Introduction
microRNAs (miRNAs), a class of short non-coding RNA
molecules, function as transcriptional or post-transcriptional
regulators of gene expression (1,2).
Deregulation of miRNAs is tightly associated with human disorders,
including obesity, cardiovascular diseases and tumorigenesis
(3,4). Recent studies have also shown that a
number of miRNAs are upregulated or downregulated and play critical
roles in osteosarcoma development (5,6). For
instance, miR-376c was shown to inhibit cell proliferation and
invasion in osteosarcoma by targeting transforming growth factor-α
(7), whereas miR-221 induces
osteosarcoma cell survival through enhancing the AKT signaling
pathway (8).
Previous studies have shown that miR-25 expression
and function is deregulated in several types of tumor (9–12).
It was shown that miR-25 is consistently highly expressed in the
serum of patients with breast cancer or hepatocellular carcinoma,
indicating that this miR may be used as a biomarker in diagnosis
and treatment (9,10). In addition, the miR-25 level was
significantly increased in human gastric cancer (GC) tissues and
cell lines (11). Overexpression
of miR-25 markedly enhanced cell proliferation, migration and
invasion in GC cells, whereas inhibition of miR-25 caused a
significant reduction in proliferation rates and a significant
increase in apoptosis (11).
However, miR-25 was found to be downregulated in human colon cancer
tissues when compared to matched, non-neoplastic mucosa tissues
(12). Functional studies revealed
that restoration of the miR-25 expression inhibits cell
proliferation and migration (12).
By contrast, miR-25 inhibition promoted cell proliferation and
migration (12). Therefore, miR-25
appears to act as either an onco-miRNA or a tumor suppressor in
different types of cancer, and plays a crucial role in cancer
biology. However, its biological functions in osteosarcoma remain
unexplored to date.
Materials and methods
Cell culture and tissue samples
Osteosarcoma cell lines (Saos-2 and U2OS) were
obtained from the American Type Culture Collection (Rockville, MD,
USA). Cells were cultured in Gibco® RPMI-1640 (Thermo
Fisher scientific, Inc., Beijing, China) supplemented with 10%
Gibco® fetal bovine serum (Thermo Fisher Scientific,
Inc.). Tumor tissues and adjacent noncancerous normal tissues were
collected from routine therapeutic surgery at the Department of
Orthopaedics, Pudong New Area Zhoupu Hospital. A total of 25
samples (male; median age, 57 years; range, 48–65 years) were
obtained with informed consent from patients with osteosarcoma, and
the procedures were approved by the Institutional Review Board of
the hospital. Subjects were excluded if they had other diseases,
including biliary obstructive diseases, and acute or chronic virus
hepatitis. The individuals with an alcohol consumption of ≥120
g/week for men at the time of the study or in the prior 6 months
were also excluded from the study.
Analysis of miRNA expression
miRNA from tissue samples and cell lines was
harvested using the Ambion® mirVana miRNA Isolation kit
(Thermo Fisher Scientific, Inc., Grand Island, NY, USA), following
the manufacturer’s instructions. All RNA samples were examined as
to their concentration and purity. RNA purity was measured using
the NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific,
Waltham, MA, USA). Based on the absorbance ratio at 260/280 nm
(mean±standard deviation = 1.86±0.03), all RNA samples were pure
and protein free. Expression of mature miRNAs was assessed with the
Applied Biosystems® TaqMan® microRNA assay
(Thermo Fisher Scientific, Inc.) with probes specific to the human
hsa-miR25 (5′-aggcggagacuugggcaauug-3′) and the small nuclear U6
snRNA, used as an internal control (5′-cauugaccauggacauacgacug-3′).
The quantitative (q)PCR reaction was performed using a TaqMan
Universal PCR Master mix on an Applied Biosystems®
7900HT Real-Time PCR system (all from Thermo Fisher Scientific,
Inc.). Briefly, PCR conditions included an initial holding period
at 95°C for 5 sec and 60°C for 30 sec for 45 cycles. Relative
quantitation analysis of the gene expression data was conducted
according to the 2−ΔΔCt method.
