Introduction
Impaired wound healing is frequently reported in
patients with diabetes. The increase in matrix metalloproteinase-9
(MMP-9) expression is associated with diabetes-associated wound
healing (1). Previous experimental
results indicate that MMP-9 expression is elevated during wounding
in diabetic rats (2). Furthermore,
exogenous MMP-9 expression is able to exacerbate chronic wounding
(3). Since increased levels of
MMP-9 are a factor contributing to poor wound healing in diabetic
foot ulcers (4,5), reagents that decrease MMP-9
expression levels may be a useful method to cure the impaired wound
healing in diabetic patients.
Pioglitazone is an agonist of the peroxisome
proliferator-activated receptor-γ (PPAR-γ) that is a
ligand-dependent transcription factor (6). The activation of PPAR-γ protects
pancreatic β-cells from cytotoxicity by preventing nuclear factor
(NF)-κB activation (7–10). Pioglitazone is an antidiabetic
agent, which improves insulin production in patients with diabetes.
Pioglitazone also increases insulin sensitivity, thus it elevates
glucose uptake and inhibits hepatic glucose output (11). It has been reported that
pioglitazone is also able to reduce oxidative stress (12–16).
Advanced glycation end-products (AGEs) are a group
of heterogeneous compounds that are derived from the non-enzymatic
reaction of reducing sugars with proteins, lipids or nucleic acids
(17). AGEs contribute to the
development of various vascular diabetic complications through
increasing the production of reactive oxygen species (ROS), the
formation of cross-links between molecules in the basement membrane
and the extracelluar matrix, and by affecting various cellular
signaling pathways through the receptor for AGEs (RAGE) (18). The binding of AGEs to RAGE triggers
oxidative stress and activates the transcription factor NF-κB,
thus, promoting the expression of pro-inflammatory mediators and
local cellular responses (19,20).
In the present study, human keratinocytes were
treated with pioglitazone in the presence of AGEs. It was
demonstrated that pioglitazone decreases the expression of MMP-9
induced by the treatment of AGEs. The results suggest that
pioglitazone may have therapeutic effects on impaired wound healing
associated with diabetes via a mechanism of inhibiting the
expression level of MMP-9.
Materials and methods
Cells and reagents
Human HaCaT keratinocytes, which were provided by
Xiangya Hospital (Changsha, China), were cultured in Dulbecco’s
modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine
serum (Gibco-BRL, Carlsbad, CA, USA), penicillin (100 U/ml) and
streptomycin (100 μg/ml). For the serum-starving experiments, cells
at 80% confluence were cultured by overnight incubation in
serum-free DMEM containing 0.5 mg/ml bovine serum albumin (BSA;
Calbiochem, La Jolla, CA, USA). Pioglitazone and AGE-BSA were
purchased from Sigma (St. Louis, MO, USA). Antibodies against MMP-9
and β-actin were purchased from Santa Cruz Biotechnology, Inc.
(Santa Cruz, CA, USA).
Cell treatments and the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
HaCaT cells at a density of 6×105
cells/well were seeded into 6-well plates in medium and were
cultured for 24 h. The cells were then treated with BSA only or
AGE-BSA (50, 100, 200, 300 and 400 μg/ml), with or without
pioglitazone (0.5 μM). At the end of each experiment, cells were
incubated with 0.5 mg/ml MTT at 37°C for 4 h. The MTT kit was
purchased from Invitrogen Life Technologies (Carlsbad, CA, USA).
The supernatants were discarded and 50 μl dimethylsulfoxide was
added into each well. The 96-well plates (Asahi Glass Corp, Tokyo,
Japan). were agitated for 10 min. The growth status and
morphological changes of the cells were detected under an inverted
microscope (Olympus, Tokyo, Japan). The absorbance was determined
at 540 nm using a Synergy HT microplate reader (Molecular Devices,
Sunnyvale, CA, USA). The viability of treated cells was expressed
relative to the control cells treated with BSA (relative
viability).
