Introduction
Appearance restoration is an important part of
treatment following cancer extirpations, including surgical
excision of head and neck tumors or breast tumors, which lead to
esthetic disfigurement. For instance, breast reconstruction
following mastectomy is being selected by a larger number of
patients (1). Given the
progression of stem cell research in recent years, regenerative
medicine has become a promising alternative for conventional
surgical reconstruction. Restoration of stable, functional and
naturally appearing tissue using different types of stem cells is
an attractive method and a hot topic of investigation in the tissue
engineering field. At present, numerous regenerative medicine
studies focus on mesenchymal stem cells (MSCs) due to their several
advantages, including easily accessible sources, differentiation
plasticity, low immunogenicity and low neoplastic risk (2). It has been previously confirmed that
fat tissue is a rich source of adult stem cells and these adult
stem cells are termed adipose-derived stem cells (ASCs) (3). Subcutaneous adipose tissue is
ubiquitous and easily accessible in large quantities through
liposuction aspiration or reduction mammoplasty. As a minimally
invasive procedure, liposuction surgery is a well-tolerated and
safe procedure yielding large quantities of aspirate. This method
is more economical and less invasive than bone marrow aspiration
for stem cell isolation. Therefore, the lipoaspirate, usually
discarded as medical waste, has become a popular starting material
for isolating adipose-derived MSCs or stromal cells. As reported,
ASCs may even be isolated from needle biopsies of human adipose
tissue or from inguinal fat pads in mice as well as from other
mammals (4–8). These and other studies have provided
solid evidence to support that adipose tissue contains a large
number of multipotent stem cells suitable for stem cell-based
therapies (9). It has been
demonstrated that stem and progenitor cells in the uncultured
stroma-vascular fraction of adipose tissue usually consists of up
to 3% of the total cells, which is 2,500-fold higher than the ratio
of stem cells in the bone marrow (4). Approximately 5,000–200,000 stem cells
can be isolated from each gram of adipose tissue (10–17).
ASCs, due to their proliferation capacity, can be
expanded for multiple passages without losing their multipotent
properties and genomic integrity in long-term cultures (15,18,19).
Numerous studies have demonstrated the plasticity of ASCs towards
chondrocytes, osteoblasts, adipocytes, cardiomyocytes, smooth
muscle cells and skeletal muscle cells (12,20–28).
In general, in vitro differentiation of ASCs is achieved by
culturing in selective media with lineage-specific induction
factors. The possible mechanism for mesodermal lineage-specific
differentiation of stem cells has been investigated in certain
studies (29–34). However, the efficiency of in
vitro differentiation of ASCs into numerous lineages, including
neuronal cells and mammary gland lineages remains inadequate for
clinical application and requires substantial improvements. Human
ASCs have been isolated from multiple donor sites, including the
abdomen and inner thigh (35–38).
In addition, the isolation of ASCs from patients with gynecomastia
was briefly mentioned in one study (38). Hanson et al previously
reported isolation of ASCs from various anatomical sites, including
breast (39). The data from this
study suggested that the cell surface profile of ASCs does not
distinguish them from normal fibroblasts, however, their
differentiation capacity and pluripotency-associated gene
expression clearly define ASCs as multipotent stem cells,
regardless of tissue isolation location (39). Nevertheless, detailed
characterization of hbASCs remains insufficient.
Platelet-rich plasma (PRP) is blood plasma with
concentrated platelets. It is enriched with multiple essential
growth factors (39). Several of
these growth factors, including platelet-derived growth factor
(PDGF), transforming growth factor β1 (TGF-β1), vascular
endothelial growth factor (VEGF), fibroblast growth factor (FGF)
and insulin-like growth factor-1 (IGF-1) are known to stimulate
cell growth, migration, mobilization or differentiation and,
therefore, have been applied in regenerative medicine. For
instance, the combined use of enhanced stromal vascular fraction
and PRP has been demonstrated to improve fat grafting maintenance
in breast reconstruction (40,41).
However, tissue regeneration is a cascade of complex, systematic
events regulated by various factors. Application of a single factor
usually cannot yield satisfactory therapeutic effects. In addition,
the application of PRP has significant advantages over using a
single growth factor and has been demonstrated to promote tissue
regeneration and the wound-healing process (42). PRP has been successfully applied to
treat tendon or cartilage injuries that were unable to be treated
by traditional therapeutic approaches (40). Furthermore, compared with using
exogenous cytokines, using autologous PRP in the clinic has fewer
safety concerns as it is extracted from the same individual’s
venous blood. In addition to its clinical application, PRP has also
been used for in vitro cell culture. It has been
demonstrated that PRP is capable of promoting in vitro
proliferation and differentiation of several cell types, including
bone marrow-derived mesenchymal cells and ASCs (43). For instance, our previous study
demonstrated that autologous PRP is capable of promoting cell
proliferation and neurogenic differentiation of hASCs in
vitro (30). These studies
greatly inspired scientists to introduce PRP into the regenerative
medicine field as the application of stem cells in regenerative
medicine commonly requires cell expansion or differentiation in
vitro, however, supplementation with exogenous cytokines or
serums into the cell culture system has security and ethical
concerns. Therefore, considering its safety and effectiveness,
autologous PRP may be a powerful tool to solve these problems, thus
facilitating the application of stem cells in regenerative
medicine.
In the present study, in order to fill the gap in
our knowledge regarding hbASCs, hbASCs were isolated from discarded
surgical fat tissue from a reduction mammoplasty procedure and a
series of experiments were conducted to characterize these hbASCs
and examine the effect of PRP on their in vitro
differentiation into mammary gland-like epithelial cells
(MGECs).
Materials and methods
Patient consent and ethical approval
The present study was approved by the institutional
ethical review board of Southern Medical University (Guangzhou,
Guangdong, China). Written informed consent was provided by the
donor patient.
Isolation and expansion of human breast
adipose-derived stem cells (hbASCs)
The hbASCs were isolated from spare fat tissue from
a patient who underwent reduction mammoplasty. The fat tissue was
cut into small sections and was washed with phosphate-buffered
saline (PBS) to eliminate red blood cells. Then, the adipose tissue
was finely minced and digested with 0.1% collagenase for 60 min at
37°C with vigorous agitation. Following centrifugation at 260 × g
for 5 min, the cell pellet, mainly consisting of hbASCs, was
resuspended with Dulbecco’s modified Eagle’s medium (DMEM) plus 15%
fetal bovine serum (FBS). The suspended cells were seeded onto
dishes to expand the hbASCs. The cultures were incubated at 37°C
with 5% carbon dioxide. The first medium change was conducted 24 h
after seeding and nonadherent cells were discarded. The possibility
of residual mammary epithelial cells remaining in the hbASC culture
was minimized through cautious procedures in the initial isolation
and expansion period. Contamination of mammary epithelial cells was
not observed in any hbASC cultures used for the following
characterization and differentiation assays. Thereafter, the medium
was replaced every 3 days. Specific differentiation medium and
culture conditions are described in the following relevant results
sections.
