Introduction
Bone marrow mesenchymal stem cells (bmMSCs) are a
type of multipotent stem cells that are capable of differentiating
into multiple cell types, including osteoblasts, chondrocytes,
adipocytes, cardiomyocytes and neurons (1,2).
Currently, bmMSCs are widely utilized in regenerative medicine. The
proliferative ability of bmMSCs is the most important determinant
for the efficiency of bmMSC-based transplantation therapy (1,3).
Previous studies have demonstrated that bmMSCs may lose their
proliferative ability with consecutive expansion (4). However, why the late passages of
bmMSCs have low proliferative ability remains unclear.
The Wnt/β-catenin signaling pathway has a critical
role in cell proliferation. The upregulation of Wnt/β-catenin
signals stimulates cell proliferation and improves cell survival
(5,6). Akt is also an important regulator for
cell proliferation and survival (7). Previous studies have demonstrated
that downregulation and deficiency of Akt impairs cell
proliferation (8,9). In the preliminary study, we observed
that β-catenin and Akt were markedly downregulated in the late
(8th) passage of bmMSCs. Therefore, it was hypothesized that the
downregulation of β-catenin and Akt signals may be important in
regulating the proliferative ability of the late passage of bmMSCs
and the present study was designed to address this hypothesis.
Materials and methods
Materials and reagents
Dulbecco’s Modified Eagle Medium (DMEM),
Lipofectamine® 2000, DNase I, RNeasy Mini kit and
SuperScript II First Strand DNA Synthesis kit were purchased from
Invitrogen Life Technologies (Carlsbad, CA, USA) and the 2X PCR
Reaction Mix was obtained from Sigma-Aldrich (St. Louis, MO, USA).
HyClone fetal bovine serum (FBS) and ECL western blotting substrate
was purchased from Thermo Fisher Scientific Inc. (Cleveland, OH,
USA). Rabbit anti-mouse β-catenin antibody was purchased from Santa
Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Phospho-Akt and
β-actin primary antibodies, as well as HRP-conjugated goat
anti-rabbit secondary antibody were obtained from Abcam (Cambridge,
MA, USA). Precision Plus Protein prestained standards were
purchased from Bio-Rad (Hercules, CA, USA). The PVDF membrane was
obtained from GE Healthcare (Pittsburgh, PA, USA).
Cell culture and study protocol
BmMSCs were obtained and cultured as previously
described (1,10). Passage 2 and passage 8 of cells
were used in the experiments. The present study was approved by the
Ethics Committee of Xinxiang Medical University (Xinxiang,
China).
Cell counting
The passage 2 and passage 8 of bmMSCs were plated in
24-well plates (4 wells/group). Cells were collected on days 2, 4,
6, 8, 10 and 12, and counted under a microscope (Olympus, Tokyo,
Japan). The cell growth curve was drawn according to the average
cell number of each group.
Western blot analysis
Western blotting was performed following the
standard procedure. Proteins were extracted from passage 2 and
passage 8 of bmMSCs and separated by sodium dodecyl
sulfate-polyacrylamide gel (SDS-PAGE) electrophoresis. Following
the electrophoresis, proteins were transferred to the
polyvinylidene difluoride (PVDF) membranes. The membranes were
blocked with 5% milk or 5% bovine serum albumin (BSA) in
Tris-buffered saline with Tween-20 (TBS-T), and then incubated with
β-catenin or phospho-Akt primary antibodies at 4°C overnight.
Following three times washing with TBS-T, the blots were incubated
with β-actin antibody at room temperature for 1 h. Then, the blots
were washed with TBS-T and incubated with HRP-conjugated secondary
antibody at room temperature for 1 h. The immunoreactive bands were
visualized by enhanced chemiluminescence, and the images were
scanned using a Gel Imaging Analysis System (Bio-Rad, Hercules, CA,
USA).
Reverse transcription polymerase chain
reaction (RT-PCR) assay
In the present study, expression of Akt1 in passage
8 of bmMSCs was measured using RT-PCR assay following transfection
of the cells with pMXs-β-catenin plasmids. The procedures of RNA
extraction and RT-PCR reaction were conducted as previously
described (10). The primers used
were 5′-GTCTCTAGGGTCCAGGGCCAAAGTC-3′ (Akt1 forward) and
5′-CATCTAAAAGGACAAGTGCTAGGAG-3′ (Akt1 reverse);
5′-TTCTTTGCAGCTCCTTCGTTGCCG-3′ (β-actin forward) and
5′-TGGATGGCTACGTACATGGCTGGG-3′ (β-actin reverse).
