Introduction
A rapid increase has occurred in the number of
patients with dialysis that are >65 years of age (1). Elderly patients are often in poor
health and physically incapacitated, susceptible to malnutrition
and have multiple complicating medical disorders in addition to
end-stage renal disease. Continuous ambulatory peritoneal dialysis
(PD) offers numerous advantages to elderly patients, including
hemodynamic stability, steady-state chemistries and no requirement
to create vascular access. Conventional PD solutions contain
glucose as an osmotic agent as it is inexpensive, safe and readily
metabolized. However, the use of glucose-based PD fluid may cause a
number of side-effects, including dehydration, hypokalemia,
hyperglycemia, metabolic alkalosis and chemical peritonitis.
Previous studies indicated that high glucose (HG) induces a complex
mixture of proinflammatory and profibrotic stimuli during
peritoneal epithelial-to-mesenchymal transition (EMT) in
vivo (2,3). EMT is a dynamic and complex process
that may require the participation of numerous growth factors or
cytokines (4). Therefore, factors
that regulate EMT are attracting increasing attention as inducers
of peritoneal fibrosis.
The expression of heat shock proteins (HSPs) is
induced in response to a wide variety of physiological and
environmental insults. Their functions as molecular chaperones
allow cells to adapt to gradual changes in their environment and to
survive in otherwise lethal conditions (5). HSP70, as one of the HSPs, is an
ATP-dependent molecular chaperone which performs house-keeping
functions; controlling the activity, turnover and trafficking of a
variety of proteins, including protein kinases, steroid receptors
and transcription factors. HSP70 exhibits an important role in
various signaling pathways that have crucial roles in growth
control, cell survival and developmental processes (6,7).
However, the role of HSP70 in the HG-induced EMT of peritoneal
mesothelial cells is unknown. The present study investigated the
regulation of HSPs and their role in cell EMT, particularly in rat
peritoneal mesothelial cells (RPMCs), and the surrounding glucose
concentrations and the molecular mechanism involved.
In the present study, whether HG influences HSP
expression and to what extent HSPs contribute to HG-induced EMT in
RPMCs was investigated.
Material and methods
Reagents
Penicillin-streptomycin (5,000 U/ml penicillin;
5,000 U/ml streptomycin), Dulbecco’s modified Eagle’s medium
(DMEM)/F12 and fetal bovine serum (FBS) were obtained from
Gibco-BRL (Grand Island, NY, USA). Triton X-100 and
dimethylsulfoxide were purchased from Sigma (St. Louis, MO, USA).
HG was obtained from Xinhua Pure Chemical Industries (Shenyang,
China). Anti-collagen type I, anti-β-actin, and anti-phospho Smad 3
and 4 antibodies were obtained from Santa Cruz Biotechnology, Inc.
(Santa Cruz, CA, USA); anti-α-SMA and anti-vimentin antibodies were
purchased from Sigma; and anti-E-cadherin antibody was obtained
from BD Biosciences (San Jose, CA, USA). An ECL kit was purchased
from Thermo Scientific Pierce (Rockford, IL, USA). A FACSCalibur
flow cytometer was purchased from BD Biosciences. All reagents used
were trace element analysis grade. All water used was glass
distilled.
Isolation and culture of RPMCs
RPMCs were isolated and cultured. Sprague-Dawley
rats were purchased from the experimental Animal Breeding Center of
Shanghai Jiao Tong University (Shanghai, China). The handling of
animals was in accordance with provisions of Medical Ethics.
Briefly, surgically resected omenta from Sprague-Dawley rats were
digested with 0.125% trypsin for 25 min at 37°C, followed by
neutralization with DMEM/F12 medium supplemented with 10% FBS. The
tissue-free cells were then centrifuged at 60 × g at 4°C for 5 min,
followed by removal of the supernatant. The cell pellet was
resuspended to a final volume of 4 ml in culture medium and then
seeded in 25-cm2 tissue culture flasks. The culture
medium consisted of DMEM/F12 medium supplemented with 10% FBS, 2
mmol/l L-glutamine, 100 IU/ml penicillin and 100 mg/ml
streptomycin. After 1–3 days incubation at 37°C, the media were
changed for the first time. Cells were then transferred to
serum-free DMEM/F12 medium for overnight starvation prior to each
experiment.
Identification of peritoneal mesothelial
cells
The RPMCs were observed under a phase contrast
inverted microscope and identified using immunocytochemistry. The
immunohistochemical antibodies used included vimentin cytokeratin
VIII factor-related antigen and leukocyte CD45 antibodies. The
cells were seeded and fixed in 10% neutral formalin for 30 min.
Subsequently, antigen retrieval and DAB color dehydration were
performed. The cultured cells were observed under a light
microscope and cells staining positive for vimentin were
characteristic mesothelial cells.
