Introduction
Cervical cancer is the second most common type of
malignancy in females, with over half a million cases occurring
annually worldwide (1). Early
stage cervical cancer can be treated with surgery or radiation with
equivalent results. For locally advanced disease; however,
radiation offers the only initial option for curative therapy
(2). The dose of radiation is
increased to enhance the effects of radiotherapy, which inhibits
cervical cancer growth and development; however, this also leads to
adverse reactions. In order to improve the local control and
diminish side effects, radiation is combined with a
radiosensitizer.
Aloe-emodin (AE), an Aloe vera leaf exudate
(3), is used in traditional
Chinese medicine for its laxative, antiviral and hepatoprotective
properties (4–6). Its ingredients are of great value for
the food, modern pharmaceutical and cosmetic industries (7). Previous studies have demonstrated
that AE exerts anti-proliferative effects by inhibiting cell cycle
progression in certain types of cancer cells, including cervical
cancer (8), KB oral cancer cells
(9), bladder cancer (10), leukemia (11), HK-2 human kidney cells (12) and nasopharyngeal carcinoma cells
(13). AE is hypothesized to
inhibit progression at the G2/M phase of the cell cycle.
In addition, AE has been reported to inhibit the proliferation of
gastric cancer and tongue squamous cancer cells by inhibiting the
cell cycle at S phase (14,15).
Radiation affects the cell cycle, and
radiosensitivity depends on the phase of the cell cycle and its
progression (16), however, the
effect of AE as a radiosensitizer in HeLa cells has not been
reported. The present study hypothesized that the combination
treatment of AE and radiation may enhance the radiosensitivity and
differentiation of cervical cancer cells. In order to assess this
hypothesis, the present study analyzed the effects of combination
treatment with AE and radiation in the HeLa human cervical cancer
cell line and examined the underlying mechanisms of the inhibition
of HeLa cells.
Materials and methods
Cells and cell culture
The HeLa cell line was purchased from Jilin Tumor
Institute (Changchun, China). Cells were cultured under a
humidified atmosphere containing 5% CO2 at 37°C in
RPMI-1640 medium supplemented with 10% heat-inactivated fetal
bovine serum, penicillin (100 U/ml) and streptomycin (100 μg/ml).
Cells were subcultured every 2–3 days. Exponentially growing cells
were used for the experiments.
Reagents and equipment
AE was purchased from Aladdin Chemical Co., Ltd.
(Shanghai, China). RPMI-1640 medium (Gibco-BRL, Carlsbad, CA, USA),
fetal bovine serum (FBS; Gibco-BRL),
methylthiazolyldiphenyl-tetrazolium bromide (MTT; Sigma, St. Louis,
MO, USA), enhanced chemiluminescence (ECL) substrate (Pierce
Biotechnology, Inc., Rockford, IL, USA), mouse anti-human β-actin
monoclonal antibody (Sigma), anti-cyclin B and anti-γ-H2AX
monoclonal antibody (Abnova Corporation, Taipei, Taiwan), goat
anti-mouse IgG/horseradish peroxidase (HRP; Beijing Dingguo
Changsheng Biotechnology Co. Ltd, Beijing, China), electrophoresis
system (Bio-Rad, Hercules, CA, USA), an alkaline phosphatase (ALP)
activity assay kit (Nanjing Jiancheng Bioengineering Institute,
Nanjing, China) and an X-ray linear accelerator (Varian Medical
Systems, Palo Alto, CA, USA) were used in the present study. The
dose rate was 200 c Gy/min and the distance to the radiation source
was 100 cm.
MTT assay for cell proliferation
HeLa cells were seeded in 96-well plates at a
density of 4.0×103 cells/well for 24 h. The cells were
treated with various concentrations of AE (0, 5, 10, 25, 50, 75,
100, 200 and 500 μM) for 24, 48 and 72 h. Dimethylsulfoxide (DMSO;
0.5%) was used as the control. MTT (5 mg/ml) was added to each well
and incubated for 4 h at 37°C, following which DMSO was added to
each well to dissolve the dark blue crystal product. The absorbance
was measured at a wavelength of 590 nm using a microplate reader
(Bio-Rad, Shanghai, China).