β-catenin overexpression in passage 8
bmMSCs
Passage 8 bmMSCs were plated in 6-well plates. As
the cells reached 80% confluency, they were transfected with
pMXs-β-catenin plasmids using Lipofectamine® 2000. The
cells transfected with empty pMXs plasmids served as controls.
Statistical analysis
Statistical analysis was performed with SPSS 11.5
software (SPSS, Inc., Chicago, IL, USA). Data are presented as the
mean ± SD from four independent experiments. Univariate comparisons
of the means were evaluated using the Student’s t-test and/or
one-way ANOVA with Tukey’s post-hoc adjustment for multiple
comparisons when appropriate. P<0.05 was considered to indicate
a statistically significant difference.
Results
Proliferation of passage 2 and passage 8
bmMSCs
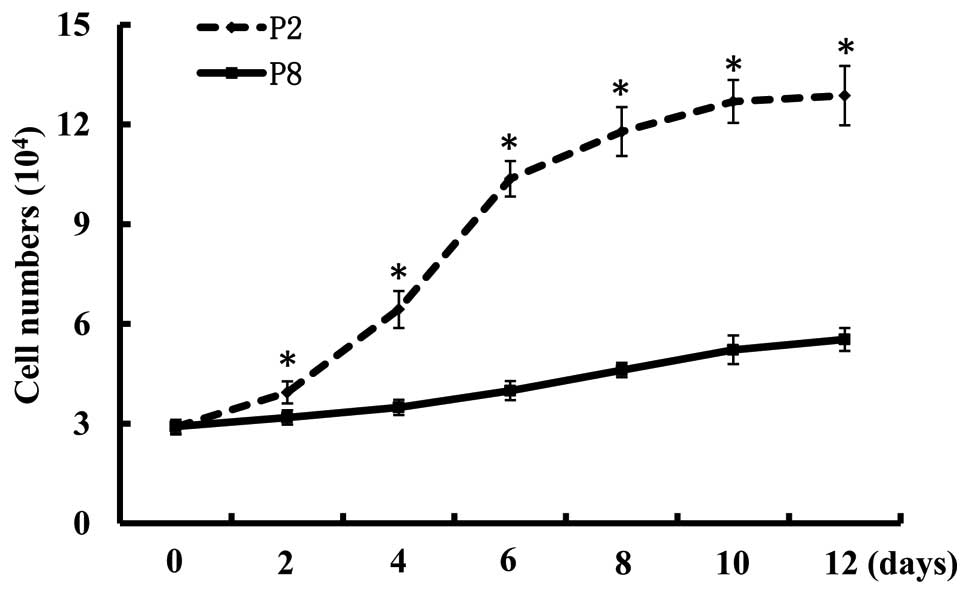
As illustrated in Fig.
1, the cell growth curve revealed that the cell numbers of
passage 8 bmMSCs following plating for 2, 4, 6, 8, 10 and 12 days
was significantly lower than the numbers of passage 2 cells
(P<0.05). This demonstrates that the late passage of bmMSCs have
a lower proliferative ability than the early passage of cells.
