Introduction
Osteosarcoma is one of the most common primary bone
malignancies and primarily affects adolescents (<20 years old)
(1). It is characterized by
frequent vascular invasion, infiltration to adjacent soft tissue, a
high rate of local recurrence and early distant metastasis
(2). Although multidisciplinary
approaches, including chemotherapy and wide resection surgery, are
able to significantly improve the clinical outcome for patients
with osteosarcoma, the prognosis of advanced cases with distant
metastasis and local recurrence remains poor, even with additional
extensive chemotherapy (3).
Accumulating studies have indicated that a variety of cellular and
molecular events may be implicated in the tumorigenesis of
osteosarcoma. Therefore, identification of novel markers is
required for the prediction of malignant behavior and prognosis of
osteosarcomas, in order to guide treatment strategies and improve
the clinical outcome of this disease.
AKT family members encode highly similar
serine-threonine protein kinases, which belong to the more general
class of AGC kinases (related to AMP/GMP kinase and protein kinase
C) (4). There are three members of
the AKT kinase family: AKT1 (protein kinase B-α, PKB-α), AKT2
(protein kinase B-β, PKB-β) and AKT3 (protein kinase B-γ, PKB-γ)
(5). All of the AKT kinases lie at
the center of the phosphoinositide 3-kinase (PI3k)/AKT signal
transduction pathway, which regulates multiple cellular processes,
including cell proliferation, growth, survival, transformation and
differentiation (6). Activation of
the AKT kinases, initiated by extracellular stimuli in a
PI3k-dependent manner, regulates downstream signaling proteins that
are involved in cell survival, growth, cell cycle progression and
apoptotic response (7).
Previously, several studies of cancer biology have demonstrated the
crucial role of AKT kinases in tumorigenesis and that it may be
possible to target them therapeutically and/or as a
chemopreventative strategy (8).
Among the three AKT kinases, AKT2 has been demonstrated to be
associated with the development of human cancers. For example, in
non-small cell lung cancer, AKT2 contributes to cell survival via
different mechanisms (9). In
breast cancer cells, ablation of AKT2 may induce cell cycle arrest
and be differentially involved in regulating cell migration and the
epithelial-to-mesenchymal transition (10). In hepatocellular carcinoma, AKT2
expression may be upregulated and be a novel independent predictor
for the development and progression of this malignancy (11). Furthermore, Zhang et al
(12) indicated that knockdown of
AKT2 expression may have therapeutic applications in enhancing the
efficacy of chemotherapy in patients with osteosarcoma. However, it
is currently unknown whether the aberrant expression of AKT2 has
relevance to the progression of osteosarcoma. The aim of this study
was to investigate the clinicopathological and prognostic value of
AKT2 in patients with osteosarcoma.
Materials and methods
Patients and tissue samples
This study was approved by the Research Ethics
Committee of Shanghai Pudong Hospital, (Shanghai, China). Written
informed consent was obtained from all patients. All specimens were
anonymous and handled according to the ethical and legal
standards.
Formalin-fixed paraffin embedded osteosarcoma and
self-paired non-cancerous bone tissue samples were obtained from
126 patients with osteosarcoma at Shanghai Pudong Hospital between
1998 and 2008. The patients ranged from 12–66 years of age (median,
19 years; mean, 25.2 years). The study cohort included 78 males and
48 females. Clinicopathological parameters, including age, gender,
tumor size, tumor site, histological subtype, local recurrence,
distant metastasis and response to the neoadjuvant chemotherapy are
summarized in Table I. All samples
were obtained from primary lesions. The biopsies were performed
prior to chemotherapy or radiotherapy for diagnostic purposes.
 | Table IAssociation between AKT2 expression
and different clinicopathological features of osteosarcoma
patients. |
Table I
Association between AKT2 expression
and different clinicopathological features of osteosarcoma
patients.