RNA interference plasmid construction and
transient transfection
A small interfering RNA (siRNA)-DNA hybrid was
formed from DNA corresponding to the rat HSP70 cDNA sequence
(5′-AAGGCCAACAAGATCACCAT-3′) and an antisense RNA strand
[5′-AUGGUGAUCUUGUUGGCCU(dTdT)-3′]. RNA interference plasmid
construction was performed by cloning the synthesized
oligonucleotide into a pSilencer 2.1-U6-neo plasmid. All these
procedures were conducted by Genechem Co. (Shanghai, China). The
target sequences of the HSP70 siRNA and control HSP70 siRNA were
BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi) searched
against the GenBank database (https://www.ncbi.nlm.nih.gov/genbank/). The HSP70
targeting sequence matched exactly with partial sequences of the
rat HSP70 gene, but not with any other genes. The control siRNA did
not match any known rat gene. Transient transfections were
performed using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA),
according to the manufacturer’s instructions. All these experiments
were performed in six-well tissue culture plates with cells plated
to reach 50–60% confluence on the day of transfection. The
transfection efficiency averaged between 50 and 70%. Cells were
allowed to recover in medium for 24 h after transfection.
HSP70 overexpression plasmid construction
and transient transfection
Plasmids expressing a HSP70-cDNA gene plasmid
(constructed by Genechem Co., Shanghai, China). were transfected
into peritoneal mesothelial cells using Lipofectamine 2000
according to the manufacturer’s instructions. All of these
experiments were performed in six-well tissue culture plates with
cells plated to reach 50–60% confluence on the day of
transfection.
Quantitative polymerase chain reaction
(qPCR)
Total RNA from the cultured cells was isolated using
TRIzol reagent (Invitrogen). RNase-free DNase I was used to
eliminate genomic DNA contamination in the RNA samples. The 260/280
absorbance ratio was measured for verification of the purity of the
RNA. The sequences of the HSP70 and β-actin genes were obtained
from the GenBank database, and specific primers for them were
designed over an exon-exon junction with Primer Premier, version
5.0 (Premier Biosoft, Palo Alto, CA, USA). The following primer
sequences were used: 5′-AGC GGG AAA TCG TCG GTG-3′ and 5′-GGG TAC
ATG GTG GTG CCG-3′ for β-actin; and 5′-TA CAT ATG GCC AAA GCC GCG
GCA GTC G-3′ and 5′-TG CTC GAG ATC TAC CTC CTC AAT GGT GGG-3′ for
HSP70. PCR reactions were performed with a Gene Amp PCR system 9700
(Perkin-Elmer, Waltham, MA, USA) and 35 cycles of amplification
were conducted. The amplified products were separated by
electrophoresis on a 2% agarose gel and visualized by ethidium
bromide staining. The expression of HSP70 was semi-quantitated
using β-actin as an internal control. Image density was quantified
with a FluorImager SI (Amersham Pharmacia Biotech, Amersham,
UK).
Western blot analysis
All cells were washed twice with cold PBS and
resuspended in five volumes of ice-cold extract buffer (20 mM
western blot analysis Hepes-KOH, 1.5 mM MgCl2, 1 mM
EDTA, 1 mM ethylene glycol tetraacetic acid, 1 mM dithiothreitol,
and 0.1 mM phenylmethanesulfonyl fluoride, pH 7.5) for 15 min at
4°C. Lysates were centrifuged at 25,000 × g for 15 min. The protein
concentrations of the supernatants were determined using the Micro
BCA kit method (Sigma). The samples (10 μg protein) were separated
by sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) with 10% and 8% polyacrylamide gel. The primary
antibodies used included anti-collagen type I (1:250), anti-α-SMA
(1:400), anti-β-actin (1:400), anti-vimentin (1:400),
anti-E-cadherin (1:400), anti-phospho Smad 3 (1:800) and
anti-phospho Smad 4 (1:800). These separated proteins were
electrotransferred to a Hybond-polyvinylidenefluoride (PVDF)
membrane. The individual SDS gel was distinguished by placing the
protein molecular weight marker (Invitrogen) in a different but
consistent position. The PVDF membrane was then soaked in a
blocking solution [5% nonfat milk in Tris-buffered saline and Tween
20 (TBST) buffer (20 mM Tris-HCl, pH 7.5, 0.5 M NaCl, 0.1% Tween
20)] for 2 h at room temperature. The soaked PVDF membrane was then
incubated in TBST containing primary antibodies overnight at 4°C,
washed with TBST buffer three times for 5 min each, and incubated
at room temperature for 2 h in TBST containing horseradish
peroxidase (HRP)-conjugated goat anti-mouse and goat anti-rabbit
IgG antibodies (Santa Cruz Biotechnology, Inc.). The membrane was
washed with TBST buffer three times for 10 min each. The membranes
were incubated in ECL reagent (Thermo Scientific Pierce) for HRP
(30 sec) and exposed to autoradiography film for visualization of
the bands. The relative amounts of various proteins were analyzed.
The results were quantified by Quantity One Software (Bio-Rad,
Hercules, CA, USA).