Quantitative polymerase chain reaction
(qPCR)
Total RNA was harvested from cells using the RNeasy
mini kit (Qiagen, Valencia, CA, USA) according to the
manufacturer’s instructions. RNA (1 μl) was reverse transcribed
into cDNA using random primers with a Reverse Transcription II
system purchased from Promega Corporation (Madison, WI, USA)
according to the manufacturer’s instructions. qPCR was conducted
using an ABI Prism Sequence Detection system (Applied Biosystems,
Foster City, CA, USA). An assay reagent containing premixed primers
and a VIC-labeled probe (Applied Biosystems; cat. no. 4310884E) was
used to quantify the expression of endogenous GAPDH mRNA. The
amplification of the MMP-9 cDNA and the endogenous GAPDH cDNA was
determined with FAM and VIC fluorescent intensities, respectively.
The relative quantity of MMP-9 transcripts was normalized to the
quantity of GAPDH mRNA at the same conditions. The primers used
were as follows: Forward: 5′-GCACGACGTCTTCCAGTACC-3′ and reverse:
5′-CAGGATGTCATAGGTCACGTAGC-3′ for MMP-9. The experiments were
repeated independently at least three times.
Immunoblotting assays
Total proteins were harvested from supernatants or
from cells, separated on 10% SDS-PAGE gels and then subjected to
immunoblot analysis. The primary antibodies against MMP-9 and
β-actin were purchased from Santa Cruz Biotechnology, Inc.
(anti-MMP-9; cat. no. sc-21733; 1:200; anti-β-actin; cat. no.
sc-130301; 1:10,000). The secondary antibodies used in the present
study were goat anti-mouse IgG conjugated to horseradish peroxidase
antibodies (cat. no. sc-2005; 1:10,000; Santa Cruz Biotechnology,
Inc.). Bound antibodies were detected using an enhanced
chemiluminescence system (Pierce Biotechnology, Inc., Rockford, IL,
USA). The experiments were repeated independently at least three
times. Image quantifications were performed using ImageQuant
software (GE Healthcare Life Sciences, Piscataway, NJ, USA).
Enzyme-linked immunosorbent assay
(ELISA)
The MMP-9 concentrations in the medium of the HaCaT
keratinocytes treated with BSA, AGE-BSA or in the presence or
absence of pioglitazone (0.5 or 1 μM) were determined using
commercially available ELISA kits (MMP-9 ELISA kit; Raybiotech,
Norcross, GA, USA). The experiment was repeated independently at
least six times. The values are expressed as the mean ± standard
deviation (SD).
Statistical analysis
The experimental data are expressed as the mean ±
SD. Statistical software (SPSS 10.0; SPSS, Inc., Chicago, IL, USA)
was used for independent sample t-tests. P<0.05 was considered
to indicate a statistically significant difference.
Results
AGEs increase the expression of MMP-9
transcripts in HaCaT cells
AGEs contribute to the development of various
vascular diabetic complications and high MMP-9 expression
exacerbates chronic wounds. To determine whether AGE-BSA affects
the expression of MMP-9 in HaCaT cells, the HaCat cells were
treated with BSA only or AGE-BSA (50, 100, 200, 300 and 400 μg/ml)
for 24 h. As shown in Fig. 1A,
AGE-BSA treatment was not observed to significantly affect the cell
viability, indicating that such dosages of AGE-BSA do not result in
non-specific cytotoxicity. However, the qPCR results (Fig. 1B) indicated that AGE-BSA at
concentrations of 300 or 400 μg/ml markedly increased the
transcript levels of MMP-9 in the cells. These results suggest that
AGEs are able to increase the level of MMP-9 mRNA in HaCaT
cells.