| Clinicopathological
feature | No. of cases | AKT2 expression | P-value |
|---|
|
|---|
| High (n, %) | Low (n, %) |
|---|
| Age |
| <20 | 80 | 45 (56.25) | 35 (43.75) | NS |
| ≥20 | 46 | 25 (54.35) | 21 (45.65) | |
| Gender |
| Male | 82 | 47 (57.32) | 35 (42.68) | NS |
| Female | 44 | 23 (52.27) | 21 (47.73) | |
| Tumor size (cm) |
| ≥5.0 | 76 | 40 (52.63) | 36 (47.37) | NS |
| <5.0 | 50 | 30 (60.00) | 20 (40.00) | |
| Tumor site |
| Femur | 66 | 37 (56.06) | 29 (43.94) | NS |
| Tibia | 50 | 28 (56.00) | 22 (43.00) | |
| Other | 10 | 5 (50.00) | 5 (50.00) | |
| Histological
type |
| Osteoblastic | 58 | 27 (46.55) | 31 (53.45) | NS |
| Chondroblastic | 30 | 20 (66.67) | 10 (33.33) | |
| Fibroblastic | 22 | 13 (59.09) | 9 (40.91) | |
| Other | 16 | 10 (62.50) | 6 (37.50) | |
| Recurrence |
| Negative | 55 | 22 (40.00) | 33 (60.00) | 0.028 |
| Positive | 71 | 48 (67.61) | 23 (32.39) | |
| Metastasis |
| Negative | 57 | 18 (31.58) | 39 (68.42) | 0.006 |
| Positive | 69 | 52 (75.36) | 17 (24.64) | |
| Response to
chemotherapy |
| Good | 66 | 28 (42.42) | 32 (57.58) | 0.015 |
| Poor | 60 | 42 (70.00) | 24 (30.00) | |
Follow-up was conducted for all 126 patients with
osteosarcoma. Patients were monitored with computed tomography
(CT), which was performed every three months during the first three
years following chemotherapy, every four months during years four
and five, and every six months thereafter. The development of local
recurrence and distant metastases were detected by CT scans or
magnetic resonance imaging. All cases were independently reviewed
by two pathologists and discrepancies resolved by consensus review.
Of 126 patients with osteosarcoma, 48 (38.10%) experienced local
recurrence and 52 (41.27%) patients had succumbed to the disease by
the last follow-up date. The median follow-up time was 42 months
(range, 2–116 months). Overall survival time was calculated from
the date of the initial surgical operation to death. Event-free
survival was calculated from the date of the initial surgical
operation to the date of secondary cancer, tumor recurrence,
distant metastases or mortality from any cause.
Immunohistochemistry analysis
Expression patterns of AKT2 proteins in 126
formalin-fixed paraffin embedded osteosarcomas and self-paired
non-cancerous bone tissue samples were detected by
immunohistochemical staining. Briefly, all tissue samples were
retrieved and divided into 3-μm sections and mounted on pre-coated
slides. Following deparaffinizing in xylene and washing in a graded
series of ethanol, the sections were submerged into EDTA antigenic
retrieval buffer (ab93680, Abcam, Cambridge, UK). and microwaved
for antigenic retrieval. The slides were incubated with the primary
antibody raised against AKT2 (dilution, 1:50, mouse polyclonal
antibody; Cell Signaling Technology, Inc., Beverly, MA, USA). All
incubations with primary antibody were conducted overnight at 4°C.
Following washing in Tris-buffered saline (Sigma, Munich, Germany),
the tissue sections were treated with biotinylated rabbit
anti-mouse secondary antibody (Zymed Laboratories, Inc., San
Francisco, CA, USA), followed by further incubation with
streptavidin-horseradish peroxidase complex (Zymed Laboratories,
Inc.). The tissue sections were immersed in 3-amino-9-ethyl
carbazole and counterstained with 10% Mayer’s hematoxylin, then
dehydrated and mounted in Crystal Mount. Negative control staining
was conducted by substituting non-immune rabbit IgG and
phosphate-buffered saline for the primary antibodies.