Analysis of cell surface protein
markers
To identify specific cellular surface markers,
hbASCs at the third passage were stained with
fluorescence-conjugated cluster of differentiation (CD)31, CD34,
CD44, CD49d, CD90 and CD105. The primary antibodies used were
monoclonal mouse anti-human (1:200; Sigma, St. Louis, MO, USA) and
the secondary antibodies were goat anti-mouse IgG-Cy3 monoclonal
antibody (CD31, CD49d and CD105) or IgG-fluorescein isothiocyanate
(CD34, CD44 and CD90; all 1:100; Sigma). hbASCs stained with CD49d
were counterstained with propidium iodide (PI), while hbASCs
stained with CD31, CD34, CD44, CD90 and CD105 were counterstained
with 4′,6-diamidino-2-phenylindole, dihydrochloride (DAPI).
Staining was performed according to the following
procedures: i) fixation with 4% paraformaldehyde for 30 min,
followed by washing with PBS three times for 5 min; ii) treatment
with 3% H2O2 and incubation at room
temperature for 10 min, followed by washing three times with PBS
for 5 min; iii) treatment with 2 mol/l hydrochloric acid and
incubation for 30–45 min at room temperature, followed by washing
three times with PBS for 5 min; iv) blocking with goat serum (1:20)
for 20 min; v) Primary antibody incubation for 16 h at 4°C,
followed by washing three times with PBS for 5 min; vi) secondary
antibody incubation for 45 min at 37°C, followed by washing three
times with PBS for 5 min; vii) counterstaining with PI or DAPI.
Preparation of PRP and measurement of
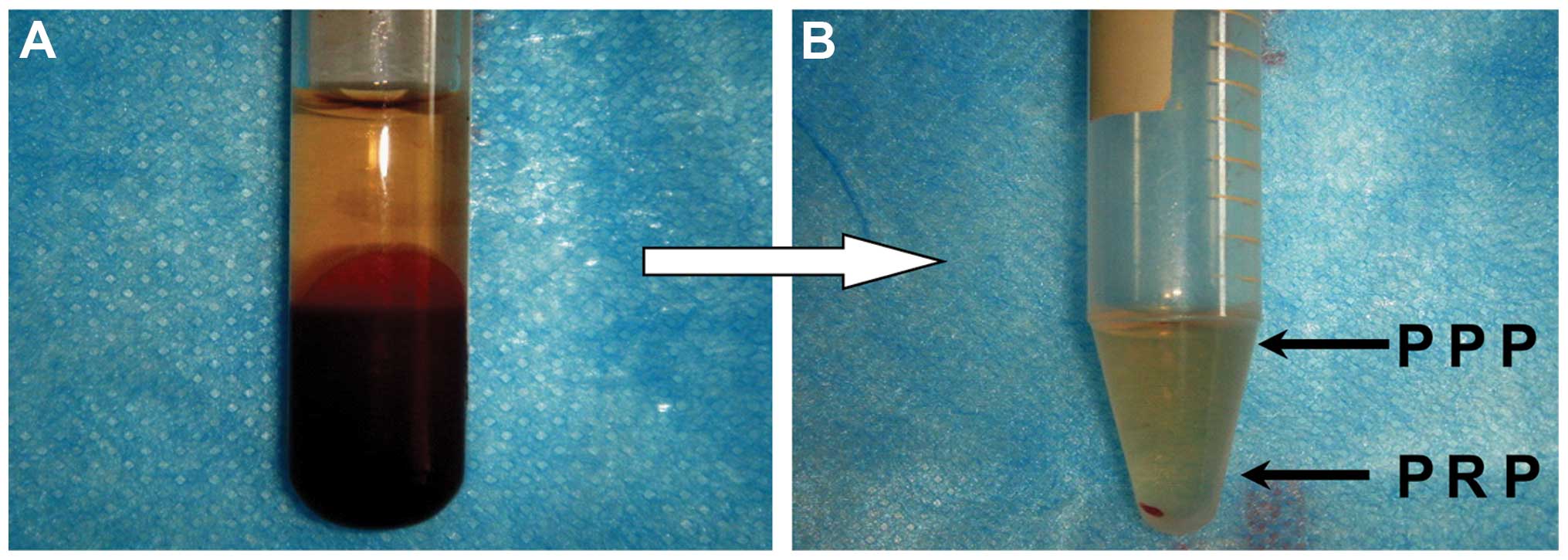
growth factor concentrations
PRP was obtained from the venous blood of the same
patient who underwent reduction mammoplasty. PRP was prepared via
double centrifugation of the blood. In brief, 9 ml of venous blood
was drawn into a polypropylene tube containing 1 ml of
anticoagulant (acid citrate dextrose; Agilent Technologies, Santa
Clara, CA, USA). The blood was centrifuged at 300 × g for 10 min at
25°C to separate out the blood cell components. The upper phase
containing PRP was transferred into a new tube and then centrifuged
at 600 × g for an additional 10 min at 25°C to separate out the
platelet-poor plasma (PPP) in the upper phase (Fig. 1). The platelet pellets were
resuspended in 1 ml of plasma and were pooled as PRP. To activate
the platelets, one part of bovine thrombin stock solution (1,000
U/ml; Sigma) was added to nine parts of PRP to yield a final
thrombin concentration of 100 U/ml. The mixture was incubated for 1
h at 37°C for clot preparation. The supernatants obtained from the
clot preparation were referred to as activated PRP. The PRP was
stored at −80°C until use. The concentrations of growth factors,
including PDGF, TGF-β, VEGF, FGF and IGF-1, were measured using
commercially available Quantikine colorimetric sandwich ELISA kits
(R&D Systems, Minneapolis, MN, USA) according to the
manufacturer’s instructions.
Application of PRP for the
differentiation of MGECs from hbASCs
The hbASCs at the third passage were seeded onto
six-well culture plates. When the cell culture reached 70–80%
confluency, the medium was aspirated and replenished with medium A
containing PRP and medium B without PRP for the test group and the
control group, respectively. Medium A contained 20 μg/ml insulin, 2
μg/ml hydrocortisone, 20 μg/ml prolactin, 20 μg/ml corporin and 10%
PRP in its base medium, low glucose-DMEM. Medium B did not contain
10% PRP and the remaining ingredients were the same as those of
medium A. The cellular morphology was examined every 24 h under an
inverted microscope in phase-contrast and three-dimensional modes
(Leica Microsystems AG, Wetzlar, Germany). The conversion rate was
calculated based on the number of MGECs among every 100 random
cells. In addition, hbASCs at passage 3 were also harvested and
seeded onto 96-well culture plates at an equal cell density with
media A and B for the proliferation assay. Cell Counting kit-8
(CCK-8) tests were performed over 7 days to establish growth curves
for the two groups.