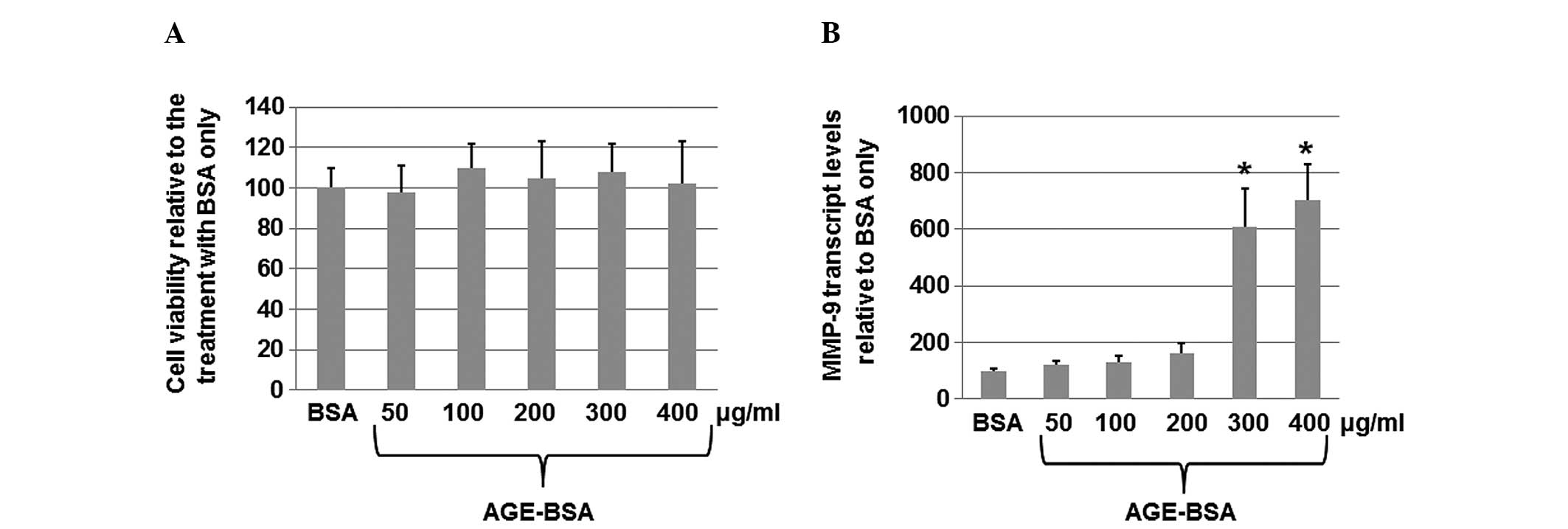 | Figure 1AGE-BSA induces the expression of
MMP-9 in HaCaT cells. HaCaT cells at a density of 6×105
cells/well were seeded into 6-well plates, serum-starved overnight
and then cultured for 24 h with various concentrations (50, 100,
200, 300 and 400 μg/ml) of AGE-BSA or unmodified BSA. (A) Cell
viability was measured using the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay
following the treatment. (B) Total RNA was harvested from HaCaT
cells treated with AGE-BSA or unmodified BSA only. Quantitative
polymerase chain reaction was performed to analyze the MMP-9 mRNA
levels in the cells treated with various concentrations (50, 100,
200, 300 and 400 μg/ml) of AGE-BSA compared with the BSA only
control. The levels (mean value) of MMP-9 transcripts in cells were
calculated. Error bars show the mean ± standard deviation
(P<0.05). The experiments were repeated at least three times.
*P<0.05 vs. the corresponding control. MMP-9, matrix
metalloproteinase-9; AGE, advanced glycation end-products; BSA,
bovine serum albumin. |
AGEs increase the levels of MMP-9 protein
secreted into the medium
To determine whether AGEs increase the levels of
MMP-9 protein secreted into the medium, HaCaT cells were seeded
into 6-well plates at a density of 6×105 cells/well,
serum-starved overnight and then cultured for 24 h with various
concentrations (50, 100, 200, 300 and 400 μg/ml) of AGE-BSA or
unmodified BSA. The medium was collected 24 h after treatment with
AGE-BSA or unmodified BSA. The levels of MMP-9 in the culture were
detected by ELISA. As shown in Fig.