Plasmid construction and
transfection
To construct the miR-25 expression plasmid, the
precursor sequence of the human miR-25 was cloned into
Ambion® pSilencer™, while the negative control (NC)
plasmid contained a scrambled sequence (both from Thermo Fisher
Scientific, Inc.). For transfection, Lipofectamine® 2000
(Thermo Fisher Scientific, Inc.) was employed following the
manufacturer’s instructions.
Bromodeoxyuridine (BrdU) assay
To assess cell proliferation, an enzyme-linked
immunosorbent assay (ELISA) was used, based on the incorporation of
BrdU during DNA synthesis, (BrdU kit; Beyotime Institute of
Biotechnology, Shanghai, China), following the manufacturer’s
protocols. All experiments were performed in triplicate. The
absorbance of the samples at 450 nm (A450) was measured
on a SpectraMax 190 ELISA reader (Molecular Devices, Sunnyvale, CA,
USA).
Western blot analysis
Cells were harvested and lysed with ice-cold lysis
buffer containing 50 mM Tris-HCl, pH 6.8, 100 mM 2-Mercaptoethanol,
2% w/v sodium dodecyl sulfate (SDS) and 10% glycerol. Following
centrifugation at 4°C, proteins in the supernatants were quantified
by a BCA quantification kit (Beyotime Institute of Biotechnology),
separated by 10% SDS-polyacrylamide gel electrophoresis (PAGE), and
transferred onto nitrocellulose membranes (Amersham Biosciences,
Buckinghamshire, UK). Anti-p27 antibody (Cell Signaling Technology,
Inc., Danvers, MA, USA) was used at 1:2,000 overnight at 4°C, and
anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody
(Cell Signaling Technology, Inc.) was used at 1:5,000 overnight at
4°C. The membranes were then incubated with the HRP-linked
secondary antibodies (Cell Signaling Technology, Inc.). The signals
were detected by SuperSignal West Pico Chemiluminescent Substrate
kit (Pierce Biotechnology, Inc., Rockford, IL, USA) according to
manufacturer’s instructions. The images were visualized by a
LAS-4000 Luminescent Image analyzer (Fujifilm, Tokyo, Japan). The
p27 protein level was quantified using Quantity One software
(Bio-Rad, Hercules, CA, USA) and was normalized to that of
GAPDH.
Luciferase reporter assay
Total cDNA from Saos-2 cells was synthesized from
total RNA using random hexamers with the Superscript III Reverse
Transcriptase kit (Invitrogen, Carlsbad, CA, USA), according to the
manufacturer’s instructions. The reactions were incubated in a
thermal cycler for 30 min at 16°C, 30 min at 42°C, 5 min at 85°C
and then held at 4°C. The cDNA was used to amplify the 3′
untranslated region (3′ UTR) of p27 by PCR. Mutations were
introduced in potential miR-25-binding sites
(5′-GCUAUUACGAAUACAUCCGUUAAC-3′ was changed into
5′-GCUAUUACGAAUACAUCCGAAUUC-3′) using the QuickChange®
Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA, USA). The
pRL-SV40 vector (Promega Corp., Madison, WI, USA) carrying the
Renilla luciferase gene was used as an internal control of
transfection efficiency. Luciferase values were determined using
the Dual-Luciferase® Reporter Assay system (Promega
Corp.).
Tumor growth assay
Male BALB/c nude mice, 4 weeks-old, were purchased
from the Shanghai Laboratory Animal Center (Shanghai, China).
Saos-2 cells (2×105) were subcutaneously injected into
the skin under the legs of the mice. The mice were observed for 5
weeks for tumor formation. Following sacrifice, the tumors were
recovered and the wet weights of each tumor were measured.
Statistical analysis
Data were expressed as the mean ± SEM from at least
three independent repetitions of the experiment. Differences
between groups were analyzed using Student’s t-tests or one way
analysis of variance (ANOVA). P<0.05 was considered to indicate
a statistically significant difference.