Following hematoxylin counterstaining,
immunostaining was scored by two independent observers, who were
blinded to the clinicopathological parameters and clinical outcomes
of the patients. The scores of the two observers were compared and
any discrepancies were trained through re-examining the stainings
by the two pathologists to achieve a consensus score. The number of
positive-staining cells exhibiting immunoreactivity on the
cytoplasm in ten representative microscopic fields was counted and
the percentage of positive cells was calculated. The frequency of
AKT2 immunoreactivity in the tissue sections was evaluated as
negative (0) when no positive cells were observed within the tumor;
weak (1) when <30% of the tumor cells were positive; moderate
(2) when 30–60% of the tumor cells were positive and strong (3)
when >60% of tumor cells were positive. The intensity of
staining was evaluated as 0, 1, 2 and 3 for no staining, weak
staining, medium staining and strong staining, respectively. An
immunostaining score (IRS) was obtained by multiplying the
frequency and intensity score for stained cells (range, 0–9). The
cut-off value for IRS of AKT2 protein was selected on the basis of
a measure of heterogeneity with the log-rank test statistic, with
respect to overall survival. An optimal cut-off value was
identified. An IRS of ≥4.5 was used to classify the tumors with
high expression and <4.5 IRS classified the tumors with low
expression of AKT2 protein.
Statistical analysis
SPSS version 16.0 software for Windows (SPSS, Inc.,
Chicago, IL, USA) and SAS 9.1 (SAS Institute, Cary, NC, USA) were
used for statistical analysis. Continuous variables were expressed
as the mean ± standard deviation. Associations between the
expression of AKT2 and clinicopathological parameters were assessed
using a χ2 test. Patient survival and their differences
were determined by Kaplan-Meier method and a log-rank test. Cox
regression (proportional hazard model) was adopted for multivariate
analysis of prognostic factors. P<0.05 was considered to
indicate a statistically significant difference.
Results
Elevated expression of AKT2 in human
osteosarcoma tissues
Expression patterns and subcellular localization of
AKT2 protein in 126 pairs of osteosarcoma and matched non-cancerous
bone tissues were detected by an immunohistochemical assay. As
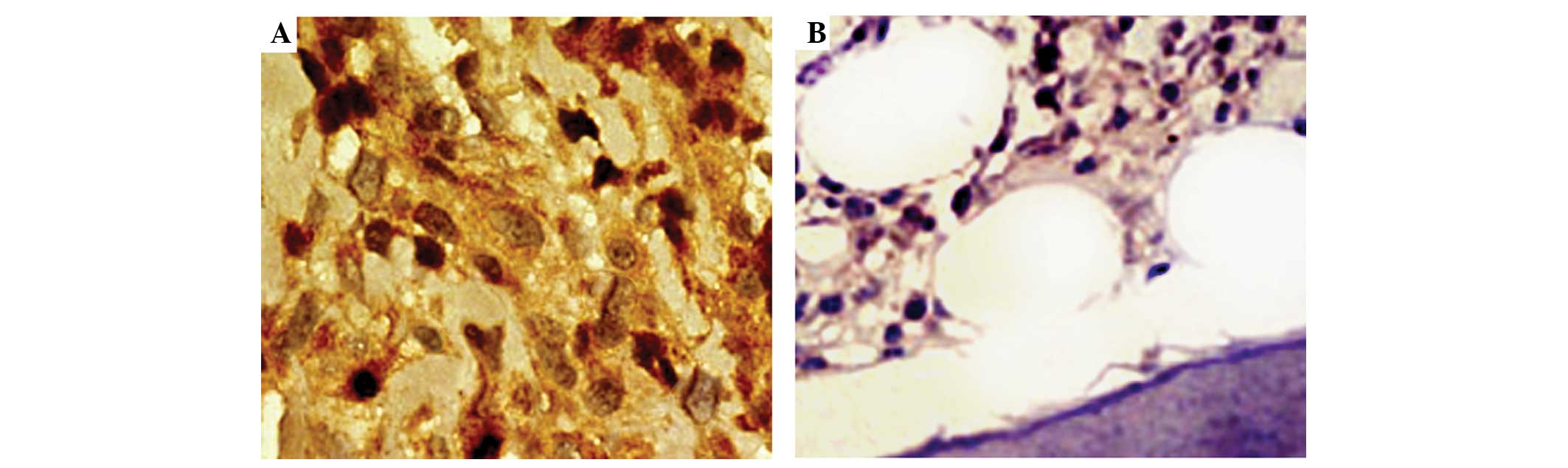
demonstrated in Fig. 1, strongly
positive immunostaining of AKT2 protein was detected in the nucleus
and/or cytoplasm of tumor cells in osteosarcoma tissues with a
uniform or interspersed granular pattern, while weakly positive or
negative immunostaining was detected in non-cancerous bone tissues.