Protein extraction and western
blotting
Following 4 weeks of MGEC differentiation culturing,
10 samples of each test and control group were harvested to prepare
whole cell extracts (WCEs). Briefly, confluent cells were washed
with ice-cold PBS and collected by scraping off the plates. Cell
pellets were sonicated in extraction buffer to obtain WCEs in which
the protein concentrations were subsequently quantified using the
Bio-Rad DC protein assay kit (Bio-Rad, Hercules, CA, USA). Equal
quantities of protein were resolved by 4–12% gradient sodium
dodecyl sulfate polyacrylamide gel electrophoresis and transferred
onto polyvinylidene fluoride membranes (Millipore, Bedford, MA,
USA). The membranes were then incubated with blocking solutions
(Pierce Biotechnology, Inc., Rockford, IL, USA) followed by primary
and secondary antibody incubation. The primary antibodies used were
monoclonal mouse anti-human cytokeratin (CK)-18 and monoclonal
mouse anti-human CK-19 (Abcam Co. Ltd., Cambridge, UK). Goat
anti-mouse monoclonal horseradish peroxidase-conjugated secondary
antibodies and enhanced chemiluminescence substrate (Super-signal
West Dura detection system; Pierce Biotechnology, Inc.) were used
for primary antibody detection.
RNA extraction and quantitative reverse
transcription polymerase chain reaction (qRT-PCR)
qRT-PCR was performed to assess the expression of
MGEC marker genes, including cytokeratin-19 (CK-19),
α-casein and β-casein. Total RNA was isolated from
monolayer cultures with the Ultraspec RNA purification kit (Biotecx
Laboratories Inc., Houston, TX, USA) according to the
manufacturer’s instructions. Reverse transcription was performed
with 2 μg of total RNA using the Superscript RT II kit (Life
Technologies, Rockville, MD, USA) and random primers. qPCR was
performed with the DyNamo SYBR Green qPCR kit using an MJ Research
Opticon 2 real-time PCR machine according to the manufacturer’s
instructions (MJ Research, Reno, NV, USA). Melting curve analysis
and agarose gel electrophoresis were performed to determine the
purity of the PCR products. β-actin was used as an internal
control. The comparative threshold cycle (Ct) method was used to
calculate the relevant concentration of target genes (42). The PCR primers are listed in
Table I.
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Gene name
(human) | Forward primer
sequence (5′ to 3′) | Reverse primer
sequence (5′ to 3′) |
|---|
| CK-19 |
CTTCCTACAGCTATCGCCAG |
TCCGTCTTGCTGATCTGCAG |
| α-casein |
GACAACCATGAAACTTCTCATC |
CTCACCACAGTGGCATAGTA |
| β-casein |
AGGAACAGCAGCAAACAG |
TTTCCAGTCGCAGTCAAT |
| GAPDH |
GGTGAAGGTCGGAGTCAACG |
CAAAGTTGTCATGGATGHACC |
Statistical analysis
qRT-PCR was repeated six times and the results are
expressed as the mean ± standard deviation. The PCR and western
blotting results were compared using the unpaired Student’s t-test.
P<0.05 was considered to indicate a statistically significant
difference. All statistical analyses were performed using
SPSS® version 16.0 software (SPSS, Inc., Chicago, IL,
USA).
Results
Characterization of hbASCs
Following initial isolation and expansion,
homogeneous hbASCs that grew in a monolayer with spindle-shaped
morphology were observed following culturing for ~2 weeks (Fig. 2A). These hbASCs presented a strong
proliferation capacity. The hbASCs reached 80–90% confluency 7 days
after initial seeding for the first passage. In subsequent
culturing, these cells reached the same confluency within 3–4 days
with a 1:3 split ratio. These observations demonstrated that hbASCs
resemble other ASCs in terms of morphology and proliferation
capacity (11,44). It has been reported that MSCs
derived from different tissue sources express similar but
nonidentical patterns of cell surface markers, possibly due to
differences in tissue source and donor age (45,46).
CD105, CD90 and CD44 are the main three positive markers for MSCs.
While CD34 is the most frequently reported negative marker. CD49d
and CD31 have also been reported as a positive marker and a
negative marker, respectively (45). In order to define the cellular
features of hbASCs, all above-mentioned cell surface markers were
analyzed in the hbASCs isolated in the present study. The hbASCs at
the third passage were subjected to immunofluorescence staining
with antibodies against these marker proteins. The
immunofluorescence staining results demonstrated that these hbASCs
express CD44, CD49d, CD90 and CD105 (>90% cells are positive for
staining), however, do not express CD31 or CD34 (<5% cells are
positive for staining; Fig.
3).
Multipotency of hbASCs
To validate the multilineage differentiation
capacity of the isolated hbASCs, subconfluent hbASCs at passage 3
were cultured for 1–3 weeks with osteogenic, adipogenic and
chondrogenic induction media as listed in Table II. The lineage-specific cell
morphology was observed following 1, 2 and 3 weeks of inductive
culturing for adipocytes, osteocytes and chondrocytes,
respectively. Positive staining of oil Red-O, alizarin red or
alcian blue typically indicate adipocytes, osteocytes or
chondrocytes, respectively. Thus, lineage-specific histological
staining was performed with these dyes and the results confirmed
that the hbASCs were differentiated into adipocytes, osteocytes and
chondrocytes following relevant inductive culturing (Fig. 2B–D). Positive staining was not
observed in any of the control groups (Fig. 2E–G). These results validated the
multipotency of hbASCs.
 | Table IIMultilineage induction of
adipose-derived stem cells. |
Table II
Multilineage induction of
adipose-derived stem cells.
| Lineage | Induction
media |
Characterization |
|---|
| Adipogenic | DMEM (high
glucose), 10% FBS, 1% antibiotic/antimycotic, 200 μM indomethacin,
0.5 mM isobutyl-methylxanthine, 1 μM dexamethasone, 10 μM
insulin, | Oil red O
staining |
| Osteogenic | DMEM (high
glucose), 10% FBS, 1% antibiotic/antimycotic, 0.1 M dexamethasone,
50 μM ascorbate-2-phosphate, 10 mM b-glycerophosphate, | Alizarin red
staining |
| Chondrogenic | DMEM (high
glucose), 1% FBS, 10 ng/ml TGF-β1, 1% antibiotic/antimycotic, 6.25
μg/ml insulin, 50 nM ascorbate-2-phosphate | Alcian blue
staining |
Effect of PRP on hbASC proliferation and
their conversion to MGECs
The PRP was prepared from the same patient’s venous
whole blood (VWB) by the double centrifugation approach as
described in the Materials and methods section (Fig. 1). The platelet density was markedly
increased in PRP compared with that in the original VWB. The mean
values of the platelet density were (136.12±21.73) ×
109/l and (892.07±41.25) × 109/l in VWB and
PRP, respectively, revealing a 655.36% increase in PRP.