Detection of intracellular reactive
oxygen species (ROS) level
To determine the levels of ROS generation within
HG-treated cells, fluorescence-activated cell sorting analysis was
performed. Cells were stained with 5 μg/ml
dihydrodichlorofluorescein diacetate (DCF-DA) for 30 min, subjected
to flow cytometry using a FACSCalibur and analyzed by CellQuest
software (BD Biosciences, San Jose, CA, USA).
Statistical analysis
Statistical analysis was performed using SPSS
software (version 18.0, SPSS, Inc., Chicago, IL, USA). Data are
expressed as the mean ± standard error of the mean. Variance was
homogenous for use of standard analysis of variance (ANOVA)
methodology. After statistical significance was established by
ANOVA, individual comparisons were made using Tukey’s multiple
comparison test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of HG on EMT of peritoneal
mesothelial cells
RPMCs were isolated and cultured. The RPMCs were
positive for cytokeratin and vimentin and negative for the VIII
factor-related antigen and leukocyte CD45 antigen. To elucidate the
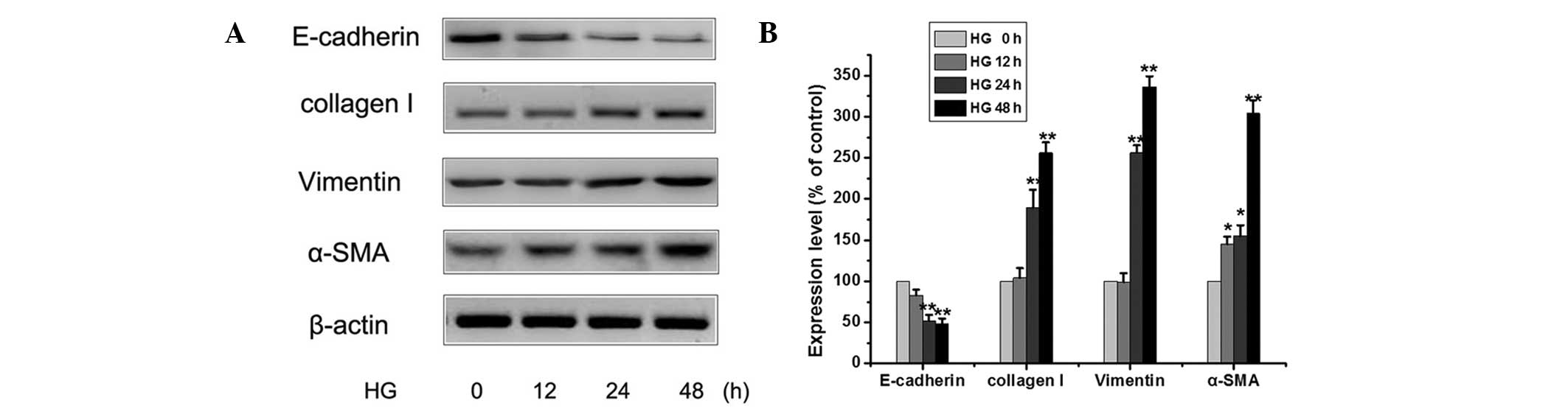
mechanism of EMT by HG, the expression levels of E-cadherin,
vimentin, collagen I and α-smooth muscle actin (SMA) were
monitored. Exposure of peritoneal mesothelial cells to HG (25 mM
glucose) for 0–48 h reduced the protein expression levels of
E-cadherin and increased the protein expression levels of the
vimentin, collagen I and α-SMA over time (Fig. 1). Using western blotting, it was
shown that there was time-dependent change in the expression levels
of EMT-related proteins in HG-treated peritoneal mesothelial cells.
These results indicated that HG could induce EMT and lead to
increased EMT events in peritoneal mesothelial cells.
Effect of HSP70 on HG-induced EMT of
peritoneal mesothelial cells
HSP70, as a ubiquitously expressed protein, is
upregulated by variable stresses, including heat, oxidative stress
and chemical injury in the cells (8). Therefore, whether the treatment of
peritoneal mesothelial cells with HG changes the expression of HSP
in the process was considered in the present study. Cells were
treated with 25 mM glucose for 0–48 h, and the expression of HSP70
was determined by western blotting. As shown by Fig. 2A and B, HG enhanced the levels of
HSP70, with induction sustained upon HG stimulation compared to the
control. To determine the role of HSP70, the effects of HSP70 were
studied by modulating HSP70 expression through siRNA knockdown and
overexpression. qPCR analysis was performed on isolated total RNA
to determine the levels of HSP70 mRNA following transfection of
peritoneal mesothelial cells with HSP70 siRNA and HSP70
overexpression plasmid for different time periods (Fig. 2C and D). From the results of the
qPCR, it was found that siRNA knockdown and HSP70 overexpression
were the most efficient following transfection for 48 h, and the
inhibition rate of siRNA knockdown reached >50% compared with
the levels of the control. Control, HSP70-siRNA knockdown and HSP70
overexpression cells were treated with 25 mM glucose, and the
expression levels of E-cadherin, vimentin, collagen I and α-SMA
were detected by western blot analysis (Fig. 2E and F). Transiently overexpressing
HSP70 significantly reduced the levels of HG-induced EMT, as
evidenced by the reduced upregulation of α-SMA, vimentin and
mesenchymal marker collagen I, and the ameliorated expression of
the epithelial protein, E-cadherin. By contrast, siRNA-mediated
suppression of HSP70 further exacerbated HG-induced EMT. Notably,
siRNA-treated or HSP70 overpression plasmid-treated cultures alone
did not induce EMT-mediated changes in the RPMCs.