In addition, the statistical analysis demonstrated that the
expression levels of AKT2 protein in osteosarcoma tissues were
significantly higher than in corresponding non-cancerous bone
tissues (IRS, 6.39±1.62 vs. 3.46±1.03; P<0.001). Of 126 patients
with osteosarcomas, 70 (55.56%) and 56 (44.44%) belonged to the
high and low AKT2 expression groups, respectively.
Elevated expression of AKT2 associates
with tumor progression of osteosarcoma tissues
Table I summarizes
the association of AKT2 expression with the clinicopathological
features of the osteosarcoma patients. The elevated expression of
AKT2 protein was significantly associated with positive recurrence
(P=0.028), the presence of metastasis (P=0.006) and poor response
to chemotherapy (P=0.015). However, the expression of AKT2 protein
was not correlated with other factors of patients including age,
gender, tumor size, tumor site and histological subtype (all
P>0.05).
Elevated expression of AKT2 associates
with poor prognosis in osteosarcoma patients
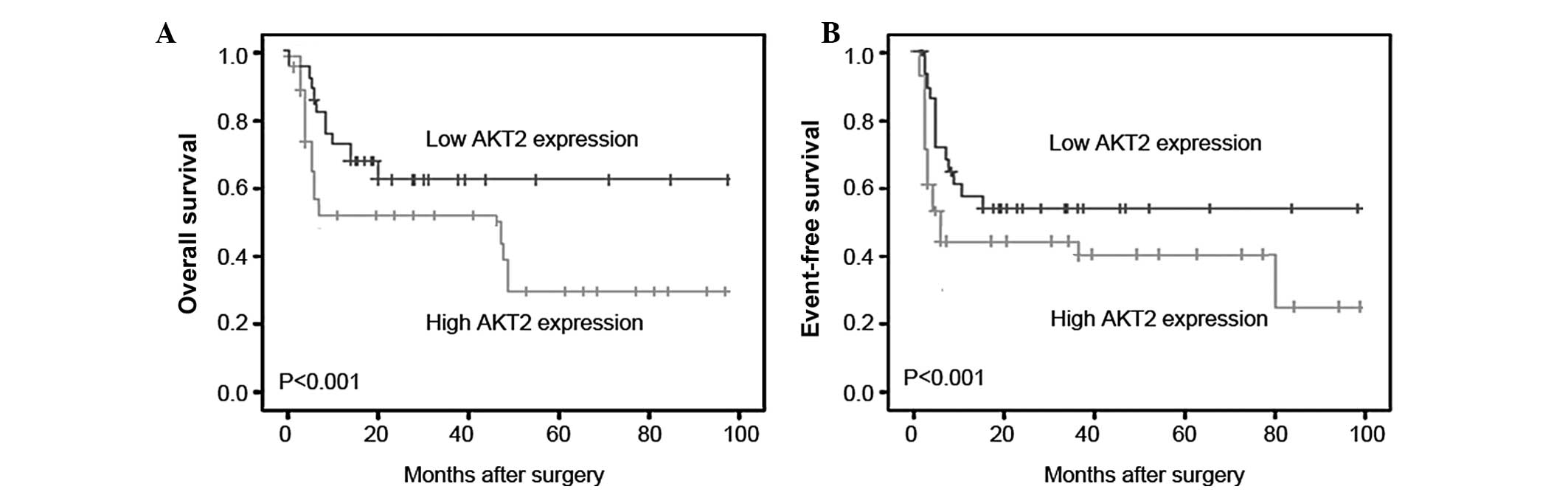
The prognostic value of AKT2 expression in human
osteosarcoma was further investigated by Kaplan-Meier analysis and
a log-rank test. As demonstrated in Fig. 2, osteosarcoma tissues with high
AKT2 expression were correlated with a shorter overall survival
(Fig. 2A; P<0.001) and shorter
event-free survival time (Fig. 2B;
P<0.001). Univariate analysis revealed that the positive
recurrence and metastasis, the high expression of AKT2 protein and
the poor response to chemotherapy were all significantly correlated
with poor overall survival (P=0.006, P<0.001, P<0.001 and
P<0.001, respectively; Table
II) and event-free survival (P=0.01, P<0.001, P<0.001 and
P<0.001, respectively; Table
II) of osteosarcoma patients. Multivariate analysis, as
summarized in Table III, further