Consistently, the concentrations of essential growth factors,
including PDGF, TGF-β1, VEGF, FGF and IGF-1 in PRP were increased
>100-fold in PRP compared with VWB (Table III).
 | Table IIIConcentrations of growth factors in
PRP and in VWB (ng/ml). |
Table III
Concentrations of growth factors in
PRP and in VWB (ng/ml).
| Growth factor | Concentration in
PRP | Concentration in
VWB |
|---|
| PDGF | 719.25±63.04 | 6.65±1.27 |
| TGF-β1 | 694.08±58.92 | 5.91±0.58 |
| VEGF | 1127.36±70.43 | 8.03±1.35 |
| FGF | 865.79±67.55 | 7.21±1.33 |
| IGF-1 | 623.17±45.37 | 5.12±0.64 |
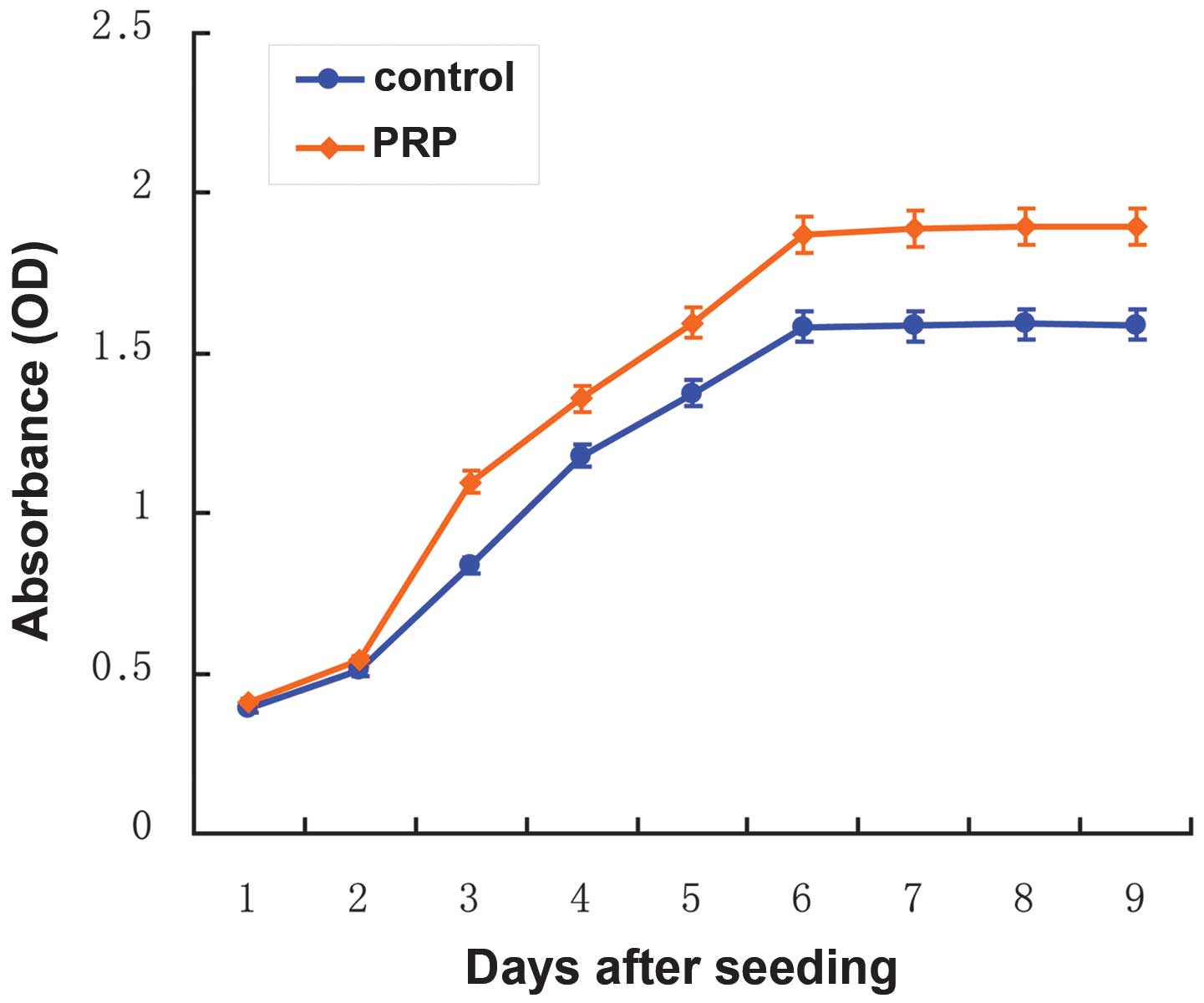
In order to examine the effect of PRP on hbASC
proliferation during MGEC differentiation, growth curves were
established using the CCK-8 for hbASCs cultured in medium A
containing 10% PRP and in control medium B without PRP, which were
designated as the test group and the control group, respectively. A
proliferation phase in the first 6 days and a subsequent
morphological conversion phase were observed during the
differentiation process for the test and the control groups
(Fig. 4). These data further
revealed that PRP significantly promoted hbASC growth in the
proliferation phase. Following initial equivalent seeding, the
absorbance values of the test group, which were positively
associated with the cell densities, were significantly greater than
those of the control group (P<0.001) at day 3 of differentiation
(Fig. 4). The proliferation
difference between the two groups continued to increase until the
cells reached the growth plateau on day 6, when morphology
conversion started. However, it is noteworthy that the timeline for
the transition between the proliferation phase and the conversion
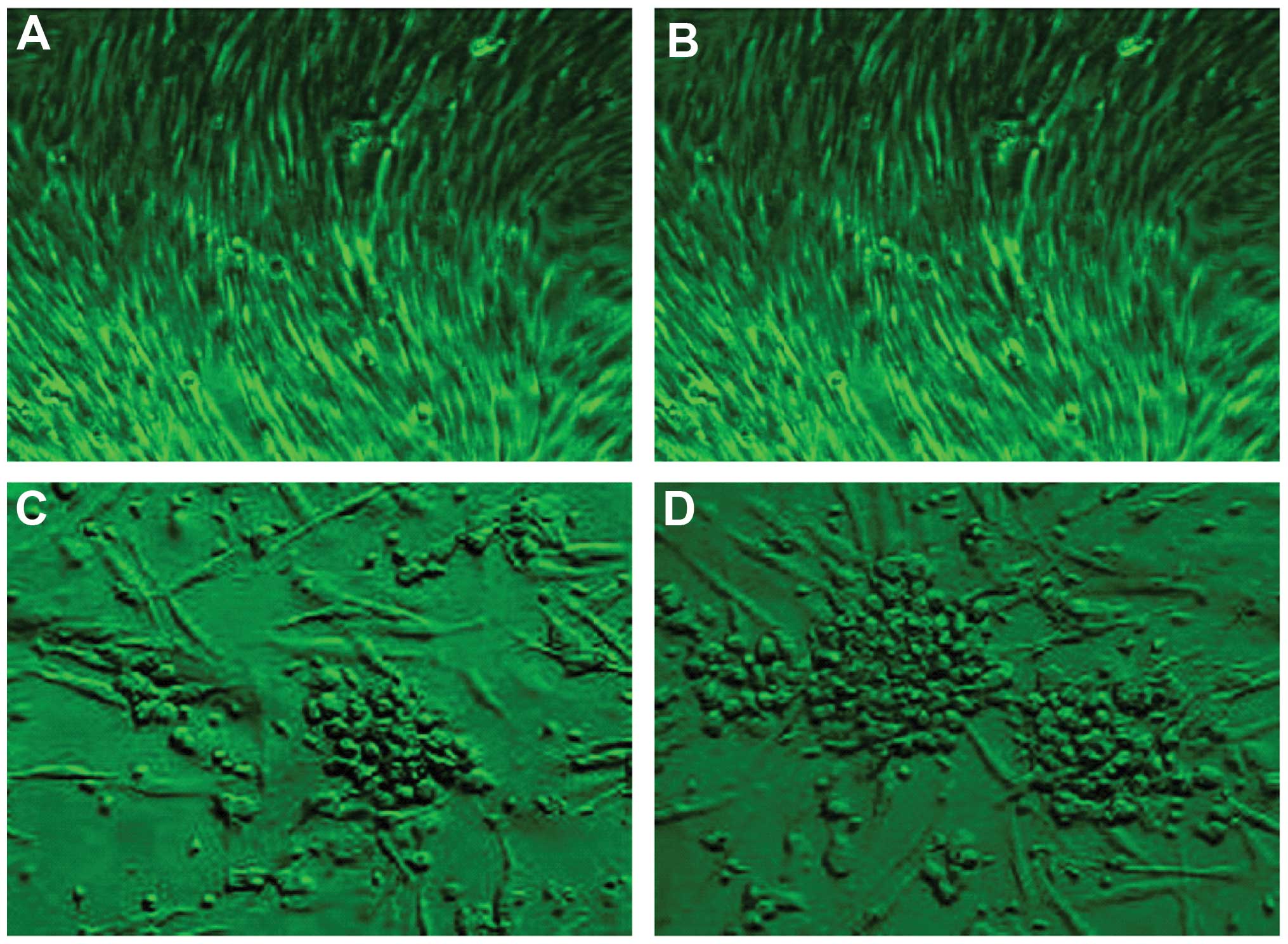
phase was the same for the two groups. On day 6 of differentiation,
the cellular morphology altered from a spindle-like shape to a
polygonal or circle-like shape in the two media groups, resembling
the morphology of MGECs (Fig. 5)
(47). Following 4 weeks of
induction culturing, the polygonal or circle-like cells formed
clusters and developed into colonies (Fig. 5). More colonies were observed in
the test group cultured with PRP than in the control group. The
conversion rate of MGECs in the test group (35.13±6.02%) was
significantly increased compared with that in the control group
(11.24±3.27%; P<0.01).
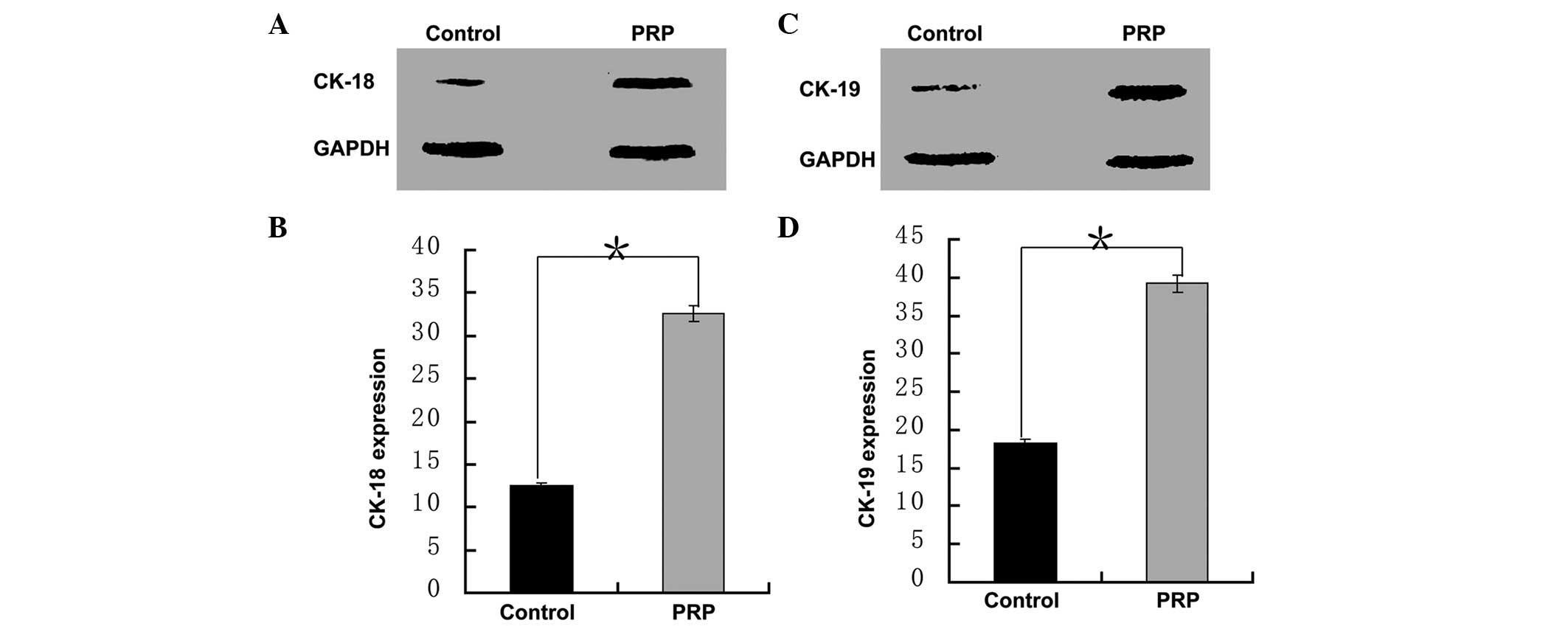
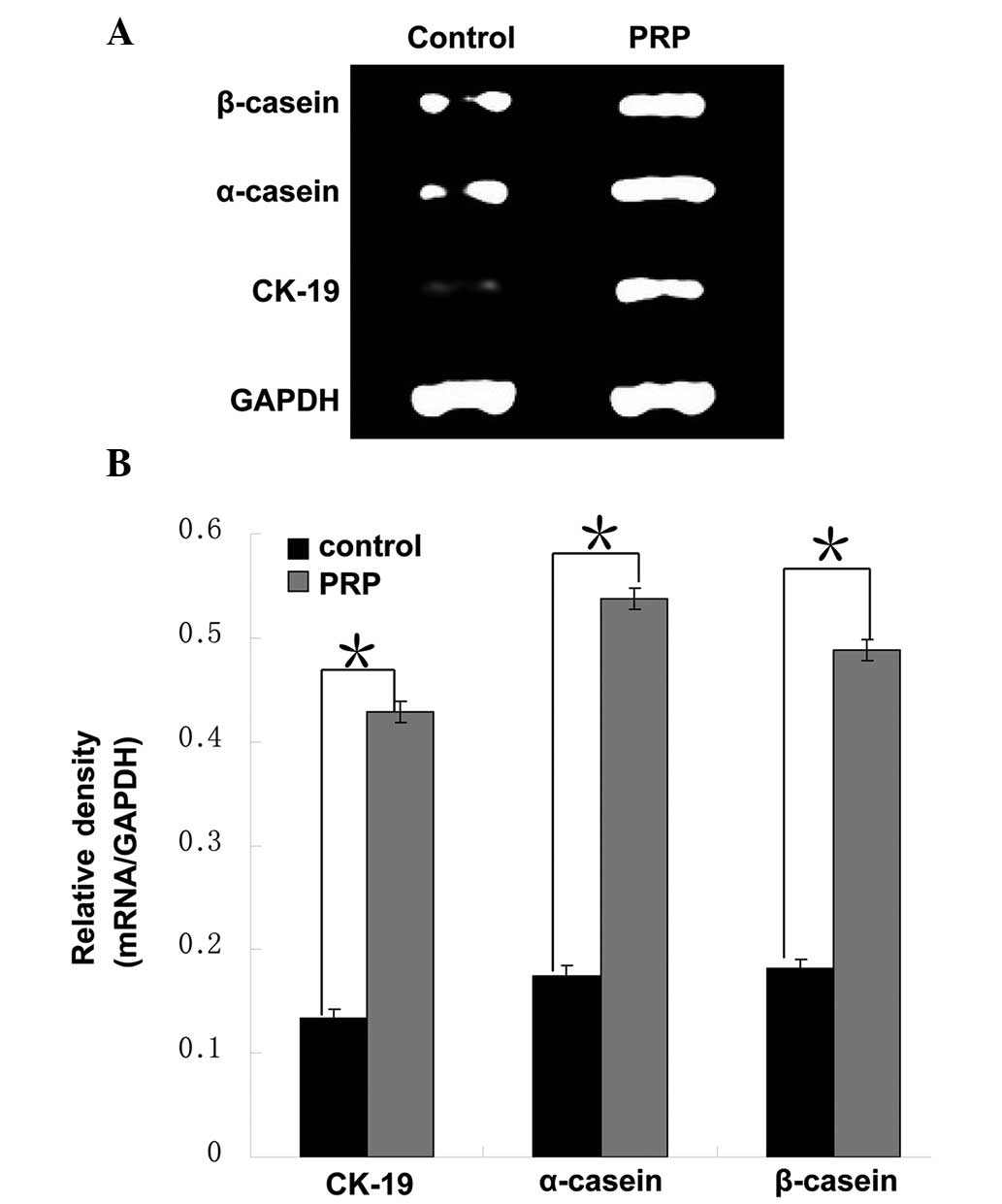
CK-18 and CK-19 are two cytokeratin proteins
expressed in MGECs (48).
Therefore, the protein expression levels of CK-18 and CK-19 in the