Colony-forming assays
A single-cell suspension was plated onto 6-well
culture plates at different densities (1×103,
2×103, 5×103 and 1×104 cells/ml).
Following incubation for 24 h, the cells were treated with AE in
medium at different concentrations (0, 50, 100 and 200 μM) and
cells were then exposed to different irradiation doses (0, 2, 4, 6,
8 and 10 Gy). The cells were washed with normal medium and
incubated in fresh, drug-free medium (10% FBS in RPMI-1640) for 14
days, 24 h after radiation treatment. The cells were then washed
with phosphate-buffered saline (PBS), fixed with methanol and
stained with Giemsa solution. The number of colonies containing at
least 50 cells was counted manually under a microscope (Olympus,
Tokyo, Japan) by two independent investigators. The plating
efficiency (PE) and survival fraction (SF) were calculated as
follows: PE = colony-forming number / inoculating number × 100. SF
= PE (tested group) / PE (control group) × 100. GraphPad Prism
software 5.01 and the multi-target single-hit model (using the
equation SF = 1− (1−e-D/D0)N were applied to
generate the dose-survival curve and calculate the radiobiological
parameters, including the mean lethal dose (D0),
quasi-threshold dose (Dq), daily fraction dose of 2 Gy
in clinical practice (SF2), extrapolation number (N),
sensitizing enhancement ratio (SER)D0 and
SERDq. SER is one of the radiobiological parameters and
AE increases the radiosensitivity in a concentration-dependent
manner. Three replicates were set at each radiation dose.
Cell cycle distribution and apoptosis
analysis by flow cytometry
Single-cell suspensions at a density of
1×106 cells/well were seeded in 6-well plates and
incubated for 24 h. The cells were treated with AE (0 and 50 μM)
for 30 min, irradiated with doses of 0 and 4 Gy and incubated for
12, 24, 48 and 72 h. The cells were harvested by trypsinization,
washed twice with PBS and sedimented by centrifugation at 1,500 × g
for 5 min. The supernatant was removed and cells were fixed in 70%
ice-cold ethanol overnight. Cells were washed twice with cold PBS,
incubated with 20 μg/ml RNase and stained with propidium iodide.
Cell cycle distribution and apoptosis were determined by flow
cytometry (FACSCalibur; BD Biosciences, Franklin Lakes, NJ,
USA).
Western blot analysis of cyclin B and
γ-H2AX expression
A single-cell suspension was incubated for 24 h,
following which the cells were treated with AE (0 and 50 μM) for 30
min followed by radiation treatment at doses of 0 and 4 Gy.
Subsequently, the cells were washed, scraped with ice-cold PBS and
centrifuged. The cell lysate was harvested and protein
concentration was determined with a protein assay kit (Ye zhou,
Shanghai, China). A total of 50 μg of protein was separated by
sodium dodecyl sulfate-polyacrylamide gel electrophoresis,
transferred onto nitrocellulose membranes (Mai bio, Shanghai,
China) and inhibited overnight with PBS containing 3% skimmed milk.
The membranes were incubated with primary antibodies against
β-actin, cyclin B and γ-H2AX for 2 h and washed with PBS. The
membranes were then treated with HRP-conjugated rabbit anti-mouse
IgG secondary antibody. Antibody-reactive bands were visualized by
the ECL detection system (Amersham Pharmacia Biotech, Piscataway,
NJ, USA). Representative data were from an individual experiment
repeated three times.
Determination of relative ALP
activity
A single-cell suspension was incubated for 24 h,
following which the cells were treated with AE (0 and 50 μM) for 30
min followed by radiation treatment at doses of 0 and 4 Gy.
Subsequently, the cells were cultured for 24, 48 and 72 h. The
cells were washed, lysed and centrifuged. The supernatant was used
for the measurement of ALP activity with an ALP activity assay kit
(Nanjing Jiancheng Bioengineering Institute). The absorbance at 520
nm was measured on a microplate reader. Three independent
experiments were performed in this analysis.