Results
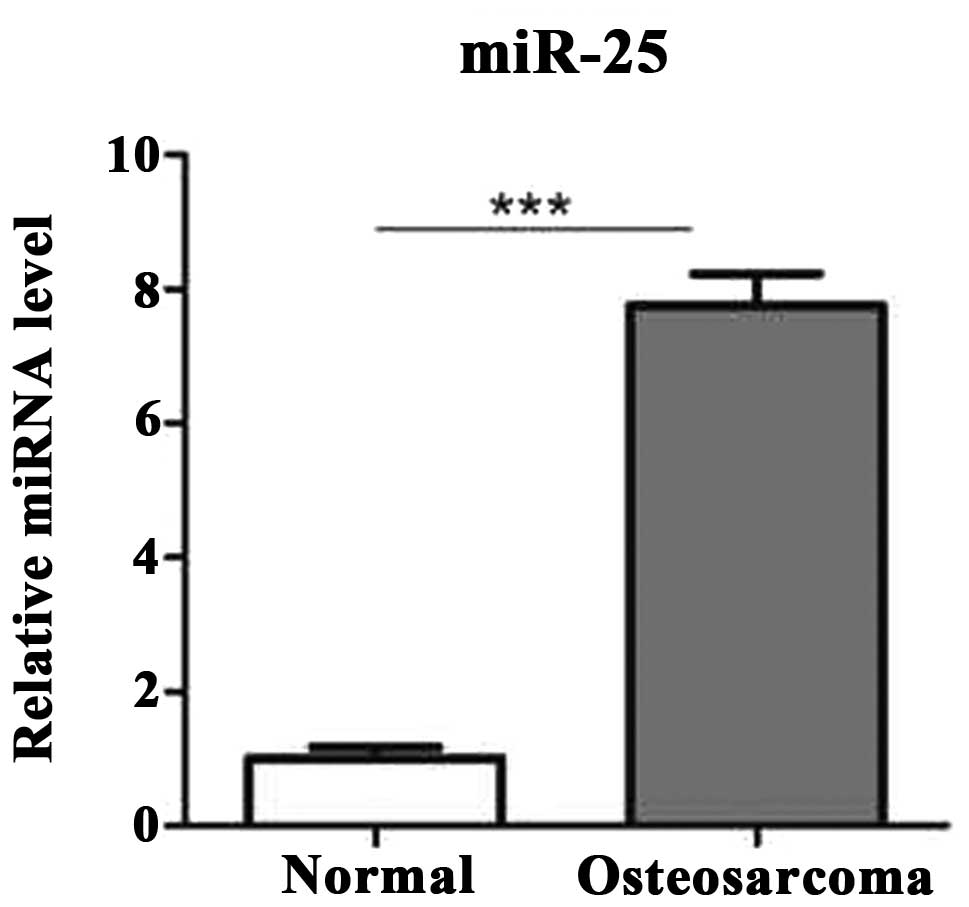
The miR-25 expression level is increased
in osteosarcoma tissues
First, in order to examine whether the miR-25 is
differentially expressed in human osteosarcoma compared to
noncancerous tissues, its expression level was determined using
reverse transcription (RT)-qPCR in 25 pairs of human osteosarcoma
tissues and pair-matched adjacent noncancerous tissues. The results
demonstrated that the expression level of miR-25 was significantly
increased in osteosarcoma tissues compared to the adjacent
noncancerous tissues (Fig. 1).