Expression of β-catenin and Akt in
passage 2 and passage 8 bmMSCs
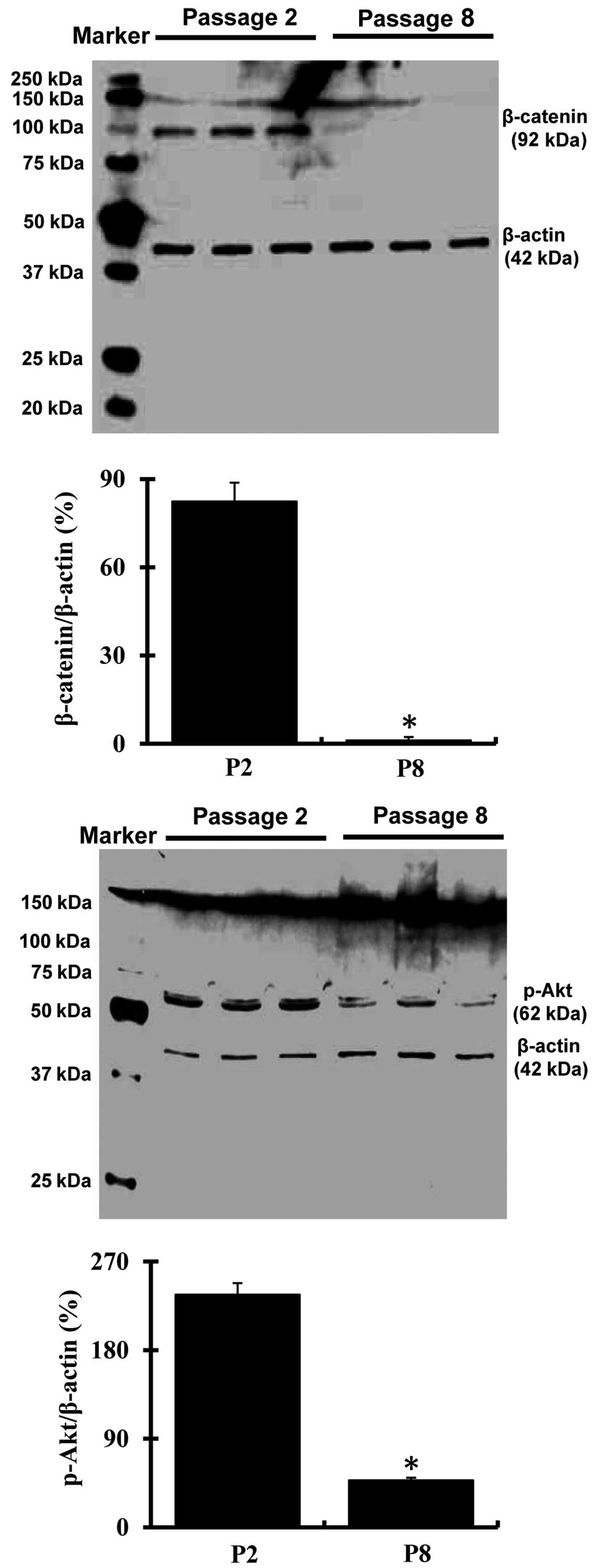
Western blotting analysis demonstrated that
expression of β-catenin and phospho-Akt was markedly decreased
(P<0.05) in passage 8 bmMSCs as compared with passage 2 cells
(Fig. 2).
Expression of β-catenin and Akt in
passage 8 bmMSCs following transfection with pMXs-β-catenin
plasmids
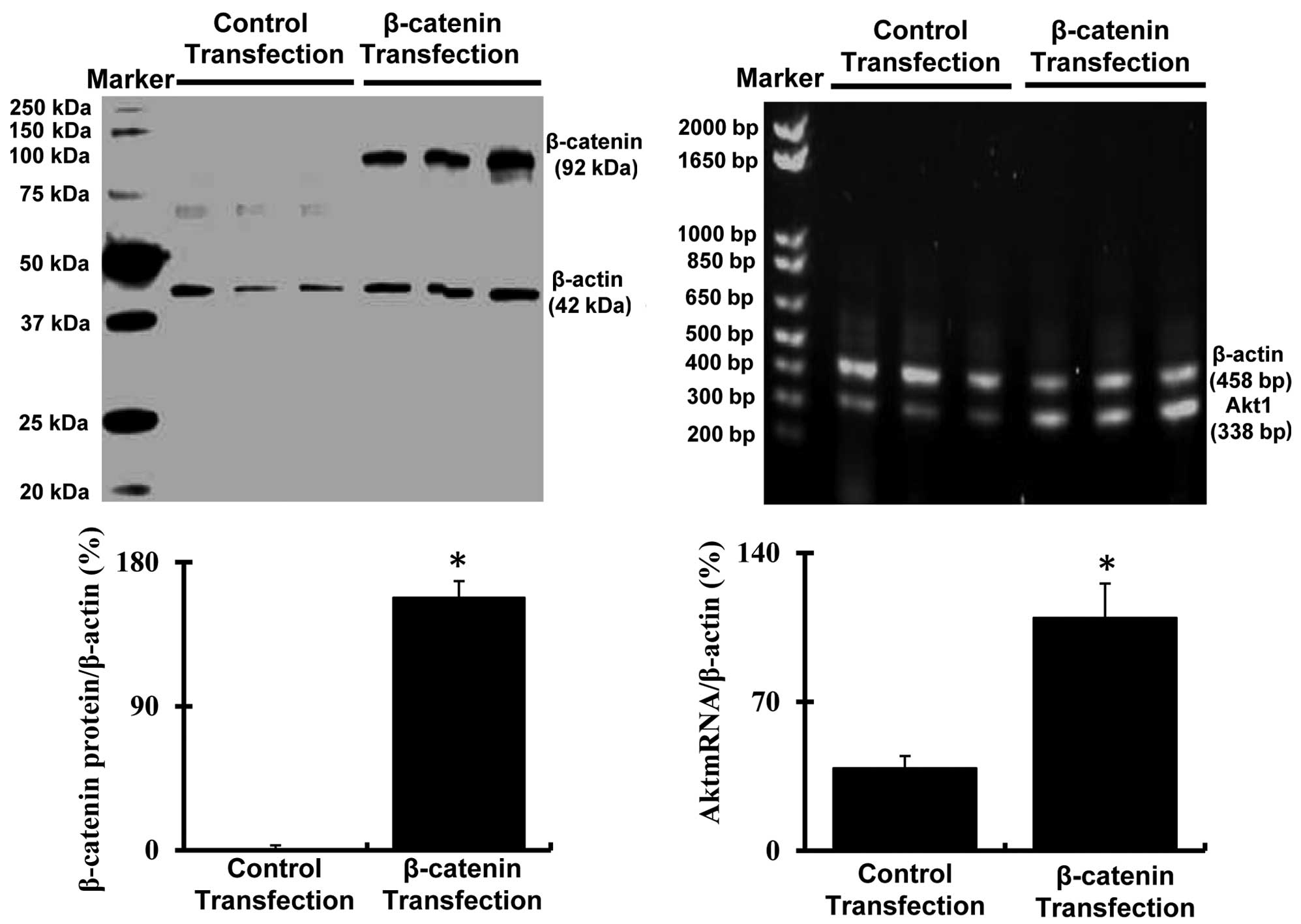
As illustrated in Fig.
3, the western blot analysis revealed that the expression of