Statistical analysis
Statistical analysis was performed using SPSS
version 18.0 (SPSS, Inc., Chicago, IL, USA). Student’s t-test or
one-way analysis of variance were used for all comparisons. Data
are presented as the mean ± standard error of three independent
experiments. P<0.05 was considered to indicate a statistically
significant difference.
Results
Growth inhibitory effects of AE on HeLa
cells
The effect of AE on the proliferation of HeLa cells
was evaluated by an MTT assay (Fig.
1). AE inhibited the proliferation of HeLa cells in a
concentration- and time-dependent manner, particularly when the
concentration of AE was >50 μM. Based on these results, 50 μM AE
was selected as the dose for subsequent experiments.
Effect of AE on the radiosensitization of
HeLa cells
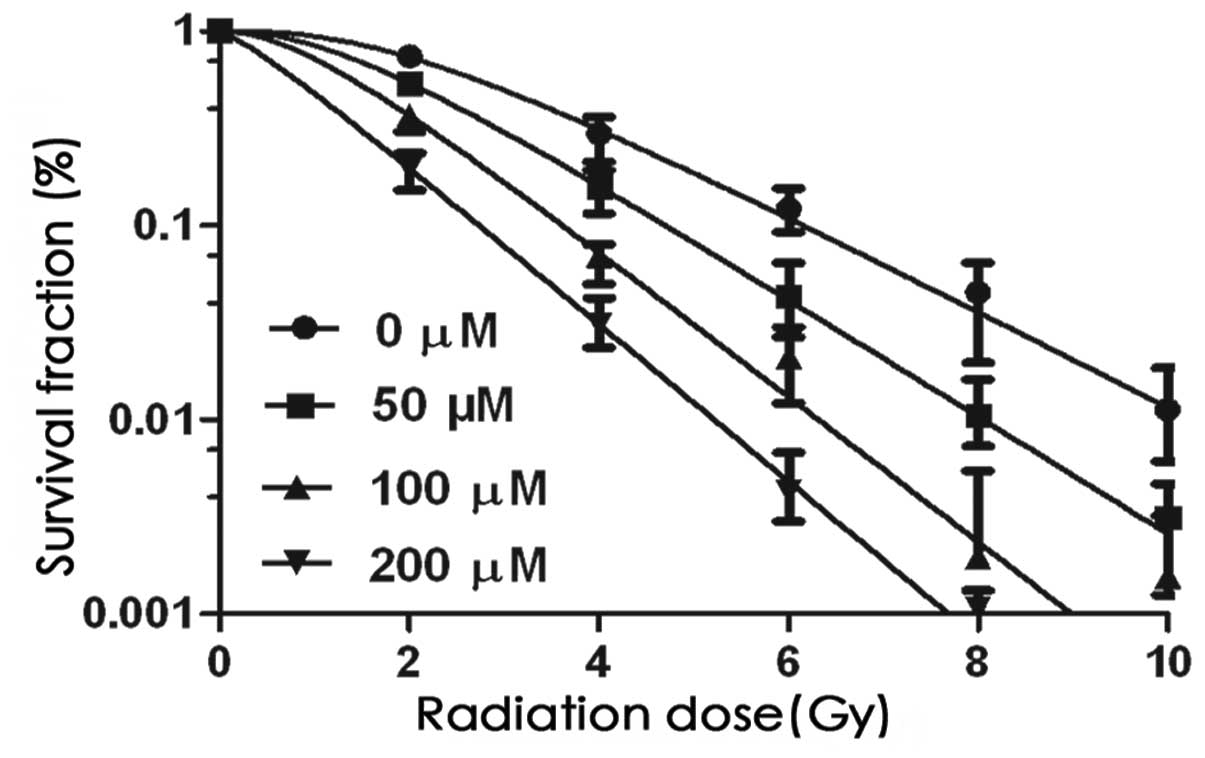
As shown in Fig. 2
and Table I, the radiobiological
parameters D0, Dq, N and SF2
decreased, and the SERD0 and SERDq increased
in response to AE in a concentration-dependent manner in
vitro. These effects were enhanced by combination treatment
compared with the effect of radiation alone.