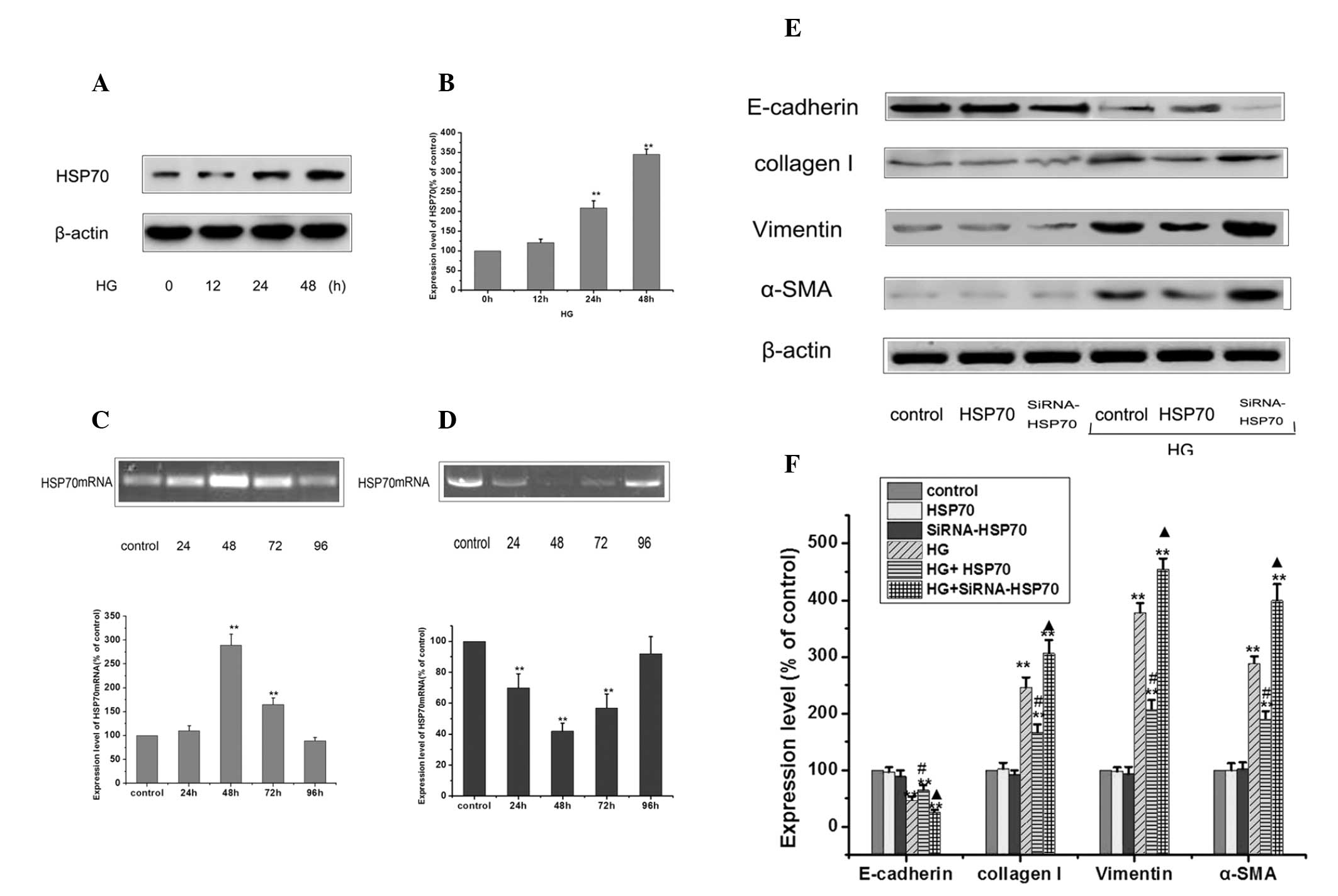 | Figure 2Effects of transfection and RNA
interference of HSP70 on the protein expression of E-cadherin,
α-SMA, vimentin and collagen I in RPMCs. (A) RPMCs were treated
with 25 mM HG at different time points to induce EMT and the
expression of HSP70 was detected by western blotting. (B) Values
represent the mean ± standard error of the mean of three
independent experiments performed. β-actin was used as the loading
control. **P<0.01 vs. HG 0 h group,
*P<0.05 vs. HG 0 h group. (C) qPCR analysis was
performed using isolated total RNA from HSP70 mRNA following
transfection with a HSP70 overexpression plasmid for different time
periods. Each value represents the mean ± standard error of the
mean (n=3). *P<0.05 vs. untreated control.