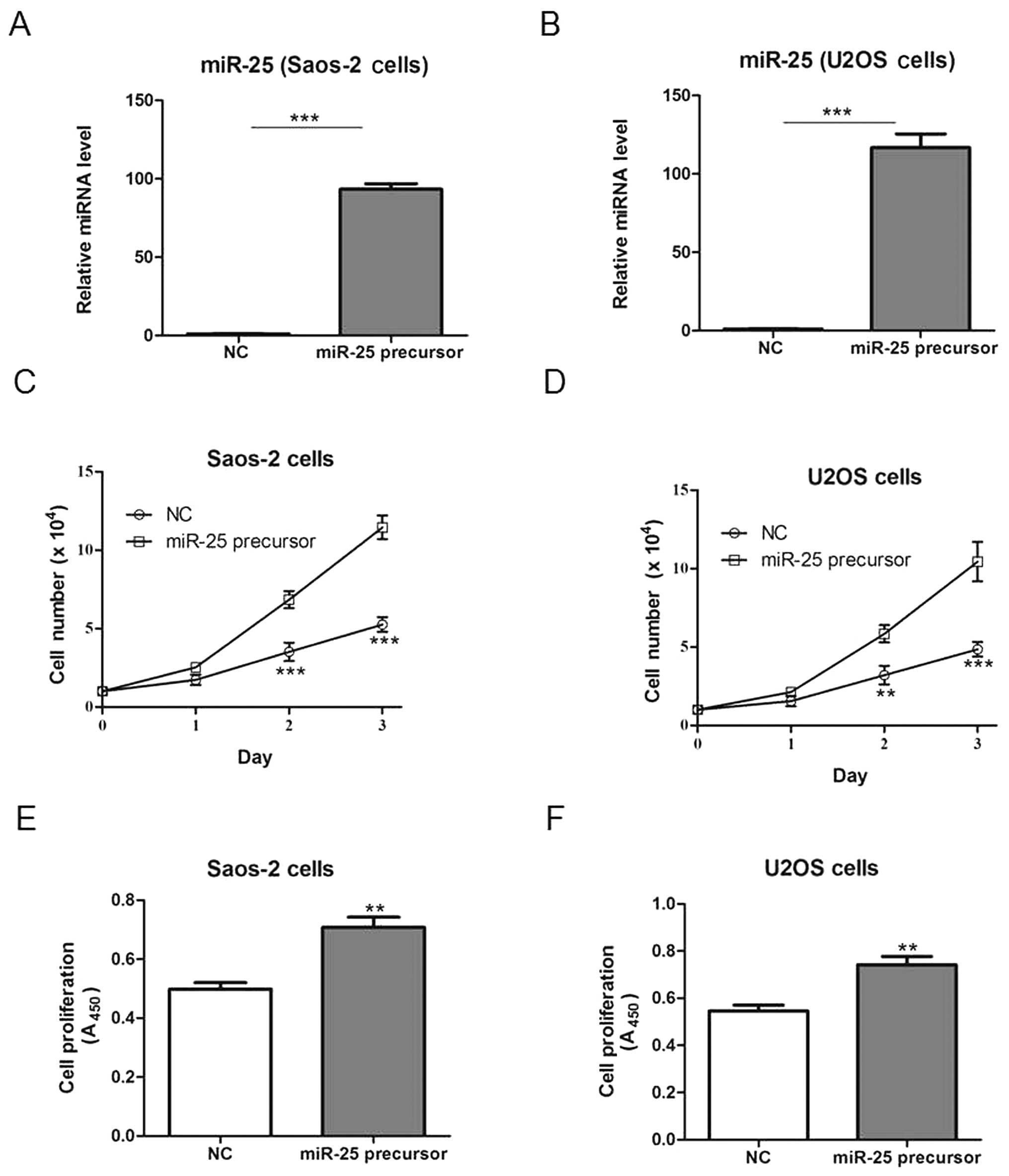
miR-25 overexpression promotes cell
proliferation in vitro
In order to assess the effects of miR-25 on
osteosarcoma cell growth, Saos-2 and U2OS cells were transfected
with a plasmid bearing the miR-25 precursor or the NC plasmid, and
cell growth was subsequently examined. Transfection with the miR-25
precursor-bearing plasmid increased miR-25 expression compared to
the NC transfection (Fig. 2A and
B), and significantly increased the cell number and
proliferation in both cell lines (Fig.