 | Table IRadiobiological parameters of the
dose-survival curve. |
Table I
Radiobiological parameters of the
dose-survival curve.
| Concentration | D0 | Dq | N | SF2 |
SERD0 |
SERDq |
|---|
| 0 μM + RT | 1.756 | 2.177 | 3.454 | 0.538 | | |
| 50 μM + RT | 1.437 | 1.444 | 2.731 | 0.435 | 1.222 | 1.508 |
| 100 μM + RT | 1.158 | 0.987 | 2.345 | 0.324 | 1.517 | 2.207 |
| 200 μM + RT | 1.072 | 0.283 | 1.302 | 0.286 | 1.638 | 7.695 |
Effects of AE and radiation on HeLa cell
cycle distribution and apoptosis assessed by flow cytometry
As shown in Fig. 3,
HeLa cells treated with radiation or AE alone and the combination
of radiation with AE demonstrated an increase in the number of
cells in the G2/M phase. Investigation demonstrated that
the S phase was increased with AE alone at 48 and 72 h and a
sub-G1 peak clearly appeared (apoptosis) at 24, 48 and
72 h in cells treated with the combination of radiation and AE.
Apoptotic peaks were significant with the extension of time.
Cyclin B and γ-H2AX expression by western
blotting
Figs. 4 and
5 show the expression of cyclin B
and γ-H2AX by western blotting. The cells treated with radiation (4
Gy) or AE (50 μM) alone and a combination of radiation and AE
expressed cyclin B and γ-H2AX. The expression of cyclin B was
highest at 12 h in cells treated with the combination and the
expression of γ-H2AX in the combination group was higher than that
following AE or radiation treatment alone.
Relative activity of ALP
As shown in Fig. 6,
the ALP activity of HeLa cells was increased by the treatment with
radiation or AE alone and the combination of radiation and AE in a
time-dependent manner. This effect was enhanced by combination
treatment compared with the effect of radiation or AE alone.
Discussion
Although the anticancer effect of AE has been
reported in numerous studies, its effect on enhancing the
radiosensitivity of cancer cells has not been investigated to date.
The present study investigated the effect of AE on the
radiosensitivity and differentiation of HeLa cells in vitro
and examined its underlying mechanisms.
The results demonstrated that AE inhibited the
proliferation of HeLa cells in vitro in a concentration- and
time-dependent manner. Notably, AE concentrations >50 μM
demonstrated significant cytotoxicity in the MTT assay. The
radiosensitivity of cells treated with AE increased in a
dose-dependent manner compared with that of cells treated without
AE in colony-forming assays. These results demonstrated that AE
enhanced the radiosensitivity of HeLa cells. It has been reported
that the G2/M and M phases are the most sensitive to
radiation in the cell cycle (17–19).
Flow cytometry results demonstrated that treatment with AE or
radiation alone and AE in combination with radiation inhibited cell
cycle progression at the G2/M phase. Furthermore, the
experiments also demonstrated that the cells arrested in the S
phase with AE alone at 48 and 72 h and that there was a
sub-G1 peak at 24, 48 and 72 h, which was particularly
clear 72 h after combination treatment with AE and radiation in a
concentration-dependent manner. The underlying mechanism of
apoptosis (sub-G1 peak) in HeLa cells caused by S phase
arrest was not further investigated in the present study. However,
previous studies have demonstrated that AE is able to inhibit the
growth of gastric cancer cells and induce apoptosis in squamous
cell carcinoma of the tongue by inhibiting cell cycle progression
at the S phase (14,15). The findings of the present study
therefore, support the results of the contribution of S phase
arrest to apoptosis. The present study hypothesized that S phase
arrest may be one of the factors triggering apoptosis in HeLa
cells, although it may not be the only one.