2A, the ELISA results suggested that AGE-BSA increased the
levels of MMP-9 in the medium. The levels of MMP-9 secreted into
the medium were upregulated by up to four-fold upon treatment with
AGE-BSA at a concentration of 400 μg/ml. The western blot analysis
results (Fig. 2B) also indicated
that AGE-BSA (300 or 400 μg/ml) significantly increased the levels
of the secreted MMP-9 proteins. These results suggest that AGEs are
able to increase the levels of MMP-9 protein secreted into the
medium.
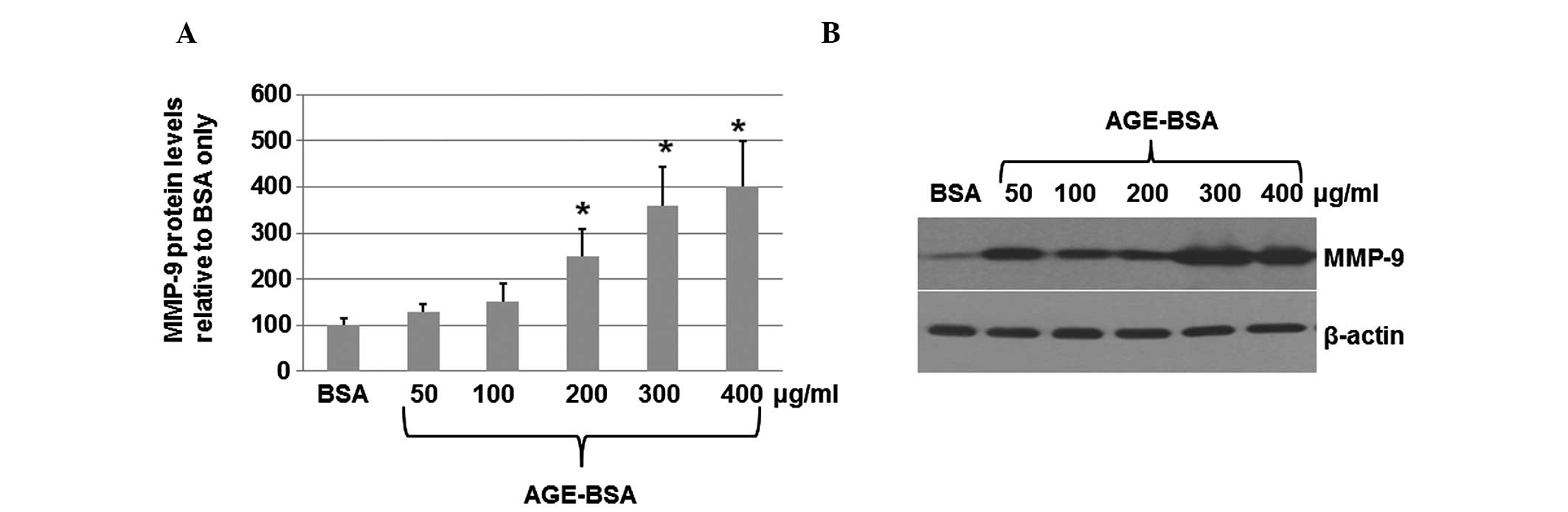 | Figure 2Levels of MMP-9 protein in the medium
as determined by ELISA and western blot analysis. HaCaT cells at a
density of 6×105 cells/well were seeded into 6-well
plates, serum-starved overnight and then cultured for 24 h with
various concentrations (50, 100, 200, 300 and 400 μg/ml) of AGE-BSA
or unmodified BSA. (A) Medium was collected 24 h post-treatment
with AGE-BSA or unmodified BSA. The levels of MMP-9 in the culture
were detected by ELISA. The data (mean ± standard deviation) are
from six independent experiments. (B) Medium was collected and
concentrated. The total proteins were isolated from the medium and
subjected to western blot analysis. Primary antibodies against
MMP-9 and β-actin were purchased from Santa Cruz Biotechnology,
Inc, Santa Cruz, CA, USA). The secondary antibodies used in the