2C–F).
miR-25 overexpression promotes cell
proliferation in vivo
To study the role of miR-25 overexpression in
tumorigenesis in vivo, we generated Saos-2 cells stably
overexpressing miR-25 and injected the cells into a xenograft mouse
model. miR-25 overexpression markedly promoted tumorigenesis in
comparison with the NC, as evidenced by tumor weights and sizes
(Fig. 3A and B).
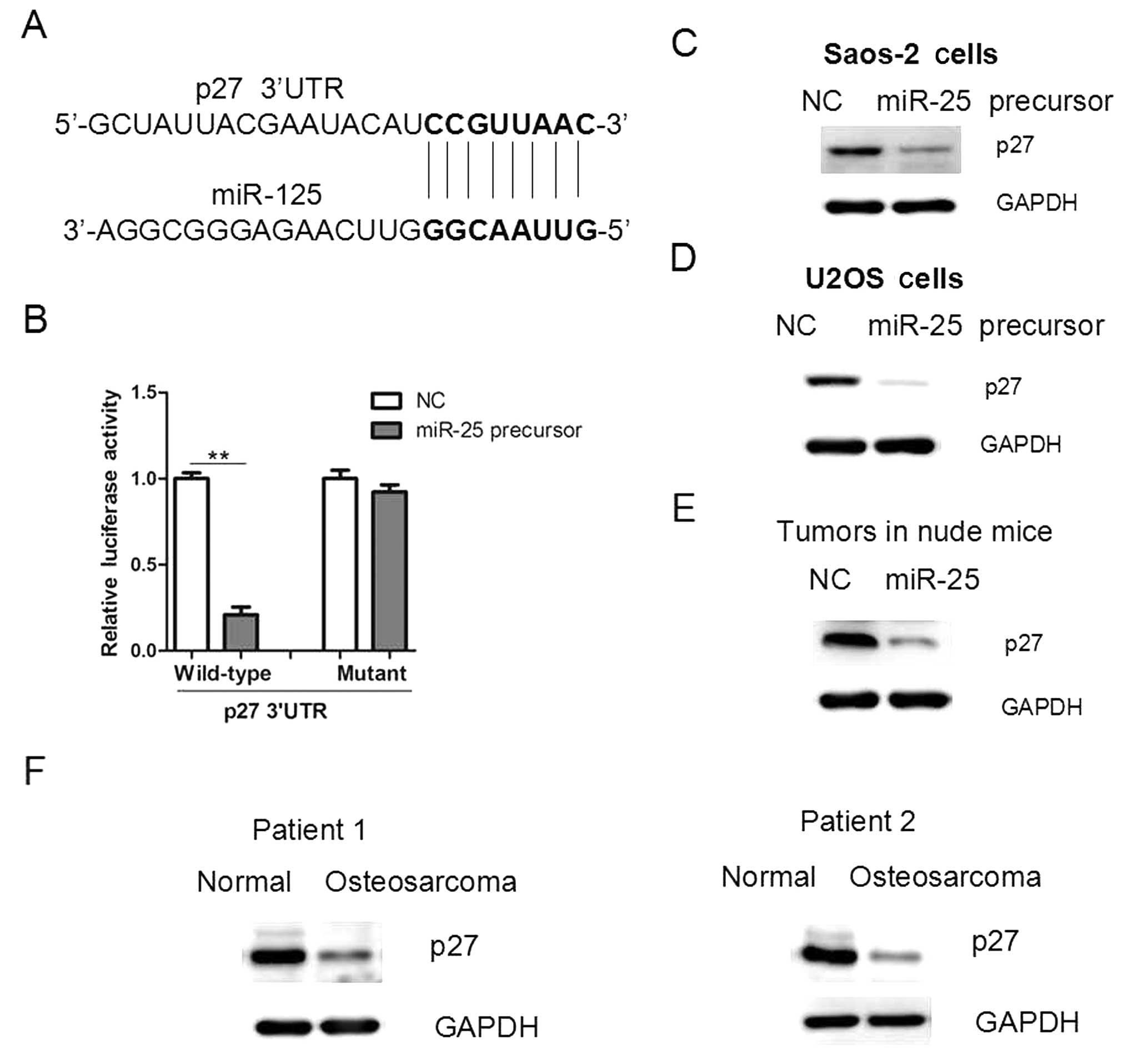
miR-25 targets the p27 in osteosarcoma
cells
Since miR-25 overexpression promoted cell
proliferation and tumor growth, we examined the underlying
mechanisms. Through a stringent bioinformatics approach (miRWalk
software; http://www.umm.uni-heidelberg.de/apps/zmf/mirwalk/),
we identified the gene p27, encoding a cell-cycle inhibitor,
as a candidate target of miR-25, since p27 bears a potential
miR-25-binding site (Fig. 4A). The
3′ UTR of p27 was cloned into a reporter luciferase system.