β-catenin was markedly increased (P<0.05) in passage 8 bmMSCs
transfected with pMXs-β-catenin plasmids as compared with the cells
transfected with empty pMXs plasmids. More notably, another cell
proliferation signal Akt (Akt1 mRNA) was also markedly upregulated
(P<0.05) in passage 8 bmMSCs transfected with pMXs-β-catenin
plasmids.
Cell proliferation following transfection
of pMXs-β-catenin plasmids
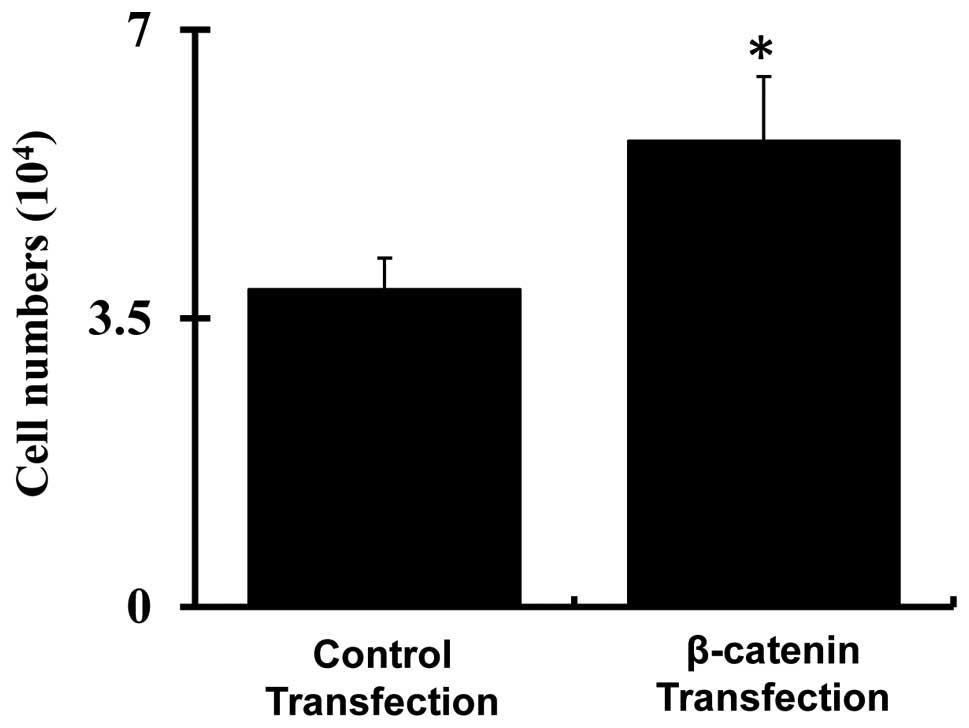
As illustrated in Fig.