cells following 4 weeks of induction culturing were analyzed by
western blot analysis to evaluate the differentiation outcomes. The
protein levels of CK-18 and CK-19 in the test group were >2-fold
greater than those in the control group (P<0.001; Fig. 6). Consistently, the mRNA levels of
the MGEC markers CK-19, α-casein and β-casein
in the test group were approximately three times of those in the
control group (P<0.01; Fig.
7).
Overall, the cell growth, morphology conversion and
expression of marker gene results consistently demonstrated that
PRP promoted the proliferation of hbASCs and subsequently increased
their conversion rate to MGECs during this differentiation
process.
Discussion
Given their proliferation and multilineage
differentiation capacity, ASCs have become an attractive subject in
regenerative medicine and have been applied in several clinical
trials regarding breast reconstruction, repair of craniofacial
defects, treatment of cardiovascular diseases and chronic wound
healing (49). However, to the
best of our knowledge, hbASCs derived from human breast adipose
tissue have neither been carefully characterized nor been actively
employed in studies. In the present study, hbASCs were isolated and
their features were analyzed, including their cellular morphology,
antigen pattern on the cell surface, proliferation capacity and
multipotency. Data from the present study demonstrated that hbASCs
resemble other ASCs in all these aspects.
The hbASCs in culture adhered to the dish surface
and appeared as a fibroblast-like spindle shape (Fig. 2A, 5A
and 5B), thus, exhibiting the typical morphology of ASCs
(11,50,51).
The hbASCs grew at a rate of 3 days per passage when cultured in
standard medium, indicating a strong proliferation capacity. As
reported, ASCs present a similar cell surface expression profile as
that of bone marrow stromal cells (39). Analysis of cell surface markers
revealed that hbASCs express the most frequently reported MSC
markers, CD105, CD90 and CD44, and do not express CD34, a
well-known hematopoietic marker. The results from the present study
clearly demonstrated that hbASCs, a subtype of ASCs, are similar to
the majority of MSCs in terms of cell surface markers. The absence
of CD34 expression indicated a high purity of hbASCs and excluded
the possibility of hematopoietic stem cell contamination in our
cultures. Of note, the hbASCs were positive for CD49d but negative
for CD31. The expression/absence of these markers has been observed
in a few reported MSCs, depending on the tissue source (45). Thus, the unique expression pattern