**P<0.01 vs. control. (D) qPCR analysis was performed
using isolated total RNA from HSP70 mRNA following transfection
with HSP70-siRNA for different time periods. Each value represents
the mean ± standard error of the mean (n=3). *P<0.05
vs. untreated control. **P<0.01 vs. control. (E)
Control, HSP70-siRNA knockdown and HSP70 overexpression cells were
treated with or without 25 mM glucose for 24 h and the expression
of E-cadherin, vimentin, collagen I and α-SMA were detected by
western blotting. (F) Values represent the mean ± standard error of
the mean of three independent experiments performed. β-actin was
used as the loading control. (**P<0.01 vs. control;
#P<0.01 HG group vs. HG+HSP70
group;.▲P<0.01 HG group vs. HG+siRNA-HSP70 group).
HSP70, heat shock protein 70; α-SMA, α-smooth muscle actin; siRNA,
small interfering RNA; HG, high glucose; RPMCs, rat peritoneal
mesothelial cells; EMT, epithelial-to-mesenchymal transition; qPCR,
quantitative polymerase chain reaction. |
Effect of HSP70 on Smad pathway in the
HG-treated RPMCs
Smad signaling pathways have been reported as
important in the EMT (9). To
investigate the possible involvement of Smad signaling in the
protective effect of HSP70 in RPMCs from HG-induced EMT, control,
HSP70-siRNA knockdown and HSP70 overexpression cells were treated
with 25 mM glucose, and the expression levels of p-Smad3 and
p-Smad4 were detected by western blot analysis. Under controlled
conditions, HSP70-siRNA knockdown and HSP70 overexpression did not
enhance p-Smad3 and 4 levels compared with those in the control
cells. However, following exposure to HG, p-Smad3 and 4 levels were
found to be significantly enhanced in HSP70-siRNA group compared
with those in the HG group. Conversely, overexpression of HSP70
significantly inhibited HG-induced phosphorylation of Smad3 and 4
(Fig. 3). Overall, these results
suggest that HSP70 may attenuate HG-induced EMT by inhibiting the
activated Smad pathway.
HSP70 inhibits ROS and attenuates EMT of
peritoneal mesothelial cells
It has been shown that oxidant/antioxidant imbalance
can activate multiple molecular pathways that culminate in the
induction of EMT in target cells: ROS could activate Smad signaling
molecules, which are crucial for EMT (10). Thus, the HG-induced ROS levels in
different treatment groups were detected. The cells were stained
with DCF-DA to detect the intracellular ROS production. To further
analyze the involvement of ROS in the course of EMT, the effect of
HG in the presence of the antioxidant, N-acetylcysteine (NAC) was
assessed. As shown by the results of Fig. 4A, HG increased the levels of
intracellular ROS production compared with those in the control
cells. However, HG-induced ROS production was significantly blocked
by pretreatment of cells with NAC (Fig. 4A and B). The western blot analysis
results demonstrated that HG reduced the expression levels of the
protein E-cadherin and increased the expression levels of the
proteins vimentin, collagen I and α-SMA, compared with those in the
control cells. HG-mediated changes in the expression levels of the
EMT proteins were abolished by NAC (Fig. 4C and D). These results indicate
that ROS generation induced by HG has an important role in EMT of
peritoneal mesothelial cells. Subsequently, the role of HSP70 in
HG-induced ROS generation was investigated. Under controlled
conditions, HSP70-siRNA knockdown and HSP70 overexpression did not
markedly change the levels of intracellular ROS production compared
with those in the control cells. However, following exposure to HG,
the ROS levels were found to be significantly enhanced in the
HSP70-siRNA group compared with those in the HG group. Conversely,
overexpression of HSP70 significantly inhibited HG-induced ROS
production and HG-induced EMT (Fig. 4E
and F). Overall, these results suggest that HSP70 may attenuate
HG-induced EMT by inhibiting ROS production.