demonstrated that AKT2 expression (P=0.029 and 0.016,
respectively), the status of recurrence (P=0.018 and 0.012,
respectively) and metastasis (P=0.020 and 0.015, respectively), and
the response to chemotherapy (P=0.011 and 0.008, respectively) were
all independent prognostic factors for overall survival and
event-free survival time.
 | Table IIUnivariate analysis of prognostic
parameters in patients with osteosarcoma by Cox regression
analysis. |
Table II
Univariate analysis of prognostic
parameters in patients with osteosarcoma by Cox regression
analysis.
| Overall survival | Event-free
survival |
|---|
|
|
|---|
| Variable | P-value | Relative risk | P-value | Relative risk |
|---|
| Age at diagnosis
(years) |
| <20 vs. ≥20 | 0.132 | 0.982 | 0.171 | 1.058 |
| Gender |
| Male vs. female | 0.162 | 0.961 | 0.189 | 1.146 |
| Tumor size (cm) |
| <5.0 vs.
≥5.0 | 0.113 | 1.268 | 0.115 | 1.321 |
| Tumor site |
| Femur vs. tibia and
other | 0.207 | 1.199 | 0.131 | 1.087 |
| Histological
type |
| Osteoblastic vs.
other | 0.106 | 1.425 | 0.129 | 1.525 |
| Recurrence |
| Negative vs.
positive | 0.006 | 3.168 | 0.01 | 3.032 |
| Metastasis |
| Negative vs.
positive | <0.001 | 6.879 | <0.001 | 6.532 |
| Response to
chemotherapy |
| Good vs. poor | <0.001 | 8.332 | <0.001 | 7.969 |
| AKT2 expression |
| Low vs. high | <0.001 | 6.501 | <0.001 | 6.089 |
 | Table IIIMultivariate analysis of prognostic
parameters in patient with osteosarcomas by Cox regression
analysis. |
Table III
Multivariate analysis of prognostic
parameters in patient with osteosarcomas by Cox regression
analysis.
| Overall
survival | Event-free
survival |
|---|
|
|
|---|
| Variable | P-value | Relative risk | P-value | Relative risk |
|---|
| Recurrence |
| Negative vs.
positive | 0.018 | 3.141 | 0.012 | 3.226 |
| Metastasis |
| Negative vs.