present study were goat anti-mouse IgG conjugated to horseradish
peroxidase. Bound antibodies were detected using an enhanced
chemiluminescence system (Pierce Biotechnology, Inc.). The
experiments were repeated independently at least three times. Image
quantifications were performed using ImageQuant software. MMP-9,
matrix metalloproteinase-9; ELISA, enzyme-linked immunosorbent
assay; AGEs, advanced glycation end-products; BSA, bovine serum
albumin. |
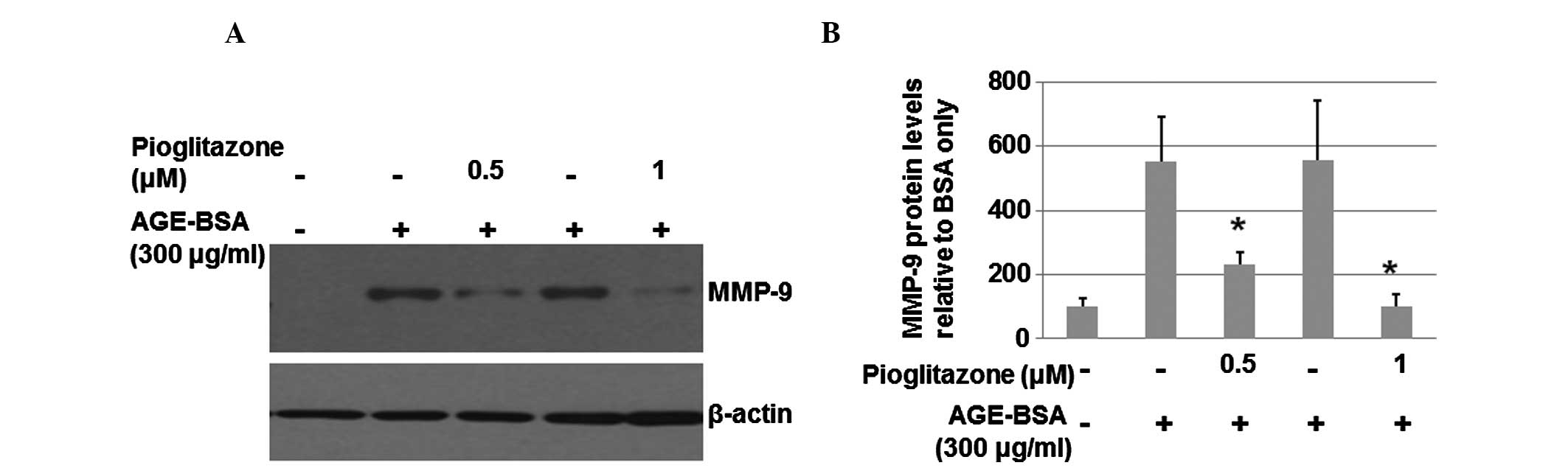
Pioglitazone is able to reduce the high
levels of MMP-9 protein induced by AGE
In order to determine whether pioglitazone is able
to decrease MMP-9 expression, HaCaT cells at a density of
6×105 cells/well were serum-starved overnight and then
cultured for 24 h with 300 μg/ml AGE-BSA in the absence or presence
of pioglitazone (0.5 or 1 μM). The total proteins were harvested
from the cells and then subjected to western blot analysis. As
shown in Fig. 3A, pioglitazone
(0.5 μM) significantly inhibited the MMP-9 level. A higher
concentration of pioglitazone (1 μM) resulted in a greater
inhibitory effect on the MMP-9 level. The levels of MMP-9 in the
medium were also measured by ELISA. As shown in Fig. 3B, pioglitazone (0.5 or 1 μM)
significantly suppressed the levels of MMP-9 in the medium. These
results suggest that pioglitazone significantly inhibits the
expression of MMP-9.