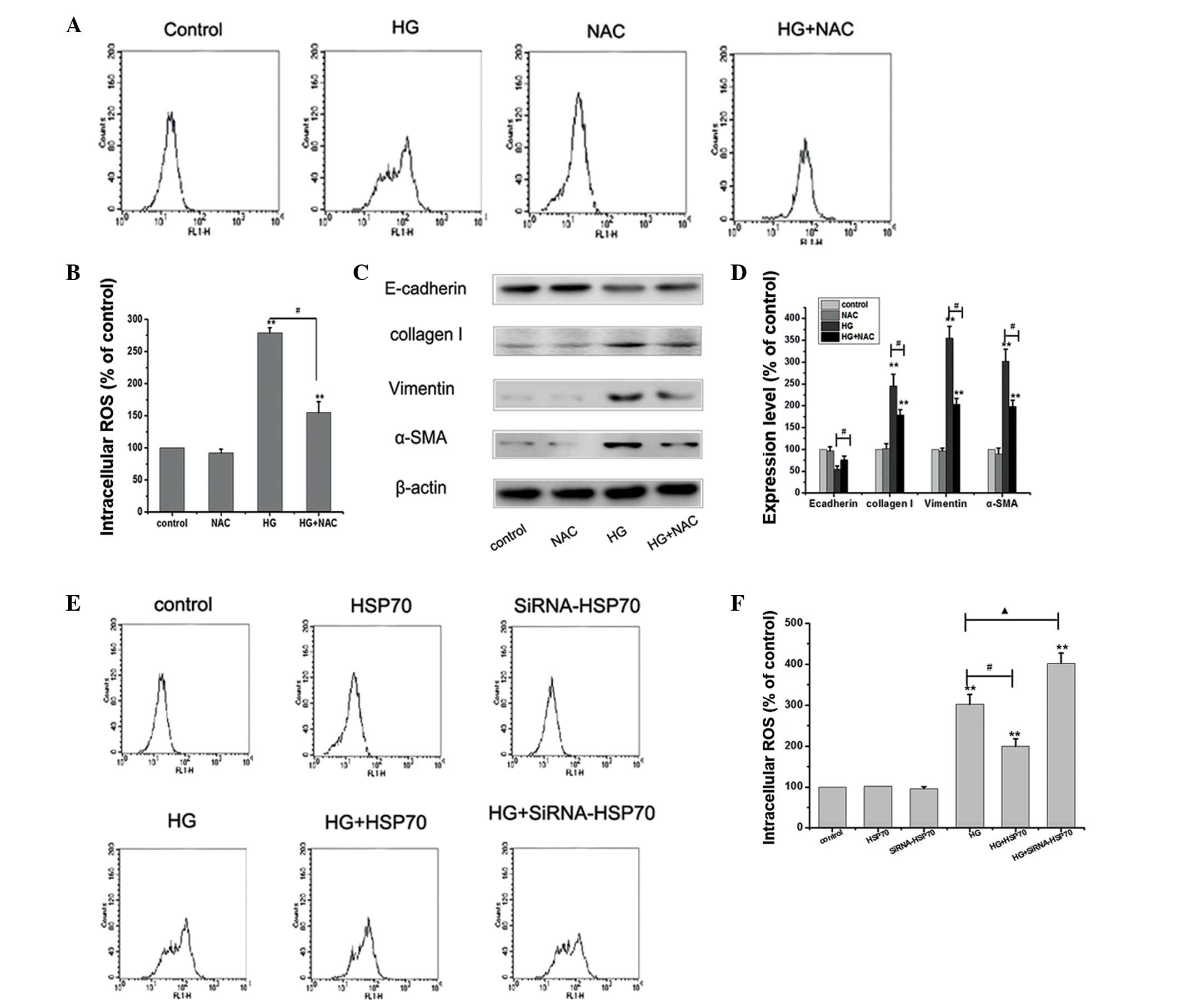 | Figure 4HSP70 reduces HG-induced ROS and
inhibits HG-induced EMT in RPMCs. (A and B) Cells were exposed to
25 mM glucose for 24 h, or a combined treatment of 25 mM glucose
and 10 mmol/l NAC for 24 h, and the cells were stained with DCF-DA
to detect the intracellular ROS production. **P<0.01
vs. control; #P<0.01 HG group vs. HG+NAC group. (C
and D) Cells were treated as previously described, and the
expression of E-cadherin, vimentin, collagen I and α-SMA were
detected by western blotting. Values represent the mean ± standard
error of the mean of three independent experiments performed.
β-actin was used as the loading control. **P<0.01 vs.
control; #P<0.01 HG group vs. HG+NAC group. (E and F)
Control, HSP70-siRNA knockdown and HSP70 overexpression cells were
treated with or without 25 mM glucose for 24 h and the cells were
stained with DCF-DA to detect the intracellular ROS production.
**P<0.01 vs. control; #P<0.01 HG group
vs. HG+HSP70 group; ▲P<0.01 HG group vs.
HG+siRNA-HSP70 group. HG, high glucose; NAC, N-acetylcysteine;
HSP70, heat shock protein 70; ROS, reactive oxygen species; α-SMA,
α-smooth muscle actin; siRNA, small interfering RNA; EMT,
epithelial-to-mesenchymal transition; RPMCs, rat peritoneal
mesothelial cells; DCF-DA, dihydrodichlorofluorescein
diacetate. |
Discussion
By the end of 2005, the number of patients with PD
displayed a rapid growth trend (10). Globally, the number of patient with
PD was ~160,000 in 2012, accounting for 11% of the total number of
patients receiving dialysis (11).
However, the ultrafiltration failure resulting from peritoneal
fibrosis is one of the main complications that occur after long
periods of PD, and compels patients to withdraw from PD. A previous
study implicates the appearance of the EMT as the main point in the
early pathogenesis of the development and progression of peritoneal
fibrosis (12). Another study
showed that HG could induce EMT in vivo (2). Therefore, the present study was
conducted to identify the factors that reduce or inhibit EMT. In
this study, it was found that HG could induce EMT and lead to
increased EMT events in peritoneal mesothelial cells.