4, cell counting demonstrated that proliferation of passage 8
bmMSCs was significantly increased following transfection with
pMXs-β-catenin plasmids as compared with the cells transfected with
empty pMXs plasmids (P<0.05).
Discussion
In the present study, it was demonstrated for the
first time, to the best of our knowledge, that β-catenin and Akt
expression are involved in the weak proliferative ability of the
late passage of bmMSCs. The results revealed that β-catenin and Akt
were markedly downregulated in the late passage (passage 8) of
bmMSCs as compared with the early passage (passage 2).
Overexpression of β-catenin in the late passage of bmMSCs increased
their proliferation and Akt expression.
bmMSCs are the promising source of seed cells for
stem cell transplant therapy. The proliferative ability of bmMSCs
is the most important determinant of bmMSC-based therapeutic
efficiency. Previous studies have demonstrated that the late
passages of MSCs will partially lose their proliferative ability
(4). As is consistent with
previous studies, we also observed that the proliferation of the
late passage (passage 8) of bmMSCs was markedly lower than the
early passage (passage 2) cells.
More importantly, we identified that the expression
of β-catenin was notably downregulated in the late passage of
bmMSCs. β-catenin is a dual function protein that mainly regulates
cell-cell adhesion and gene transcription. The expression of
Wnt/β-catenin signals has also been implicated in the proliferation
of numerous cell types, including MSCs (11,12).
It has been demonstrated that stimulation of Wnt/β-catenin
signaling with its agonists promotes proliferation of MSCs
(13). Downregulation or
deficiency of β-catenin was also identified to inhibit the
proliferative ability of other cell types (14). In the present study, we
hypothesized that the downregulation of β-catenin may be
responsible for the weak proliferation of the late passage of
bmMSCs. Our further experiments confirmed this, which suggests that
overexpression of β-catenin in the late passage of bmMSCs enhances
their proliferative ability.
Akt is a serine/threonine-specific protein kinase
that is important in numerous cell physiological processes,
including cell metabolism, proliferation, migration, autophagy and
apoptosis (7,15,16).
Akt is known as a cell survival signal and activation of the
PI3K/Akt pathway has been observed to promote proliferation of
bmMSCs (17). In the present
study, we identified that phospho-Akt expression was significantly
decreased in the late passage of bmMSCs following the expression of
β-catenin. Previous studies have demonstrated that Akt regulates
expression of β-catenin and the β-catenin signaling also
participates in the regulation of Akt expression (18–20).
Furthermore, it appears β-catenin regulates cell proliferation via
activation of the PI3K/Akt pathway (21). In the present study, overexpression
of β-catenin in the late passage of bmMSCs also enhanced Akt1
expression.
In conclusion, these data revealed that β-catenin
and Akt were markedly downregulated in passage 8 bmMSCs and that
the proliferative ability of passage 8 bmMSCs was significantly
lower than passage 2 bmMSCs. Transfection of β-catenin cDNA in
passage 8 bmMSCs enhanced the proliferation of these cells and
increased Akt expression. These results indicate that Wnt/β-catenin
and Akt signals have an important role in the regulation of bmMSC
growth.
Acknowledgements
This study was supported by a grant from the
National Natural Science Foundation of China (no. 81370428).
References
|
1
|
Zhang F, Wang C, Jing S, et al:
Lectin-like oxidized LDL receptor expresses in mouse bone
marrow-derived mesenchymal stem cells and stimulates their
proliferation. Exp Cell Res. 319:1054–1059. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferroni L, Gardin C, Tocco I, et al:
Potential for neural differentiation of mesenchymal stem cells. Adv
Biochem Eng Biotechnol. 129:89–115. 2013.PubMed/NCBI
|
|
3
|
Meligy FY, Shigemura K, Behnsawy HM, et
al: The efficiency of in vitro isolation and myogenic
differentiation of MSCs derived from adipose connective tissue,
bone marrow, and skeletal muscle tissue. In Vitro Cell Dev Biol
Anim. 48:203–215. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Boroujeni ME, Gowda P, Johnson J, et al:
The proliferation and differentiation capacity of bone marrow
derived-human mesenchymal stem cells in early and late doubling.