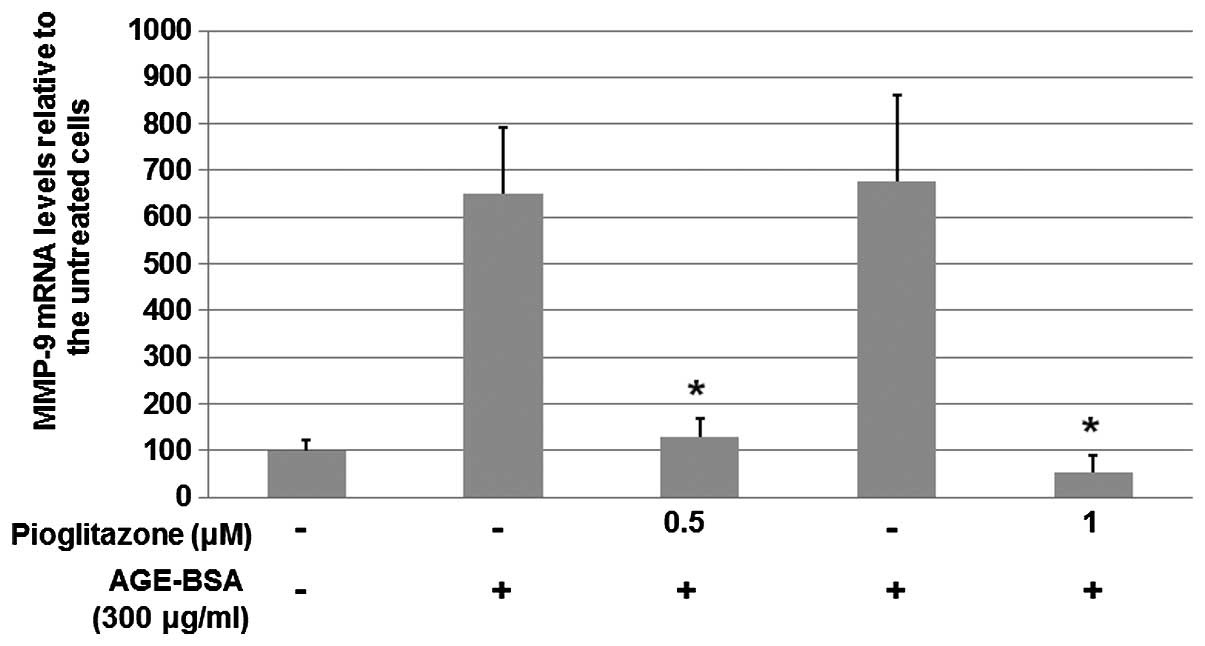
Pioglitazone reduces the protein expression of
MMP-9 induced by AGEs.
To further investigate the mechanisms underlying the
inhibitory effect of pioglitazone on the increased MMP-9 expression
induced by AGEs, HaCaT cells at a density of 6×105
cells/well were serum-starved overnight and then cultured for 24 h
with 300 μg/ml AGE-BSA in the absence or presence of pioglitazone
(0.5 or 1 μM). Total RNA was harvested from HaCaT cells and qPCR
was performed to analyze the mRNA levels of MMP-9. As shown in
Fig. 4, the qPCR results indicated
that pioglitazone at concentrations of 0.5 and 1 μM significantly
suppressed the levels of MMP-9 mRNA to 20 and 8%, respectively.
These results suggest that pioglitazone suppresses the expression
of MMP-9 via a transcriptional mechanism.
Discussion
As a PPAR agonist, pioglitazone has demonstrated
promise as a therapeutic agent due to its ability to increase the
functional recovery of wounds and decrease lesion sizes following
injury (21). The therapeutic
effects of pioglitazone are considered to be a result of the
regulation of multiple pathways (11–16).