positive | 0.020 | 2.817 | 0.015 | 3.962 |
| Response to
chemotherapy |
| Good vs. poor | 0.011 | 3.309 | 0.008 | 4.556 |
| AKT2
expression |
| Low vs. high | 0.029 | 2.261 | 0.016 | 3.198 |
Discussion
Despite the advancement of therapeutic strategies,
it is extremely difficult to improve the prognosis of patients with
osteosarcoma. Furthermore, as 75% of osteosarcoma cases occur in
10–20-year-old patients, this disease is a significant burden to
families and society (13). For
these reasons, the screening of efficient molecular markers is of
great significance to differentiate the malignant progression,
determine the clinicopathological characteristics and predict the
clinical outcome of patients with osteosarcoma. In the present
study, it was demonstrated that elevated expression of AKT2 may be
associated with the progression of osteosarcoma. The results of the
immunohistochemical assay reveal that AKT2 is upregulated in
primary osteosarcoma specimens. Furthermore, the expression level
of AKT2 significantly correlated with aggressive disease severity
in the patients. The statistical analyses revealed that the
patients with high expression of AKT2 had a poorer prognosis. Based
on these findings, it was suggested that the expression of AKT2 may
be of value in predicting the progression and prognosis of patients
with osteosarcoma.
Activation of specific AKT family members promotes
tumor cell cycling, survival and invasiveness, enhances telomerase
activity and modulates angiogenesis (14). These processes are all considered
to be hallmarks of cancer. In particular, accumulating evidence
indicates that alterations in AKT2 are common in numerous types of
human cancer. For example, Cheng et al (15) identified the amplification and
overexpression of AKT2 in human ovarian carcinoma tissues and cell
lines and Roy et al (16)
demonstrated that the overexpression of AKT2 may be an early event
during colon tumorigenesis. Furthermore, Cheng et al
(17) indicated that the
overexpression of AKT2 may be capable of transforming NIH3T3 cells,
and AKT2 antisense RNA may inhibit the tumorigeneic phenotype of
cancer cells exhibiting amplified AKT2. In the osteosarcoma
literature, the activation of AKT2 has been demonstrated to be
capable of promoting a chemoresistant phenotype and the inhibition
of this kinase may sensitize chemoresistant cancer cells (12). Consistent with these previous
findings, data from the present study demonstrated that the
expression of AKT2 was significantly increased in osteosarcoma
tissues compared with non-cancerous bone tissues. Notably, it was
identified that the elevated expression of AKT2 protein may be
closely associated with the status of recurrence and metastasis,
the response to chemotherapy and the clinical outcome of patients
with osteosarcoma, implicating that AKT2 protein may have an
oncogenic role during the progression of this malignancy.
In previous years, a variety of mechanisms regarding
the oncogenic role of AKT2 have been delineated, but not fully
elucidated. It is well established that the activation of AKT
kinases is a multi-step process that involves membrane
translocation and phosphorylation, and is triggered by the
engagement of receptor tyrosine kinases by peptide growth factors
and cytokines (18,19). AKT kinases belong to the PI3K/AKT
signaling pathway, including the upstream PI3K, PTEN and LKB1, and
the downstream tuberous sclerosis complex 2 and eukaryotic
initiation factor 4E. All these components have been linked to
tumorigenesis (20). The critical
step in the signaling cascade leading to AKT activation is
stimulation of the growth factor receptor-associated PI3K that
forms a direct axis with AKT (21). Aside from this, several
serine-threonine phosphatases, including protein phosphatase 2A,
may also be involved in the inactivation of AKT in vivo
(22). However, the precise
molecular mechanisms for the altered expression of AKT2 in human
osteosarcoma remain unclear thus far.
In conclusion, to the best of our knowledge, this is
the first study to indicate that the elevated expression of AKT2
may be associated with aggressive clinical behavior and poor
outcome in patients with osteosarcoma. Therefore, AKT2 may be a
candidate marker of unfavorable prognosis in osteosarcoma. Further
studies of the molecular mechanisms by which AKT2 contributes to
the initiation and progression of osteosarcoma are warranted to
facilitate further clinical application of this evidence.
References
|
1
|
Dorfman HD and Czerniak B: Bone cancers.