When the reporter construct contained the p37 3′ UTR,
overexpression of miR-25 led to a reduction in luciferase activity
(Fig. 4B). By contrast, mutation
of the conserved miR-25-binding motif abrogated the reduced
luciferase expression (Fig. 4B).
In addition, overexpression of miR-25 in osteosarcoma cells led to
a reduction in the protein level of p27 (Fig. 4C and D). In addition, the p27
protein level was reduced in miR-25-overexpressing tumors and human
osteosarcoma tissues (Fig. 4E and
F), further supporting that p27 may be a target of
miR-25 in osteosarcoma cells.
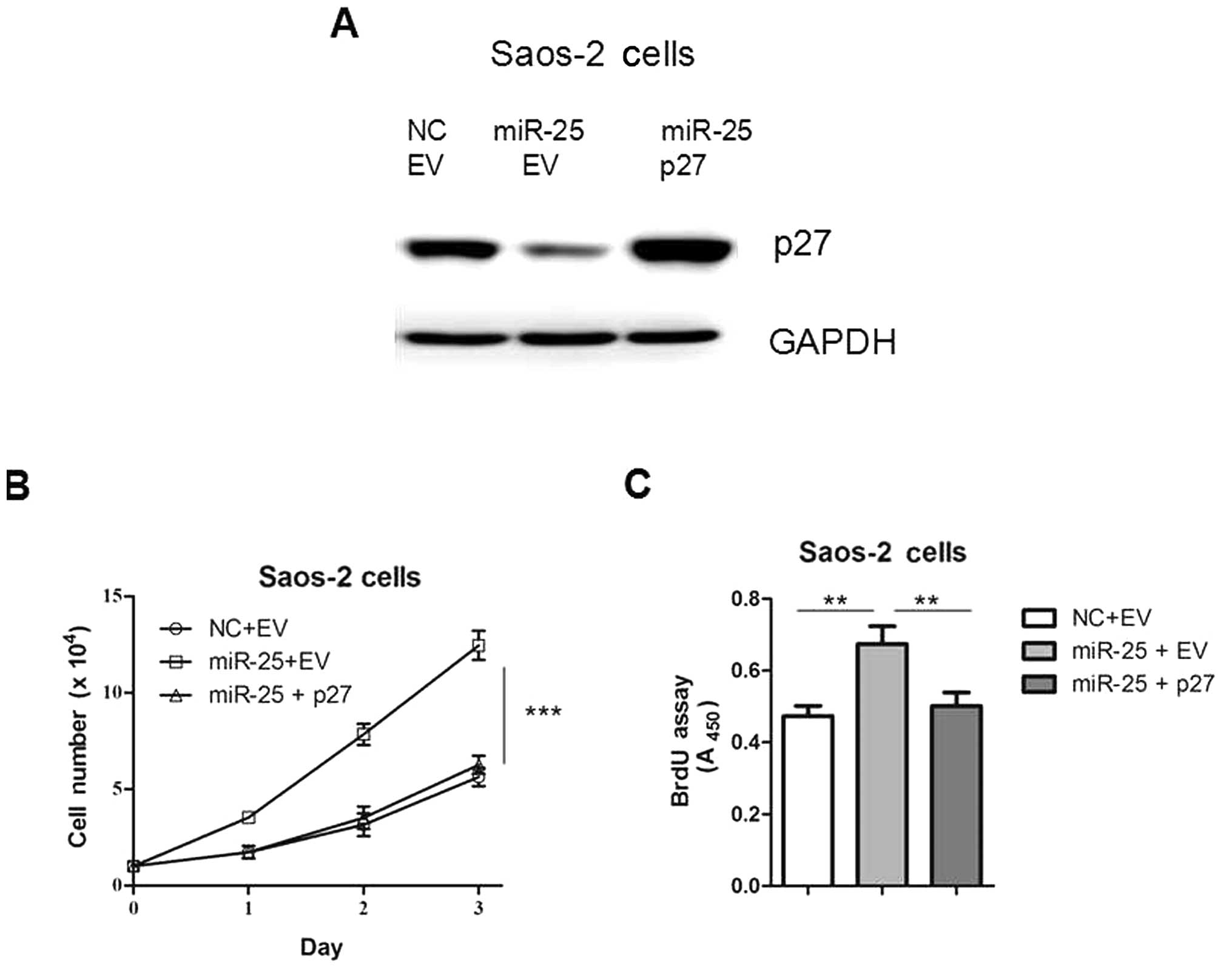
p27 restoration attenuates the promoting
effect of miR-25 overexpression on cell proliferation
In order to confirm the functional connection
between miR-25 and p27, Saos-2 cells were transfected with
p27 expression plasmids following transfection with miR-25
(Fig. 5A). As shown in Fig. 5B and C, the re-introduction of
p27 reversed miR-25-induced cell proliferation, indicating
that the interaction between miR-25 and p27 is involved in
this process. Taken together, our results suggest that the gene
p27 is an important target of miR-25 in osteosarcoma
cells.
Discussion
In this study, we demonstrate that miR-25 expression
is increased in osteosarcoma tissues. Overexpression of miR-25 by
means of cell transfection promoted cell proliferation in Saos-2
and U2OS cells. Therefore, our study provided evidence, for the
first time to the best of our knowledge, that miR-25 may act as an
onco-miRNA, promoting the progression of osteosarcoma. It is