Since the increased expression of MMP-9 contributes to poor wound
healing in diabetic foot ulcers (4,5), the
present study examined whether pioglitazone acts via a mechanism
associated with the regulation of the expression of MMP-9 in human
keratinocytes treated with AGEs. The results revealed that
pioglitazone at concentrations of 0.5 or 1 μM suppressed the levels
of MMP-9 mRNA to 20 or 8%, respectively. Since the increased
expression of MMP-9 contributes to poor wound healing (4,5), the
results of the present study suggest that pioglitazone may have
therapeutic effects on impaired wound healing associated with
diabetes via a mechanism of inhibiting MMP-9 expression. This
finding provides novel evidence for the application of pioglitazone
in the field of wound healing.
It is reported that pioglitazone may inhibit the
TGF-β-induced myofibroblast differentiation (22). Pioglitazone also attenuates
TGF-β-induced type I collagen and fibronectin mRNA and protein
production, which are involved in burn wound healing (22,23).
It was revealed that PPAR-γ-dependent and PPAR-γ-independent
mechanisms were involved in the action of pioglitazone (22–24),
although PPAR-γ agonists do not prevent the activation of quiescent
hepatic stellate cells in vitro, nor hepatic fibrogenesis in
mice (24).
In addition to the diabetes-associated wound
healing, pioglitazone may be useful for other types of wound
healing, including gastric ulcer healing. The involvement of PPAR-γ
in inflammatory responses during pioglitazone-mediated gastric
ulcer healing has been reported (25). In the present study, the findings
suggest that use of pioglitazone has potential in the wound healing
therapy and it may be a promising approach upon further study. The
results provide novel evidence for understanding the molecular
mechanisms underlying the action of pioglitazone.
Acknowledgements
This study was supported by the Inner Mongolia
Institute of Burn.
References
|
1
|
Gill SE and Parks WC: Metalloproteinases
and their inhibitors: regulators of wound healing. Int J Biochem
Cell Biol. 40:1334–1347. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang C, Zhu P, Yan L, et al: Dynamic
changes in matrix metalloproteinase 9 and tissue inhibitor of
metalloproteinase 1 levels during wound healing in diabetic rats. J
Am Podiatr Med Assoc. 99:489–496. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Reiss MJ, Han YP, Garcia E, et al: Matrix
metalloproteinase-9 delays wound healing in a murine wound model.
Surgery. 147:295–302. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu Y, Min D, Bolton T, et al: Increased
matrix metalloproteinase-9 predicts poor wound healing in diabetic
foot ulcers. Diabetes Care. 32:117–119. 2009. View Article : Google Scholar
|
|
5
|
Rayment EA, Upton Z and Shooter GK:
Increased matrix metalloproteinase-9 (MMP-9) activity observed in
chronic wound fluid is related to the clinical severity of the
ulcer. Br J Dermatol. 158:951–961. 2008. View Article : Google Scholar
|
|
6
|
Bell DS: Beta-cell rejuvenation with
thiazolidinediones. Am J Med. 115:20S–23S. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Saitoh Y, Chun-ping C, Noma K, Ueno H,
Mizuta M and Nakazato M: Pioglitazone attenuates fatty acid-induced
oxidative stress and apoptosis in pancreatic beta cells. Diabetes
Obes Metab. 10:564–573. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu X, Luo D, Zheng M, Hao Y, Hou L and
Zhang S: Effect of pioglitazone on insulin resistance in
fructose-drinking rats correlates with AGEs/RAGE inhibition and
block of NADPH oxidase and NF kappa B activation. Eur J Pharmacol.