of these two markers plus the MSC-resembling expression pattern of
the aforementioned four markers in hbASCs constitute the
hbASC-specific expression pattern of cell surface markers, which is
useful for defining hbASCs and developing a flow cytometric sorting
approach in future hbASC studies.
hbASCs, being a subtype of MSCs, presumably possess
a certain range of differentiation potency as that of MSCs. A
series of proof-of-concept differentiation experiments were
performed in the present study to examine the multipotency of
hbASCs. Similar to other MSCs, hbASCs have the potential to
differentiate into adipocytes, osteocytes and chondrocytes
following appropriate induction (Fig.
2B–D; Table III). Notably,
hbASCs were able to differentiate into adipocytes significantly
faster than into other lineages (one week versus three weeks). This
phenomenon possibly reflected the fact that certain epigenetic
modifications have been established in these adult stem cells so as
to attribute them a greater propensity to their designated cellular
fate than into other lineages. It further indicated that hbASCs may
be a better stem cell resource for breast reconstruction than other
types of ASCs as hbASCs are derived from breast adipose tissue and
are presumably designated to produce breast tissues. These
differentiation data, although preliminary, indicated that hbASCs
have a similar, if not identical, multipotency to that of MSCs and
implied that hbASCs may be utilized as alternative stem cell
sources for MSCs in numerous clinical aspects. To complete the
multipotency characterization of hbASCs, it may be noteworthy to
investigate whether hbASCs are able to differentiate toward other
lineages, including myogenic, neurogenic and angiogenic lineages
that are able to be induced from bone marrow-derived MSCs and
several types of ASCs.
ASCs are able to differentiate into epithelial
lineage cells with a limited efficiency (52,53).
In order to assess whether hbASCs have the same capacity, the
differentiation of hbASCs into MGECs was conducted. Two phases were
observed during this differentiation process: a proliferation phase
between day 0 and day 6 with fast cell growth; and a subsequent
conversion phase with an alteration in cellular morphology and
negligible cell growth (Fig. 4).
Furthermore, the effect of activated PRP from the same patient on
the conversion of hbASCs to MGECs was examined in the present
study. More than 100-fold enrichment of the critical growth factors
was detected in the PRP preparation, indicating its good quality.
