Introduction
Hepatocellular carcinoma (HCC) is a complex disease
initiated by chronic hepatitis B and C infections, exposure to
environmental chemicals or alcohol or metabolic liver diseases.
These interactions lead to a multistep carcinogenic process,
although the molecular and cellular mechanisms that underlie HCC
pathogenesis remain to be elucidated (1). It is widely accepted that tumor cell
growth is an important factor in controlling tumor cell cycle
progression, resulting from genetic anomalies in cancer cells that
affect numerous cell growth regulatory pathways as well as tumor
cell interactions with microenvironmental factors. Hypoxia has been
proposed to participate in the genesis and progression of HCC as
this tumor type is typically accompanied by neovascularization and
hyper-vascularity (2). There is
evidence that hypoxia stimulates HCC cell growth and the expression
of specific target genes such as hexokinase II (3).
Angiogenesis and the production of angiogenic
factors are essential for tumor growth, invasion and metastasis
(4). Tumor angiogenesis has been
associated with an imbalance in the equilibrium between positive
and negative regulators (5) and is
primarily triggered by the release of endothelial cell-specific
growth factors by neoplastic cells that stimulate the growth of
host blood vessels (6). This
imbalance depends on an increased production of one or more
positive regulators of angiogenesis, including vascular endothelial
growth factor (VEGF), which are hypoxia-inducible factor 1
(HIF-1)-dependent genes that are exported from tumor cells,
mobilized from the extracellular matrix or released from host cells
recruited to the tumor site (7).
Tumor angiogenesis is complex and comprises a number or processes
alongside the upregulation of angiogenic activity, and has thus
been regarded as the result of a net balance of positive and
negative regulators (5).
Adrenomedullin (AMD) is an angiogenic peptide that
was originally isolated from extracts of human pheochromocytomas
(8). This peptide has also been
shown to be a mitogen and a hypoxia survival factor of tumor cells
that promotes tumor proliferation and inhibits apoptosis through a
number of signaling pathways (9).
The detection of high levels of AMD expression in various types of
cancer, including prostatic carcinoma (10), cervical cancer (11) and HCC (12), suggests that this peptide is
involved in tumor growth.
To further determine the role of ADM in HCC, the
expression of this protein in HCC cells and normal tissue was
compared in the present study. Intracellular levels of ADM were
assessed in tissue samples from high-grade tumors and compared with
those from low-grade tumors or normal tissue. ADM has been reported
to be involved in vascular invasion and N-cadherin expression due
to hypoxia through a pathway of Akt activation (12). In the present study, the secretion
of the ADM peptide by HCC cells under normal and hypoxic conditions
was investigated. Furthermore, it was investigated whether
decreasing ADM expression using RNA interference would impact the
growth of HCC cells, and the interventional therapeutic efficacy of
a combination of cisplatin and ADM-knockdown on tumor growth was
assessed in vivo.
Materials and methods
Patient population
A total of 64 patients with HCC who underwent
curative liver resection at the First Affiliated Hospital of
Xinxiang Medical University (Xinxiang, China), between April 2010
and July 2012 were enrolled in the present study. The participants
provided their written informed consent prior to participation, and
the study protocol was approved by the Institutional Review Board
of the Cancer Institute of Xinxiang Medical University. The present
clinical investigation was conducted according to the principles
expressed in the Declaration of Helsinki. The mean age of the
patients was 50 years (range, 38–69 years), and 48 of them were
male. Samples of normal liver tissue near the tumors were collected
for use as controls. All the samples were classified by two
experienced pathologists based on histology and cytology using the
Edmondson-Steiner grading system (13). The patients with HCC were divided
into four groups as follows: 18 cases were stage I, 24 cases were
stage II, 13 cases were stage III and nine were stage IV. None of
the patients received any preoperative anticancer treatment. The
HCC and non-tumorous liver tissue samples were excised and
snap-frozen in liquid nitrogen for subsequent RNA and protein
extraction.
Cell culture and reagents
Human HCC HepG2, SMMC-7721 and Bel-7402 cells were
obtained from the American Type Culture Collection (Rockville, MD,
USA). These cell lines were cultured in RPMI-1640 supplemented with
15% fetal bovine serum under standard culture conditions (20%
O2 and 5% CO2 at 37°C). A primary anti-ADM
antibody was obtained from Abcam (Cambridge, MA, USA). The
anti-VEGF and anti-HIF-1α antibodies were purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). An anti-GAPDH antibody
was purchased from Cell Signaling Technology, Inc. (Beverly, MA,
USA). All of the chemicals were purchased from Sigma-Aldrich (St.