629:153–158. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ao C, Huo Y, Qi L, Xiong Z, Xue L and Qi
Y: Pioglitazone suppresses the lipopolysaccharide-induced
production of inflammatory factors in mouse macrophages by
inactivating NF-kappaB. Cell Biol Int. 34:723–730. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wan H, Yuan Y, Qian A, Sun Y and Qiao M:
Pioglitazone, a PPARgamma ligand, suppresses NFkappaB activation
through inhibition of IkappaB kinase activation in cerulein-treated
AR42J cells. Biomed Pharmacother. 62:466–472. 2008. View Article : Google Scholar
|
|
11
|
Bell DS: Beneficial effects resulting from
thiazolidinediones for treatment of type 2 diabetes mellitus.
Postgrad Med. 8:35–44. 2003.PubMed/NCBI
|
|
12
|
Gumieniczek A, Hopkała H and Zabek A:
Protective effects of a PPARgamma agonist pioglitazone on
anti-oxidative system in testis of diabetic rabbits. Pharmazie.
63:377–378. 2008.PubMed/NCBI
|
|
13
|
Collino M, Aragno M, Mastrocola R, et al:
Modulation of the oxidative stress and inflammatory response by
PPAR-gamma agonists in the hippocampus of rats exposed to cerebral
ischemia/reperfusion. Eur J Pharmacol. 530:70–80. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gumieniczek A, Krzywdzińska M and Nowak M:
Modulation of nitrosative/oxidative stress in the lung of
hyperglycemic rabbits by two antidiabetics, pioglitazone and
repaglinide. Exp Lung Res. 35:371–379. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Somi MH, Hajipour B, Asl NA, et al:
Pioglitazone attenuates ischemia/reperfusion-induced liver injury
in rats. Transplant Proc. 41:4105–4109. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bernardo A, Bianchi D, Magnaghi V and
Minghetti L: Peroxisome proliferator-activated receptor-gamma
agonists promote differentiation and antioxidant defenses of
oligodendrocyte progenitor cells. J Neuropathol Exp Neurol.
68:797–808. 2009. View Article : Google Scholar
|
|
17
|
Fukami K, Yamagishi SI, Sakai K, Kaida Y,
Adachi T, Ando R and Okuda S: Potential inhibitory effects of
L-carnitine supplementation on tissue advanced glycation end
products in patients with hemodialysis. Rejuvenation Res.
16:460–466. 2013. View Article : Google Scholar
|
|
18
|
Goldin A, Beckman JA, Schmidt AM and
Creager MA: Advanced glycation end products: sparking the
development of diabetic vascular injury. Circulation. 114:597–605.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bierhaus A and Nawroth PP: Multiple levels
of regulation determine the role of the receptor for AGE (RAGE) as
common soil in inflammation, immune responses and diabetes mellitus
and its complications. Diabetologia. 52:2251–2263. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wautier JL and Guillausseau PJ: Advanced
glycation end products, their receptors and diabetic angiopathy.
Diabetes Metab. 27:535–542. 2001.PubMed/NCBI
|
|
21
|
Yonutas HM and Sullivan PG: Targeting PPAR
isoforms following CNS injury. Curr Drug Targets. 14:733–742. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pan HW, Xu JT and Chen JS: Pioglitazone
inhibits TGFβ induced keratocyte transformation to myofibroblast
and extracellular matrix production. Mol Biol Rep. 38:4501–4508.
2011.
|
|
23
|
Dong X, Geng Z, Zhao Y, Chen J and Cen Y:
Involvement of mast cell chymase in burn wound healing in hamsters.
Exp Ther Med. 5:643–647. 2013.PubMed/NCBI
|
|
24
|
Da Silva Morais A, Abarca-Quinones J,
Horsmans Y, Stärkel P and Leclercq IA: Peroxisome
proliferated-activated receptor gamma ligand, Pioglitazone, does
not prevent hepatic fibrosis in mice. Int J Mol Med. 19:105–112.
2007.PubMed/NCBI
|
|
25
|
Lahiri S, Sen T and Palit G: Involvement
of glucocorticoid receptor and peroxisome proliferator activated
receptor-gamma in pioglitazone mediated chronic gastric ulcer
healing in rats. Eur J Pharmacol. 609:118–125. 2009. View Article : Google Scholar
|