HSP70, as a ubiquitously expressed protein, is
upregulated by variable stresses, including heat, oxidative stress,
anticancer chemotherapy and chemical injury in the cells (13). Thus, we hypothesized that HSP70 may
be involved in HG-induced EMT. In the present study, it was
demonstrated that HG lead to enhanced levels of HSP70, with
induction sustained upon HG stimulation compared with those of the
control. To determine the role of HSP70, the effects of HSP70 were
studied by modulating HSP70 expression through siRNA knockdown and
overexpression. This study found that overexpressing HSP70
attenuated HG-induced upregulation of collagen I and α-SMA and
ameliorated E-cadherin expression in the RPMCs, while
siRNA-mediated suppression of HSP70 further exacerbated HG-induced
EMT.
It is well-established that EMT is stimulated
through the Smad signaling pathway and inhibition of Smad signaling
is a central mechanism in the prevention of peritoneal fibrosis
(14,15). In order to further confirm the
effect of HSP70 on Smad pathways in HG-induced EMT in RPMCs, the
present study investigated the expression of HG-induced
phosphorylated Smad3 and Smad4 by HSP70 overexpression. It was
found that overexpressed HSP70 significantly inhibited HG-induced
phosphorylation of Smad3 and 4. These results suggest that HSP70
may reduce EMT by inhibiting the activation of HG-induced Smad
pathways.
ROS have been widely considered as critical cellular
signaling molecules involved in various biological processes,
including cell growth, differentiation, proliferation, apoptosis
and angiogenesis (16,17). The homeostasis of ROS is critical
to maintain normal biological processes. A previous study has shown
that ROS is important in the regulation of EMT (18). In the present study, ROS levels
were found to be significantly enhanced in the HSP70-siRNA group
exposed to HG, compared with those of the HG group. Conversely,
overexpression of HSP70 significantly inhibited HG-induced ROS
production. High concentrations of glucose may alter the
intracellular redox state and this effect is mediated by activation
of a phosphokinase C (polyol pathway) or by altering the NADH/NAD
ratio responsible for pseudohypoxia conditions (19). In the present study, ROS expression
induced by HG promoted EMT in RPMCs. A number of studies reported
that HSP70 has an antioxidative effect (20,21).
In the present study, it was found that overexpression of HSP70
suppressed HG-induced ROS upregulation, as demonstrated by flow
cytometric analysis. We consider that HSP70 could be identified as
a promising therapeutic target of peritoneal fibrosis, as selective
blockade of HSP70 exacerbated the EMT-mediated changes.
Taken together, the data of the present study
demonstrate that HSP70 is critically involved in the regulation of
EMT induced by HG and contributes to reduced EMT events in
peritoneal mesothelial cells. Therefore, the use of HSP70
upregulation, potentially achieved via Smad inhibition, to reduce
ROS release and improve clinical efficacy may represent a novel
therapeutic strategy for PD patients with peritoneal fibrosis.
Abbreviations:
|
RPMCs
|
rat peritoneal mesothelial cells
|
|
HG
|
high glucose
|
|
CAPD
|
continuous ambulatory peritoneal
dialysis
|
|
ESRD
|
end-stage renal disease
|
|
DMEM
|
Dulbecco’s modified Eagle’s medium
|
|
FITC
|
fluorescein isothiocyanate
|
|
RIPA
|
radioimmunoprecipitation assay
|
|
PBS
|
phosphate-buffered saline
|
|
DCF-DA
|
2,7-dichlorofluorescein-diacetate
|
|
TBS
|
Tris-buffered saline
|
|
PDF
|
peritoneal dialysis fluid
|
|
PI
|
propidium iodide
|
|
EMT
|
epithelial-to-mesenchymal
transition
|
|
SMA
|
smooth muscle actin
|
|
HSP70
|
heat shock protein 70
|
|
ROS
|
reactive oxygen species
|
References
|
1
|
Jager KJ, van Dijk PC, Dekker FW, Stengel
B, Simpson K and Briggs JD; ERA-EDTA Registry Committee. The
epidemic of aging in renal replacement therapy: an update on
elderly patients and their outcomes. Clin Nephrol. 60:352–360.
2003. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee YJ and Han HJ: Troglitazone
ameliorates high glucose-induced EMT and dysfunction of SGLTs
through PI3K/Akt, GSK-3β, Snail1, and β-catenin in renal proximal
tubule cells. Am J Physiol Renal Physiol. 5:F1263–F1275.
2010.PubMed/NCBI
|
|
3
|
Alisson-Silva F, Freire-de-Lima L, Donadio
JL, Lucena MC, Penha L, Sá-Diniz JN, Dias WB and Todeschini AR:
Increase of O-glycosylated oncofetal fibronectin in high
glucose-induced epithelial-mesenchymal transition of cultured human
epithelial cells. PLoS One. 8:e604712013. View Article : Google Scholar
|
|
4
|
Naber HP, Drabsch Y, Snaar-Jagalska BE,
ten Dijke P and van Laar T: Snail and Slug, key regulators of
TGF-β-induced EMT, are sufficient for the induction of single-cell
invasion. Biochem Biophys Res Commun. 435:58–63. 2013.
|
|
5
|
Lu X and Kakkar V: The role of heat shock
protein (HSP) in atherosclerosis: Pathophysiology and clinical
opportunities. Curr Med Chem. 17:957–973. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mayer MP and Bukau B: Hsp70 chaperones:
cellular functions and molecular mechanism. Cell Mol Life Sci.