The cell cycle is a strictly ordered process that is
controlled by multiple cell cycle regulatory proteins, including
cyclin and cyclin dependent kinase (CDK) protein kinases. The main
cell cycle regulatory proteins that control the G2/M
phase are known as cyclin B and CDK1. The levels of CDK1 remain
relatively constant during the cell cycle, while the levels of
cyclin B undergo cyclical changes. Cyclin B is synthesized at late
G1 phase and its expression levels increase as the cycle
progresses, reaching a maximum value at the G2 phase. As
cyclin B accumulates, the activity of CDK1 kinase is observed and
reaches a maximum value at G2 phase, which is maintained
throughout the M phase. Cyclin B is rapidly degraded at late M
phase, concomitant with the inactivation of CDK1, and the cell
enters the next cycle (20,21).
CDK1 regulates the cell cycle by modulating the expression of
cyclin B. Therefore, cyclin B was selected for analysis in the
present study. Western blot analysis demonstrated that cyclin B
expression was upregulated in cells treated with AE or radiation
alone. Furthermore, combination treatment with AE and radiation
further promoted cyclin B expression at 12 h. This result was in
line with the flow cytometry results. AE in combination with
radiation-arrested cells in the G2/M phase, inhibited cells with
injured DNA entering into the M phase and dividing, thus reducing
tumor cell survival and enhancing cell radiosensitivity.
DNA double strand breaks (DSBs) are the most
important biological effects of radiation. The histone protein H2AX
is a key protein involved in the repair of DNA DSBs, which is a
member of the H2A histone family (22). H2AX is rapidly phosphorylated on
serine 4 residues at the carboxyl terminus to form rH2AX at
burgeoning DSB sites (23,24), which is responsible for the
recruitment of DNA repair proteins, including BRCA1, 53bp1 and
Nbs1-Mre11-Rad50, to the sites of DNA damage (25–27).
Immediately following the formation of DSBs, large numbers of rH2AX
molecules accumulate around the break sites (23,24).
rH2AX detection has the potential to function as a diagnostic tool
and an indicator of treatment efficacy (23). Similarly previous studies have
demonstrated that YY1 and certain genes, including RBL2, E2F2, CDK6
and CCNE1 may be potential targets for cervical cancer detection,
monitoring and therapy (28,29).
Western blot analysis demonstrated that γ-H2AX is expressed in
cells treated with AE or radiation alone. Furthermore, combination
treatment with AE and radiation further upregulated γ-H2AX
expression at 1 and 6 h, indicating the further increase of DSBs.
Histone H2AX phosphorylation is crucial in apoptosis and the
appearance of strong H2AX phosphorylation is concurrent with the
initiation of DNA fragmentation (30). Flow cytometry also demonstrated
sub-G1 peaks (apoptosis) at 24, 48 or 72 h.
Cellular ALP is increasingly recognized as an
important marker for monitoring tumor cell differentiation
(31–33). AE inhibited the growth of cervical
cancer cells, KB oral cancer cells and gastric cancer cells by
inducing cancer cell differentiation (8,9,14).
The present study demonstrated that the activity of ALP in HeLa
cells is increased by treatment with radiation or AE alone and
further increased by a combination of radiation and AE in a
time-dependent manner. The differentiation of HeLa cells may also
be improved by combination treatment with radiation and AE compared
with the treatment with radiation or AE alone.
An ideal radiosensitizer enhances the
radiosensitivity of tumor cells and is harmless to or protects
normal tissue. Previous studies demonstrated that AE has no toxic
effects on normal cells (34).
However, whether or not it protects normal tissue remains to be
determined.
The present findings, together with those of
previous studies, suggest that AE has the following antitumor
effects: i) AE inhibits the growth of HeLa cells; ii) AE arrests
HeLa cells at the S and G2/M phases, inducing tumor cell
apoptosis; iii) AE increases the radiation sensitivity of HeLa
cells by inducing G2/M phase arrest; iv) AE increases
radiation-induced DNA damage; and v) the combination of radiation
and AE further improves the differentiation of HeLa cells. The
present results are consistent with the hypothesis that combination
treatment with AE enhances the effects of radiotherapy on HeLa
cells in vitro. The exposure of cells to the combination of
two cytotoxic modalities, AE and radiation, demonstrated an
increase in the cell death of HeLa cells. This indicated that AE
may be an effective radiosensitizer and a potential therapeutic
agent for the inhibition of tumor cell proliferation. Further in
vivo studies are required to clarify the anticancer effect of
AE and its mechanisms with the intention of clinical
application.