notable that miR-25 was shown to enhance cell proliferation,
migration and invasion in GC cells, but inhibit cell proliferation
and migration in colon cancer cells (11,12).
Although the reasons for this inconsistence remain unexplored, we
hypothesize that the biological functions of miR-25 in
tumorigenesis may be cell- or tissue-specific. Therefore, the
efficiency and safety associated with the use of miR-25 in gene
therapy should be seriously considered in future studies.
Our study explored the mechanisms underlying the
miR-25 effects, and revealed that p27 maybe a target of
miR-25 in osteosarcoma cells. p27 encodes an enzyme
inhibitor that belongs to the Cip/Kip family of cyclin-dependent
kinase (CDK) inhibitor proteins (13). Through binding to the CDK2 and CDK4
complexes, it prevents their activation, and controls the
cell-cycle progression at the G1 phase (14,15).
Transcriptional or post-transcriptional downregulation of the gene
has been observed in a number of human malignancies, including
osteosarcoma (16,17). For instance, p27 was shown
to be negatively regulated by miR-24, miR-200 and miR-222 in human
cancer (18–20). Therefore, our results provide a
novel mechanism for the downregulation of p27 in
osteosarcoma.
Taken together, the key finding of the present study
is that miR-25 can promote osteosarcoma cell proliferation in
vitro and in vivo by targeting p27, suggesting
that miR-25 may be used as a molecular target for the treatment of
osteosarcoma. However, the roles of this miRNA in osteosarcoma need
to be further investigated.