PRP did not interfere with the timeline of the two phases during
differentiation. However, PRP significantly promoted the growth
rate and augmented cell expansion in the proliferation phase.
Eventually, the greater conversion rate of MGECs, based on the
alteration in cellular morphology, was observed in the
differentiation culture supplemented with 10% PRP compared with
that in the control group (35 vs. 11%; Fig. 5). The enhanced conversion rate
stimulated by PRP was further confirmed by analyzing the expression
levels of the mammary gland epithelial markers CK-18, CK-19,
α-casein and β-casein (Figs. 6 and
7). These results consistently
demonstrated that activated autologous PRP is capable of promoting
MGEC differentiation from hbASCs. The fact that the timeline for
the transition from the proliferation phase to the conversion phase
was not affected by PRP indicated that inherent cellular events are
required for cell fate conversion, which PRP cannot interfere with,
at least not in the proliferation phase. It is not clear whether
the eventual greater conversion rate in the test group with PRP was
caused by stimulation of PRP in the conversion phase or whether
this greater conversion rate was simply an outcome secondary to the
increased cell density caused by PRP in the proliferation phase.
This question may be answered in a future study by increasing the
initial hbASC density without using PRP and determining whether a
similarly enhanced conversion rate (not an absolute number) is able
to be achieved as with PRP addition. Overall, the data from the
present study demonstrated that hbASCs are able to differentiate
into MGECs and that activated autologous PRP was able to
significantly increase the differentiation efficiency. These
results may aid the development of approaches to utilize hbASCs in
mammary gland regeneration.
In conclusion, to the best of our knowledge, the
present study was the first to provide comprehensive
characterization of hbASCs and demonstrated that hbASCs, being a
new member of the ASC family, are generally similar to other types
of ASCs in terms of cellular morphology, cell surface antigen
pattern, proliferation capacity and multilineage differentiation
potency. It was also revealed that activated autologous PRP is able
to significantly improve the differentiation of hbASCs into MGECs,
providing an efficient approach for hbASC differentiation. Overall,
the information from the present study filled the gap in our
knowledge regarding hbASCs and provided a fundamental basis for the
application of hbASCs in future studies.
Acknowledgements
This work was financially supported by the China
Postdoctoral Science Foundation (No. 20090450910) and the Medical
Scientific Research Foundation of Guangdong Province, China (No.
A2012814). The authors thank the Research Center of Tissue
Engineering, Southern Medical University for their support, and
special thanks are owed to Professor Shan Jiang.
References
|
1
|
Cordeiro PG: Breast reconstruction after
surgery for breast cancer. N Engl J Med. 359:1590–1601. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ren G, Chen X, Dong F, et al: Concise
review: mesenchymal stem cells and translational medicine: emerging
issues. Stem Cells Transl Med. 1:51–58. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dubois SG, Floyd EZ, Zvonic S, Kilroy G,
Wu X, Carling S, Halvorsen YD, Ravussin E and Gimble JM: Isolation
of human adipose-derived stem cells from biopsies and liposuction
specimens. Methods Mol Biol. 449:69–79. 2008.PubMed/NCBI
|
|
4
|
Neupane M, Chang CC, Kiupel M and
Yuzbasiyan-Gurkan V: Isolation and characterization of canine
adipose-derived mesenchymal stem cells. Tissue Eng Part A.
14:1007–1015. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Peptan IA, Hong L and Mao JJ: Comparison
of osteogenic potentials of visceral and subcutaneous
adipose-derived cells of rabbits. Plast Reconstr Surg.
117:1462–1470. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tholpady SS, Katz AJ and Ogle RC:
Mesenchymal stem cells from rat visceral fat exhibit multipotential
differentiation in vitro. Anat Rec A Discov Mol Cell Evol Biol.
272:398–402. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vidal MA, Kilroy GE, Lopez MJ, Johnson JR,
Moore RM and Gimble JM: Characterization of equine adipose
tissue-derived stromal cells: adipogenic and osteogenic capacity
and comparison with bone marrow-derived mesenchymal stromal cells.
Vet Surg. 36:613–622. 2007. View Article : Google Scholar
|
|
8
|
Safford KM, Hicok KC, Safford SD, et al:
Neurogenic differentiation of murine and human adipose-derived
stromal cells. Biochem Biophys Res Commun. 294:371–379. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jurgens WJ, Oedayrajsingh-Varma MJ, Helder
MN, Zandiehdoulabi B, Schouten TE, Kuik DJ, Ritt MJ and van
Milligen FJ: Effect of tissue-harvesting site on yield of stem
cells derived from adipose tissue: implications for cell-based
therapies. Cell Tissue Res. 332:415–426. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Grimes BR, Steiner CM, Merfeld-Clauss S,
et al: Interphase FISH demonstrates that human adipose stromal
cells maintain a high level of genomic stability in long-term
culture. Stem Cells Dev. 18:717–724. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zuk PA, Zhu M, Ashjian P, et al: Human
adipose tissue is a source of multipotent stem cells. Mol Biol
Cell. 13:4279–4295. 2002.PubMed/NCBI
|
|
12
|
Gimble JM and Guilak F: Differentiation
potential of adipose derived adult stem (ADAS) cells. Curr Top Dev
Biol. 58:137–160. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gimble J and Guilak F: Adipose-derived
adult stem cells: isolation, characterization, and differentiation
potential. Cytotherapy. 5:362–369. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Strem BM, Hicok KC, Zhu M, et al:
Multipotential differentiation of adipose tissue-derived stem
cells. Keio J Med. 54:132–141. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fraser JK, Schreiber R, Strem B, et al:
Plasticity of human adipose stem cells toward endothelial cells and
cardiomyocytes. Nat Clin Pract Cardiovasc Med. 3(Suppl 1): S33–S37.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jack GS, Almeida FG, Zhang R, Alfonso ZC,
Zuk PA and Rodríguez LV: Processed lipoaspirate cells for tissue
engineering of the lower urinary tract: implications for the
treatment of stress urinary incontinence and bladder
reconstruction. J Urol. 174:2041–2045. 2005. View Article : Google Scholar
|
|
17
|
Rodríguez LV, Alfonso Z, Zhang R, Leung J,
Wu B and Ignarro LJ: Clonogenic multipotent stem cells in human
adipose tissue differentiate into functional smooth muscle cells.
Proc Natl Acad Sci USA. 103:12167–12172. 2006.PubMed/NCBI
|
|
18
|
Mizuno H, Zuk PA, Zhu M, Lorenz HP,
Benhaim P and Hedrick MH: Myogenic differentiation by human
processed lipoaspirate cells. Plast Reconstr Surg. 109:199–209.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zaman WS, Makpol S, Sathapan S and Chua
KH: Long-term in vitro expansion of human adipose-derived stem
cells showed low risk of tumourigenicity. J Tissue Eng Regen Med.
8:67–76. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee JH and Kemp DM: Human adipose-derived
stem cells display myogenic potential and perturbed function in
hypoxic conditions. Biochem Biophys Res Commun. 341:882–888. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu TM, Martina M, Hutmacher DW, Hui JH,
Lee EH and Lim B: Identification of common pathways mediating
differentiation of bone marrow- and adipose tissue-derived human
mesenchymal stem cells into three mesenchymal lineages. Stem Cells.