References
|
1
|
Shepherd JH: Cervical cancer. Best Pract
Res Clin Obstet Gynaecol. 26:293–309. 2012. View Article : Google Scholar
|
|
2
|
Yashar CM, Spanos WJ, Taylor DD and
Gercel-Taylor C: Potentiation of the radiation effect with
genistein in cervical cancer cells. Gynecol Oncol. 99:199–205.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dutta A, Bandyopadhyay S, Mandal C and
Chatterjee M: Aloe vera leaf exudate induces a caspase-independent
cell death in Leishmania donovani promastigotes. J Med
Microbiol. 56:629–636. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Krumbiegel G and Schulz HU: Rhein and
aloe-emodin kinetics from senna laxatives in man. Pharmacology.
47:120–124. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Andersen DO, Weber ND, Wood SG, Hughes BG,
Murray BK and North JA: In vitro virucidal activity of selected
anthraquinones and anthraquinone derivatives. Antiviral Res.
16:185–196. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Arosio B, Gagliano N, Fusaro LM,
Parmeggiani L, Tagliabue J, Galetti P, De Castri D, Moscheni C and
Annoni G: Aloe-Emodin quinone pretreatment reduces acute liver
injury induced by carbon tetrachloride. Pharmacol Toxicol.
87:229–233. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Eshun K and He Q: Aloe vera: a valuable
ingredient for the food, pharmaceutical and cosmetic industries - a
review. Crit Rev Food Sci Nutr. 44:91–96. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guo JM, Xiao BX, Liu Q, Zhang S, Liu DH
and Gong ZH: Anticancer effect of aloe-emodin on cervical cancer
cells involves G2/M arrest and induction of differentiation. Acta
Pharmacol Sin. 28:1991–1995. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xiao B, Guo J, Liu D and Zhang S:
Aloe-emodin induces in vitro G2/M arrest and alkaline phosphatase
activation in human oral cancer KB cells. Oral Oncol. 43:905–910.
2007. View Article : Google Scholar
|
|
10
|
Lin JG, Chen GW, Li TM, Chouh ST, Tan TW
and Chung JG: Aloe-emodin induces apoptosis in T24 human bladder
cancer cells through the p53 dependent apoptotic pathway. J Urol.
175:343–347. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen HC, Hsieh WT, Chang WC and Chung JG:
Aloe-emodin induced in vitro G2/M arrest of cell cycle in human
promyelocytic leukemia HL-60 cells. Food Chem Toxicol.
42:1251–1257. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Suboj P, Babykutty S, Srinivas P and
Gopala S: Aloe emodin induces G2/M cell cycle arrest and apoptosis
via activation of caspase-6 in human colon cancer cells.
Pharmacology. 89:91–98. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin ML, Lu YC, Chung JG, Li YC, Wang SG,
NGSH, Wu CY, Su HL and Chen SS: Aloe-emodin induces apoptosis of
human nasopharyngeal carcinoma cells via caspase-8-mediated
activation of the mitochondrial death pathway. Cancer Lett.