Asian J Biochem. 7:27–36. 2012. View Article : Google Scholar
|
|
5
|
Masckauchán TN, Shawber CJ, Funahashi Y,
et al: Wnt/beta-catenin signaling induces proliferation, survival
and interleukin-8 in human endothelial cells. Angiogenesis.
8:43–51. 2005.PubMed/NCBI
|
|
6
|
Sarkar S, Swiercz R, Kantara C, et al:
Annexin A2 mediates up-regulation of NF-κB, β-catenin, and stem
cell in response to progastrin in mice and HER-293 cells.
Gastroenterology. 140:583–595. 2011.PubMed/NCBI
|
|
7
|
Xu J, Qian J, Xie X, et al: High density
lipoprotein cholesterol promotes the proliferation of bone-derived
mesenchymal stem cells via binding scavenger receptor-B type I and
activation of PI3K/Akt, MAPK/ERK1/2 pathwys. Mol Cell Biochem.
371:55–64. 2012. View Article : Google Scholar
|
|
8
|
Skeen JE, Bhaskar PT, Chen CC, et al: Akt
deficiency impairs normal cell proliferation and suppresses
oncogenesis in a p53-independent and mTORC1-dependent manner.
Cancer Cell. 10:269–280. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Priore R, Dailey L and Basilico C:
Downregulation of Akt activity contributes to the growth arrest
induced by FGF in chondrocytes. J Cell Physiol. 207:800–808. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang F, Jing S, Ren T, et al:
MicroRNA-10b promotes migration of mouse bone marrow-derived
mesenchymal stem cells and downregulates E-cadherin expression. Mol
Med Rep. 8:1084–1088. 2013.PubMed/NCBI
|
|
11
|
Chen BY, Wang X, Chen LW and Luo ZJ:
Molecular targeting regulation of proliferation and differentiation
of bone marrow-derived mesenchymal stem cells or mesenchymal
stromal cells. Curr Drug Targets. 13:561–571. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Y, Chen X, Zhu W, et al: Growth
inhibition of mesenchymal stem cells by aspirin: involvement of the
WNT/beta-catenin signal pathway. Clin Exp Pharmacol Physiol.
33:696–701. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hoffman MD and Brnoit DS: Agonism of
Wnt-β-catenin signaling promotes mesenchymal stem cell (MSC)
expansion. J Tissue Eng Regen Med. Apr 1–2013.(Epub ahead of
print).
|
|
14
|
Foo C, Frey S, Yang HH, et al:
Downregulation of beta-catenin and transdifferentiation of human
osteoblast to adipocytes under estrogen deficiency. Gynecol
Endocrinol. 23:535–540
|
|
15
|
Zhang F, Hong Y, Liang W, et al:
Co-culture with Sertoli cells promotes proliferation and migration
of umbilical cord mesenchyaml stem cells. Biochem Biophys Res
Commun. 427:86–90. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang RC, Wei Y, An Z, et al: Akt-mediated
regulation of autophagy and tumorigenesis through Beclin 1
phosphorylation. Science. 338:956–959. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu J, Qian J, Xie X, et al: High density
lipoprotein cholesterol promotes the proliferation of bone-derived
mesenchymal stem cells via binding scavenger receptor-B type I and
activation of PI3K/Akt, MAPK/ERK1/2 pathwys. Mol Cell Biochem.
371:55–64. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang J, Shemezis JR, McQuinn ER, et al:
Akt activation by N-cadherin regulates beta-catenin signaling and
neuronal differentiation during cortical development. Neural Dev.
8:72013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Z, Havasi A, Gall JM, et al:
Beta-catenin promotes survival of renal epithelial cells by
inhibiting Bax. J Am Soc Nephrol. 20:1919–1928. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dihlmann S, Kloor M, Fallsehr C, et al:
Regulation of AKT1 expression by beta-catenin/Tcf/Lef signaling in
colorectal cancer cells. Carcinogenesis. 26:1503–1512. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang Y, Yu J, Shi C, et al: Regulatory
effect of β-catenin on proliferation of hair follicle stem cells
involves PI3K/Akt pathway. J Appl Biomed. 11:131–141. 2013.
|