25:750–760. 2007.
|
|
22
|
Li HX, Luo X, Liu RX, Yang YJ and Yang GS:
Roles of Wnt/beta-catenin signaling in adipogenic differentiation
potential of adipose-derived mesenchymal stem cells. Mol Cell
Endocrinol. 291:116–124. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Karbiener M, Fischer C, Nowitsch S, et al:
microRNA miR-27b impairs human adipocyte differentiation and
targets PPARgamma. Biochem Biophys Res Commun. 390:247–251. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
James AW, Leucht P, Levi B, et al: Sonic
Hedgehog influences the balance of osteogenesis and adipogenesis in
mouse adipose-derived stromal cells. Tissue Eng Part A.
16:2605–2616. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Davis LA and Zur Nieden NI: Mesodermal
fate decisions of a stem cell: the Wnt switch. Cell Mol Life Sci.
65:2658–2674. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schäffler A and Buchler C: Concise review:
adipose tissue-derived stromal cells - basic and clinical
implications for novel cell-based therapies. Stem Cells.
25:818–827. 2007.PubMed/NCBI
|
|
27
|
Cousin B, André MM, Arnaud E, Pénicaud L
and Casteilla L: Reconstitution of lethally irradiated mice by
cells isolated from adipose tissue. Biochem Biophys Res Commun.
301:1016–1022. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Miranville A, Heeschen C, Sengenès C,
Curat CA, Busse R and Bouloumié A: Improvement of postnatal
neovascularization by human adipose tissue-derived stem cells.
Circulation. 110:349–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Corre J, Barreau C, Cousin B, et al: Human
subcutaneous adipose cells support complete differentiation but not
self-renewal of hematopoietic progenitors. J Cell Physiol.
208:282–288. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li H, Han Z, Liu D, Zhao P, Liang S and Xu
K: Autologous platelet-rich plasma promotes neurogenic
differentiation of human adipose-derived stem cells in vitro. Int J
Neurosci. 123:184–190. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu J, Pan Z, Cheng M, et al: Ginsenoside
Rg1 facilitates neural differentiation of mouse embryonic stem
cells via GR-dependent signaling pathway. Neurochem Int. 62:92–102.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shi AW, Gu N, Liu XM, Wang X and Peng YZ:
Ginsenoside Rg1 enhances endothelial progenitor cell angiogenic
potency and prevents senescence in vitro. J Int Med Res.
39:1306–1318. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang L and Kisaalita WS: Administration of
BDNF/ginsenosides combination enhanced synaptic development in
human neural stem cells. J Neurosci Methods. 194:274–282. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shi AW, Wang XB, Lu FX, Zhu MM, Kong XQ
and Cao KJ: Ginsenoside Rg1 promotes endothelial progenitor cell
migration and proliferation. Acta Pharmacol Sin. 30:299–306. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Moshtagh PR, Emami SH and Sharifi AM:
Differentiation of human adipose-derived mesenchymal stem cell into
insulin-producing cells: an in vitro study. J Physiol Biochem.
69:451–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Scuderi N, Ceccarelli S, Onesti MG, et al:
Human adipose-derived stromal cells for cell-based therapies in the
treatment of systemic sclerosis. Cell Transplant. 22:779–795. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kisiel AH, McDuffee LA, Masaoud E, Bailey
TR, Esparza Gonzalez BP and Nino-Fong R: Isolation,
characterization, and in vitro proliferation of canine mesenchymal
stem cells derived from bone marrow, adipose tissue, muscle, and
periosteum. Am J Vet Res. 73:1305–1317. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Heneidi S, Simerman AA, Keller E, Singh P,
Li X, Dumesic DA and Chazenbalk G: Awakened by cellular stress:
isolation and characterization of a novel population of pluripotent
stem cells derived from human adipose tissue. PLoS One.
8:e647522013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hanson SE, Kim J and Hematti P:
Comparative analysis of adipose-derived mesenchymal stem cells
isolated from abdominal and breast tissue. Aesthet Surg J.
33:888–898. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
|
|
41
|
Smith SE and Roukis TS: Bone and wound
healing augmentation with platelet-rich plasma. Clin Podiatr Med
Surg. 26:559–588. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gentile P, Orlandi A, Scioli MG, Di
Pasquali C, Bocchini I and Cervelli V: Concise review:
adipose-derived stromal vascular fraction cells and platelet-rich
plasma: basic and clinical implications for tissue engineering
therapies in regenerative surgery. Stem Cells Transl Med.
1:230–236. 2012. View Article : Google Scholar
|
|
43
|
Mardani M, Kabiri A, Esfandiari E,
Esmaeili A, Pourazar A, Ansar M and Hashemibeni B: The effect of
platelet rich plasma on chondrogenic differentiation of human
adipose derived stem cells in transwell culture. Iran J Basic Med
Sci. 16:1163–1169. 2013.PubMed/NCBI
|
|
44
|
Gaiba S, França LP, França JP and Ferreira
LM: Characterization of human adipose-derived stem cells. Acta Cir
Bras. 27:471–476. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mafi P, Hindocha S, Mafi R, Griffin M and
Khan WS: Adult mesenchymal stem cells and cell surface
characterization - a systematic review of the literature. Open
Orthop J. 5:253–260. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hass R, Kasper C, Böhm S and Jacobs R:
Different populations and sources of human mesenchymal stem cells
(MSC): A comparison of adult and neonatal tissue-derived MSC. Cell
Commun Signal. 9:122011.PubMed/NCBI
|
|
47
|
Dimri G, Band H and Band V: Mammary
epithelial cell transformation: insights from cell culture and
mouse models. Breast Cancer Res. 7:171–179. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Péchoux C, Gudjonsson T, Ronnov-Jessen L,
Bissell MJ and Petersen OW: Human mammary luminal epithelial cells
contain progenitors to myoepithelial cells. Dev Biol. 206:88–99.
1999.PubMed/NCBI
|
|
49
|
Tobita M, Orbay H and Mizuno H:
Adipose-derived stem cells: current findings and future
perspectives. Discov Med. 11:160–170. 2011.PubMed/NCBI
|
|
50
|
Zuk PA, Zhu M, Mizuno H, et al:
Multilineage cells from human adipose tissue: implications for
cell-based therapies. Tissue Eng. 7:211–228. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Halvorsen YD, Franklin D, Bond AL, et al:
Extracellular matrix mineralization and osteoblast gene expression
by human adipose tissue-derived stromal cells. Tissue Eng.
7:729–741. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Baer PC, Döring C, Hansmann ML, Schubert R
and Geiger H: New insights into epithelial differentiation of human
adipose-derived stem cells. J Tissue Eng Regen Med. 7:271–278.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Baer PC, Brzoska M and Geiger H:
Epithelial differentiation of human adipose-derived stem cells.
Methods Mol Biol. 702:289–298. 2011. View Article : Google Scholar : PubMed/NCBI
|