62:670–684. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chatterjee M, Andrulis M, Stühmer T,
Müller E, Hofmann C, Steinbrunn T, Heimberger T, Schraud H,
Kressmann S, Einsele H and Bargou RC: The PI3K/Akt signaling
pathway regulates the expression of Hsp70, which critically
contributes to Hsp90-chaperone function and tumor cell survival in
multiple myeloma. Haematologica. 98:1132–1141. 2013. View Article : Google Scholar
|
|
8
|
Mikuriya T, Sugahara K, Takemoto T, Tanaka
K, Takeno K, Shimogori H, Nakai A and Yamashita H:
Geranylgeranylacetone, a heat shock protein inducer, prevents
acoustic injury in the guinea pig. Brain Res. 1065:107–114. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Margetts PJ, Bonniaud P, Liu L, Hoff CM,
Holmes CJ, West-Mays JA and Kelly MM: Transient overexpression of
TGF-{beta}1 induces epithelial mesenchymal transition in the rodent
peritoneum. J Am Soc Nephrol. 16:425–436. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rhyu DY, Yang Y, Ha H, et al: Role of
reactive oxygen species in TGF-beta1-induced mitogen-activated
protein kinase activation and epithelial-mesenchymal transition in
renal tubular epithelial cells. J Am Soc Nephrol. 16:667–675. 2005.
View Article : Google Scholar
|
|
11
|
Grassmann A, Gioberge S, Moeller S and
Brown G: End-stage renal disease: global demographics in 2005 and
observed trends. Artif Organs. 30:895–897. 2006.PubMed/NCBI
|
|
12
|
Fang CC, Huang JW, Shyu RS, Yen CJ, Shiao
CH, Chiang CK, Hu RH and Tsai TJ: Fibrin-induced
epithelial-to-mesenchymal transition of peritoneal mesothelial
cells as a mechanism of peritoneal fibrosis: effects of
pentoxifylline. PLoS One. 7:e447652012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Grune T, Catalgol B, Licht A, Ermak G,
Pickering AM, Ngo JK and Davies KJ: HSP70 mediates dissociation and
reassociation of the 26S proteasome during adaptation to oxidative
stress. Free Radic Biol Med. 51:1355–1364. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yao Q, Pawlaczyk K, Ayala ER, Styszynski
A, Breborowicz A, Heimburger O, Qian JQ, Stenvinkel P, Lindholm B
and Axelsson J: The role of the TGF/Smad signaling pathway in
peritoneal fibrosis induced by peritoneal dialysis solutions.
Nephron Exp Nephrol. 109:e71–e78. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Neil JR, Johnson KM, Nemenoff RA and
Schiemann WP: Cox-2 inactivates Smad signaling and enhances EMT
stimulated by TGF-beta through a PGE2-dependent mechanisms.
Carcinogenesis. 29:2227–2235. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Loor G, Kondapalli J, Schriewer JM,
Chandel NS, Vanden Hoek TL and Schumacker PT: Menadione triggers
cell death through ROS-dependent mechanisms involving PARP
activation without requiring apoptosis. Free Radic Biol Med.
49:1925–1936. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen Z, Pittman EF, Romaguera J, Fayad L,
Wang M, Neelapu SS, McLaughlin P, Kwak L and McCarty N: Nuclear
translocation of B-cell-specific transcription factor, BACH2,
modulates ROS mediated cytotoxic responses in mantle cell lymphoma.
PLoS One. 8:e691262013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu Y, He K, Wang D, Yuan X, Liu Y, Ji H
and Song J: TMEPAI regulates EMT in lung cancer cells by modulating
the ROS and IRS-1 signaling pathways. Carcinogenesis. 34:1764–1772.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Henningsen C, Zahner G and Thaiss F: High
glucose induces type 1 hexokinase gene expression in isolated
glomeruli of diabetic rats and in mesangial cells. Nephron Physiol.
93:p67–p75. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Di Domenico F, Sultana R, Tiu GF, Scheff
NN, Perluigi M, Cini C and Butterfield DA: Protein levels of heat
shock proteins 27, 32, 60, 70, 90 and thioredoxin-1 in amnestic
mild cognitive impairment: an investigation on the role of cellular
stress response in the progression of Alzheimer disease. Brain Res.
1333:72–81. 2010.PubMed/NCBI
|
|
21
|
Scarpeci TE, Zanor MI and Valle EM:
Investigating the role of plant heat shock proteins during
oxidative stress. Plant Signal Behav. 3:856–857. 2008. View Article : Google Scholar : PubMed/NCBI
|