References
|
1
|
Sun K and Lai EC: Adult-specific functions
of animal microRNAs. Nat Rev Genet. 14:535–548. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
van Kouwenhove M, Kedde M and Agami R:
MicroRNA regulation by RNA-binding proteins and its implications
for cancer. Nat Rev Cancer. 11:644–656. 2011.PubMed/NCBI
|
|
3
|
Ameres SL and Zamore PD: Diversifying
microRNA sequence and function. Nat Rev Mol Cell Biol. 14:475–488.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kasinski AL and Slack FJ: Epigenetics and
genetics. MicroRNAs en route to the clinic: progress in validating
and targeting microRNAs for cancer therapy. Nat Rev Cancer.
11:849–864. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liang W, Gao B, Fu P, Xu S, Qian Y and Fu
Q: The miRNAs in the pathgenesis of osteosarcoma. Front Biosci
(Landmark Ed). 18:788–794. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cai CK, Zhao GY, Tian LY, et al: miR-15a
and miR-16-1 downregulate CCND1 and induce apoptosis and cell cycle
arrest in osteosarcoma. Oncol Rep. 28:1764–1770. 2012.PubMed/NCBI
|
|
7
|
Jin Y, Peng D, Shen Y, et al:
MicroRNA-376c inhibits cell proliferation and invasion in
osteosarcoma by targeting to transforming growth factor-alpha. DNA
Cell Biol. 32:302–309. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao G, Cai C, Yang T, et al: MicroRNA-221
induces cell survival and cisplatin resistance through PI3K/Akt
pathway in human osteosarcoma. PLoS One. 8:e539062013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li LM, Hu ZB, Zhou ZX, et al: Serum
microRNA profiles serve as novel biomarkers for HBV infection and
diagnosis of HBV-positive hepatocarcinoma. Cancer Res.
70:9798–9807. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hu Z, Dong J, Wang LE, et al: Serum
microRNA profiling and breast cancer risk: the use of miR-484/191
as endogenous controls. Carcinogenesis. 33:828–834. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao H, Wang Y, Yang L, Jiang R and Li W:
MiR-25 promotes gastric cancer cells growth and motility by
targeting RECK. Mol Cell Biochem. 385:207–213. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li Q, Zou C, Zou C, et al: MicroRNA-25
functions as a potential tumor suppressor in colon cancer by
targeting Smad7. Cancer Lett. 335:168–174. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chu IM, Hengst L and Slingerland JM: The
Cdk inhibitor p27 in human cancer: prognostic potential and
relevance to anticancer therapy. Nat Rev Cancer. 8:253–267. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Frescas D and Pagano M: Deregulated
proteolysis by the F-box proteins SKP2 and beta-TrCP: tipping the
scales of cancer. Nat Rev Cancer. 8:438–449. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mitrea DM, Yoon MK, Ou L and Kriwacki RW:
Disorder-function relationships for the cell cycle regulatory
proteins p21 and p27. Biol Chem. 393:259–274. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kitagawa K and Kitagawa M: The SCF
ubiquitin ligases involved in hematopoietic lineage. Curr Drug
Targets. 13:1641–1648. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Maran A, Shogren KL, Benedikt M, Sarkar G,
Turner RT and Yaszemski MJ: 2-methoxyestradiol-induced cell death
in osteosarcoma cells is preceded by cell cycle arrest. J Cell
Biochem. 104:1937–1945. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Giglio S, Cirombella R, Amodeo R, Portaro
L, Lavra L and Vecchione A: MicroRNA miR-24 promotes cell
proliferation by targeting the CDKs inhibitors p27Kip1 and
p16INK4a. J Cell Physiol. 228:2015–2023. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fu Y, Liu X, Zhou N, et al: MicroRNA-200b
stimulates tumour growth in TGFBR2-null colorectal cancers by
negatively regulating p27/kip1. J Cell Physiol. 229:772–782. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kedde M, van Kouwenhove M, Zwart W, Oude
Vrielink JA, Elkon R and Agami R: A Pumilio-induced RNA structure
switch in p27-3′ UTR controls miR-221 and miR-222 accessibility.
Nat Cell Biol. 12:1014–1020. 2010.PubMed/NCBI
|