Cancer. 75(Supp l): 203–210. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jaffe N: Adjuvant chemotherapy in
osteosarcoma: an odyssey of rejection and vindication. Cancer Treat
Res. 152:219–37. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kong C and Hansen MF: Biomarkers in
osteosarcoma. Expert Opin Med Diagn. 3:13–23. 2009. View Article : Google Scholar
|
|
4
|
Martelli AM, Tabellini G, Bressanin D,
Ognibene A, Goto K, Cocco L and Evangelisti C: The emerging
multiple roles of nuclear Akt. Biochim Biophys Acta.
1823:2168–2178. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hemmings BA and Restuccia DF: PI3K-PKB/Akt
pathway. Cold Spring Harb Perspect Biol. 4:a0111892012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Alessi DR and Cohen P: Mechanism of
activation and function of protein kinase B. Curr Opin Genet Dev.
8:55–62. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Radisavljevic Z: AKT as locus of cancer
positive feedback loops and extreme robustness. J Cell Physiol.
228:522–524. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Burris HA III: Overcoming acquired
resistance to anticancer therapy: focus on the PI3K/AKT/mTOR
pathway. Cancer Chemother Pharmacol. 71:829–842. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee MW, Kim DS, Lee JH, et al: Roles of
AKT1 and AKT2 in non-small cell lung cancer cell survival, growth,
and migration. Cancer Sci. 102:1822–1828. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kirkegaard T, Witton CJ, Edwards J, et al:
Molecular alterations in AKT1, AKT2 and AKT3 detected in breast and
prostatic cancer by FISH. Histopathology. 56:203–211. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu X, Sakon M, Nagano H, et al: Akt2
expression correlates with prognosis of human hepatocellular
carcinoma. Oncol Rep. 11:25–32. 2004.PubMed/NCBI
|
|
12
|
Zhang G, Li M, Zhu X, Bai Y and Yang C:
Knockdown of akt sensitizes osteosarcoma cells to apoptosis induced
by Cisplatin treatment. Int J Mol Sci. 12:2994–3005. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen D, Zhang YJ, Zhu KW and Wang WC: A
systematic review of vascular endothelial growth factor expression
as a biomarker of prognosis in patients with osteosarcoma. Tumour
Biol. 34:1895–1899. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Busaidy NL, Farooki A, Dowlati A, et al:
Management of metabolic effects associated with anticancer agents
targeting the PI3K-Akt-mTOR pathway. J Clin Oncol. 30:2919–2928.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cheng JQ, Godwin AK, Bellacosa A, et al:
AKT2, a putative oncogene encoding a member of a subfamily of
protein-serine/threonine kinases, is amplified in human ovarian
carcinomas. Proc Natl Acad Sci USA. 89:9267–9271. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Roy HK, Olusola BF, Clemens DL, Karolski
WJ, Ratashak A, Lynch HT and Smyrk TC: AKT proto-oncogene
overexpression is an early event during sporadic colon
carcinogenesis. Carcinogenesis. 23:201–205. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cheng JQ, Altomare DA, Klein MA, Lee WC,
Kruh GD, Lissy NA and Testa JR: Transforming activity and
mitosis-related expression of the AKT2 oncogene: evidence
suggesting a link between cell cycle regulation and oncogenesis.
Oncogene. 14:2793–2801. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sheppard K, Kinross KM, Solomon B, Pearson
RB and Phillips WA: Targeting PI3 kinase/AKT/mTOR signaling in
cancer. Crit Rev Oncog. 17:69–95. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mahajan K and Mahajan NP: PI3K-independent
AKT activation in cancers: a treasure trove for novel therapeutics.
J Cell Physiol. 227:3178–3184. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hafsi S, Pezzino FM, Candido S, et al:
Gene alterations in the PI3K/PTEN/AKT pathway as a mechanism of
drug-resistance (review). Int J Oncol. 40:639–644. 2012.PubMed/NCBI
|
|
21
|
Toker A: Achieving specificity in Akt
signaling in cancer. Adv Biol Regul. 52:78–87. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shimura T: Acquired radioresistance of
cancer and the AKT/GSK3β/cyclin D1 overexpression cycle. J Radiat
Res. 52:539–544. 2011.PubMed/NCBI
|