291:46–58. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guo J, Xiao B, Zhang S, Liu D, Liao Y and
Sun Q: Growth inhibitory effects of gastric cancer cells with an
increase in S phase and alkaline phosphatese activity repression by
aloe-emodin. Cancer Biol Ther. 6:85–88. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chiu TH, Lai WW, Hsia TC, et al:
Aloe-emodin induces cell death through S-phase arrest and
caspase-dependent pathways in human tongue squamous cancer SCC-4
Cells. Anticancer Res. 29:4503–4511. 2009.PubMed/NCBI
|
|
16
|
Pawlik TM and Keyomarsi K: Role of the
cell cycle in mediating sensitivity to radiotherapy. Int J Radiat
Oncol Biol Phys. 59:928–942. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Terasima T and Tolmach LJ: X-ray
sensitivity and DNA synthesis in synchronous populations of HeLa
cells. Science. 140:490–492. 1963. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sinclair WK and Morton RA: X-ray
sensitivity during the cell generation cycle of cultured Chinese
hamster cells. Radiat Res. 29:450–474. 1966. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sinclair WK: Cyclic x-ray responses in
mammalian cells in vitro. Radiat Res. 33:620–643. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chu HL, Mao H, Feng W, Liu JW and Geng Y:
Effects of sulfated polysaccharide from Masson pine (Pinus
massoniana) pollen on the proliferation and cell cycle of HepG2
cells. Int J Biol Macromol. 55:104–108. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Westendorf JM, Swenson KI and Ruderman JV:
The role of cyclin B in meiosis I. J Cell Biol. 108:1431–1444.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
West MH and Bonner WM: Histone 2A, a
heteromorphous family of eight protein species. Biochemistry.
19:3238–3245. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bonner WM, Redon CE, Dickey JS, Nakamura
AJ, Sedelnikova OA, Solier S and Pommier Y: Gamma H2AX and cancer.
Nat Rev Cancer. 8:957–967. 2008.PubMed/NCBI
|
|
24
|
Rogakou EP, Boon C, Redon C and Bonner WM:
Megabase chromatin domains involved in DNA double-strand breaks
in vivo. J Cell Biol. 146:905–916. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sedelnikova OA, Pilch DR, Redon C and
Bonner WM: Histone H2AX in DNA damage and repair. Cancer Biol Ther.
2:233–235. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Celeste A, Fernandez-Capetillo O, Kruhlak
MJ, Pilch DR, Staudt DW, Lee A, Bonner RF, Bonner WM and
Nussenzweig A: Histone H2AX phosphorylation is dispensable for the
initial recognition of DNA breaks. Nat Cell Biol. 5:675–679. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ward IM, Minn K, Jorda KG and Chen J:
Accumulation of checkpoint protein 53BP1 at DNA breaks involves its
binding to phosphorylated histone H2AX. J Biol Chem.
278:19579–19582. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Baritaki S, Sifakis S, Huerta-Yepez S, et
al: Overexpression of VEGF and TGF-beta1 mRNA in Pap smears
correlates with progression of cervical intraepithelial neoplasia
to cancer: implication of YY1 in cervical tumorigenesis and HPV
infection. Int J Oncol. 31:69–79. 2007.
|
|
29
|
Arvanitis DA and Spandidos DA:
Deregulation of the G1/S phase transition in cancer and squamous
intraepithelial lesions of the uterine cervix: a case control
study. Oncol Rep. 20:751–760. 2008.PubMed/NCBI
|
|
30
|
Rudel T and Bokoch GM: Membrane and
morphological changes in apoptotic cells regulated by
caspase-mediated activation of PAK2. Science. 276:1571–1574. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xiao B, Guo J, Lou Y, Meng D, Zhao W,
Zhang L, Yan C and Wang D: Inhibition of growth and increase of
alkaline phosphatase activity in cultured human oral cancer cells
by all-trans retinoic acid. Int J Oral Maxillofac Surg. 35:643–648.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dabare AA, Nouri AM, Cannell H, Moss T,
Nigam AK and Oliver RT: Profile of placental alkaline phosphatase
expression in human malignancies: effect of tumour cell activation
on alkaline phosphatase expression. Urol Int. 63:168–174. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Leng B, Liu XD and Chen QX: Inhibitory
effects of anticancer peptide from Mercenaria on the BGC-823 cells
and several enzymes. FEBS Lett. 579:1187–1190. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pecere T, Gazzola MV, Mucignat C, Parolin
C, Vecchia FD, Cavaggioni A, Basso G, Diaspro A, Salvato B, Carli M
and Palù G: Aloe-emodin is a new type of anticancer agent with
selective activity against neuroectodermal tumors. Cancer Res.
60:2800–2804. 2000.PubMed/NCBI
|