lung cancer. Onkologie. 33:704–709.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Baselga J and Arteaga CL: Critical update
and emerging trends in epidermal growth factor receptor targeting
in cancer. J Clin Oncol. 23:2445–2459. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Herbst RS and Sandler AB: Overview of the
current status of human epidermal growth factor receptor inhibitors
in lung cancer. Clin Lung Cancer. 6(Suppl 1): S7–S19. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ciardiello F, Caputo R, Borriello G, et
al: ZD1839 (IRESSA), an EGFR-selective tyrosine kinase inhibitor,
enhances taxane activity in bcl-2 overexpressing,
multidrug-resistant MCF-7 ADR human breast cancer cells. Int J
Cancer. 98:463–469. 2002. View Article : Google Scholar
|
|
19
|
Park JK, Lee SH, Kang JH, Nishio K, Saijo
N and Kuh HJ: Synergistic interaction between gefitinib (Iressa,
ZD1839) and paclitaxel against human gastric carcinoma cells.
Anticancer Drugs. 15:809–818. 2004. View Article : Google Scholar
|
|
20
|
Sirotnak FM, Zakowski MF, Miller VA, Scher
HI and Kris MG: Efficacy of cytotoxic agents against human tumor
xenografts is markedly enhanced by coadministration of ZD1839
(Iressa), an inhibitor of EGFR tyrosine kinase. Clin Cancer Res.
6:4885–4892. 2000.PubMed/NCBI
|
|
21
|
Shih C, Chen VJ, Gossett LS, et al:
LY231514, a pyrrolo[2,3-d]pyrimidine-based antifolate that inhibits
multiple folate-requiring enzymes. Cancer Res. 57:1116–1123.
1997.
|
|
22
|
Chattopadhyay S, Moran RG and Goldman ID:
Pemetrexed: biochemical and cellular pharmacology, mechanisms, and
clinical applications. Mol Cancer Ther. 6:404–417. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Scagliotti GV, Parikh P, von Pawel J, et
al: Phase III study comparing cisplatin plus gemcitabine with
cisplatin plus pemetrexed in chemotherapy-naive patients with
advanced-stage non-small-cell lung cancer. J Clin Oncol.
26:3543–3551. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ciuleanu T, Brodowicz T, Zielinski C, et
al: Maintenance pemetrexed plus best supportive care versus placebo
plus best supportive care for non-small-cell lung cancer: a
randomised, double-blind, phase 3 study. Lancet. 374:1432–1440.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chou TC and Talalay P: Quantitative
analysis of dose-effect relationships: the combined effects of
multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 22:27–55.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rinaldi DA, Kuhn JG, Burris HA, et al: A
phase I evaluation of multitargeted antifolate (MTA, LY231514),
administered every 21 days, utilizing the modified continual
reassessment method for dose escalation. Cancer Chemother
Pharmacol. 44:372–380. 1999. View Article : Google Scholar
|
|
27
|
Thödtmann R, Depenbrock H, Dumez H, et al:
Clinical and pharmacokinetic phase I study of multitargeted
antifolate (LY231514) in combination with cisplatin. J Clin Oncol.
17:3009–3016. 1999.PubMed/NCBI
|
|
28
|
D’Addario G, Pintilie M, Leighl NB, Feld
R, Cerny T and Shepherd FA: Platinum-based versus
non-platinum-based chemotherapy in advanced non-small-cell lung
cancer: a meta-analysis of the published literature. J Clin Oncol.
23:2926–2936. 2005.PubMed/NCBI
|
|
29
|
Pfister DG, Johnson DH, Azzoli CG, et al:
American Society of Clinical Oncology treatment of unresectable
non-small-cell lung cancer guideline: update 2003. J Clin Oncol.
22:330–353. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ardizzoni A and Tiseo M: Second-line
chemotherapy in the treatment of advanced non-small cell lung
cancer (NSCLC). J Chemother. 16(Suppl 4): 104–107. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Maemondo M: Timing the change of
chemotherapy for non small cell lung cancer. Gan To Kagaku Ryoho.
39:1316–1319. 2012.(In Japanese).
|
|
32
|
Oxnard GR, Arcila ME, Chmielecki J,
Ladanyi M, Miller VA and Pao W: New strategies in overcoming
acquired resistance to epidermal growth factor receptor tyrosine
kinase inhibitors in lung cancer. Clin Cancer Res. 17:5530–5537.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Van Schaeybroeck S, Kyula J, Kelly DM, et
al: Chemotherapy-induced epidermal growth factor receptor
activation determines response to combined gefitinib/chemotherapy
treatment in non-small cell lung cancer cells. Mol Cancer Ther.
5:1154–1165. 2006.
|
|
34
|
Li T, Ling YH, Goldman ID and Perez-Soler
R: Schedule-dependent cytotoxic synergism of pemetrexed and
erlotinib in human non-small cell lung cancer cells. Clin Cancer
Res. 13:3413–3422. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Giovannetti E, Lemos C, Tekle C, et al:
Molecular mechanisms underlying the synergistic interaction of
erlotinib, an epidermal growth factor receptor tyrosine kinase
inhibitor, with the multitargeted antifolate pemetrexed in
non-small-cell lung cancer cells. Mol Pharmacol. 73:1290–1300.
2008. View Article : Google Scholar
|
|
36
|
Davies AM, Ho C, Lara PN Jr, Mack P,
Gumerlock PH and Gandara DR: Pharmacodynamic separation of
epidermal growth factor receptor tyrosine kinase inhibitors and
chemotherapy in non-small-cell lung cancer. Clin Lung Cancer.
7:385–388. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ruvolo PP, Deng X and May WS:
Phosphorylation of Bcl-2 and regulation of apoptosis. Leukemia.
15:515–522. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Diel P, Smolnikar K and Michna H: The pure
antiestrogen ICI 182780 is more effective in the induction of
apoptosis and down regulation of BCL-2 than tamoxifen in MCF-7
cells. Breast Cancer Res Treat. 58:87–97. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sordella R, Bell DW, Haber DA and
Settleman J: Gefitinib-sensitizing EGFR mutations in lung cancer
activate anti-apoptotic pathways. Science. 305:1163–1167. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Amann J, Kalyankrishna S, Massion PP, et
al: Aberrant epidermal growth factor receptor signaling and
enhanced sensitivity to EGFR inhibitors in lung cancer. Cancer Res.
65:226–235. 2005.PubMed/NCBI
|
|
41
|
Van Schaeybroeck S, Karaiskou-McCaul A,
Kelly D, et al: EGFR activity determines response of colorectal
cancer cells to gefitinib (Iressa) alone and in combination with
chemotherapy. Clin Cancer Res. 11:7480–7489. 2005.PubMed/NCBI
|