Louis, MO, USA).
Induction of hypoxia in HCC cells
HepG2, Bel-7402 and SMMC-7721 cells were seeded in
six-well plates at a density of 2×104 cells/well,
allowing for an exponential growth rate. Each cell line was divided
into four groups: 6 h hypoxia, 12 h hypoxia, 24 h hypoxia and a
normal control group, and each group included three triplicate
wells. Initially, the 24-h hypoxia groups were exposed to hypoxic
conditions (1% O2, 5% CO2 and 94%
N2 at 37°C). After 12 and 18 h of incubation,
respectively, the 12- and 6-h hypoxia groups were exposed to the
same hypoxic conditions. The normal control group was incubated
under normal culture conditions in parallel. After 24 h, the cells
were digested with trypsin, and total RNA and protein were
extracted for further assays.
Generation of an SMMC-7721 cell line
stably expressing ADM-short hairpin RNA (shRNA)
SMMC-7721 cells from the sixth passage were seeded
in six-well plates (1×105 cells/well) and allowed to
adhere for 24 h prior to transfection. The cells were transfected
with plasmids containing shRNA directed against human ADM
(ADM-shRNA; Santa Cruz Biotechnology, Inc.) or a non-targeting
vector-control shRNA (NC-shRNA; Santa Cruz Biotechnology, Inc.). In
addition, the green fluorescence protein control plasmid (Santa
Cruz Biotechnology, Inc.) was used to monitor and optimize
transfection efficiency. At 72 h post-transfection, puromycin (7.0
mg/ml) was added to the culture medium for selection and further
characterization. The transfection efficiency of SMMC-7721 cells
expressing ADM-shRNA was assessed using flow cytometry.
Western blot analysis
Cells or tissue samples were lysed for 20 min on ice
and centrifuged at 14,000 × g for 10 min at 4°C. The supernatant
was collected, and the protein concentration was determined using a
Bradford assay (Bio-Rad, Hercules, CA, USA). Proteins were
separated using 12% SDS-PAGE and transferred to a polyvinylidene
fluoride membrane (Bio-Rad). The membranes were then blocked with
5% skimmed milk for 1 h and incubated overnight with the following
primary antibodies (all diluted 1:1,000): anti-ADM (ab69117;
Abcam), anti-VEGF (sc-152; Santa Cruz Biotechnology, Inc.),
anti-HIF-1α (sc-10790; Santa Cruz Biotechnology, Inc.) and
anti-GAPDH (2118; Cell Signaling Technology, Inc.). The membranes
were then incubated with a secondary antibody for 1.5 h at room
temperature. Membrane-bound antibodies were visualized using a
chemiluminescent substrate (Pierce, Rockford, IL, USA) and exposed
to X-OMAT film (Kodak, Rochester, NY, USA). Immunoreactive protein
bands were quantified by densitometry using QuantityOne software
(Bio-Rad).
RNA extraction and quantitative
polymerase chain reaction (qPCR)
Total RNA was extracted from cells and tissues using
the RNAiso Plus Kit (Takara, Dalian, China). A total of 1 μg total
RNA was used for first-strand DNA synthesis using the PrimeScript
RT kit reagents (Takara), and qPCR was performed using primers
specific for the respective human genes and the SYBR®
Green Premix kit (Takara). For each primer pair, the annealing
temperature was optimized by gradient PCR. The expression of each
target mRNA relative to the GAPDH mRNA was calculated based on Ct
as 2−Δ(ΔCt). The primer sequences used are shown in
Table I.
 | Table IPrimers used for quantitative
polymerase chain reaction. |
Table I
Primers used for quantitative
polymerase chain reaction.
| Gene | Forward primer | Reverse primer |
|---|
| ADM |
5′-acttggcagatcactctcttagca-3′ |
5′-atcagggcgacggaaacc-3′ |
| VEGF |
5′-gcacccatggcagaagg-3′ |
5′-ggggtacccctcaccgcctcggcttgtc-3′ |
| HIF-1α |
5′-gaaagcgcaagtcctcaaag-3′ |
5′-tgggtaggagatggagatgc-3′ |
| GAPDH |
5′-caaattccatggcaccgtc-3′ |
5′-cccatctgattttggaggga-3′ |
Flow cytometry
Apoptosis in SMMC-7721 cells was assessed using
annexin V/propidium iodide (PI) staining. Following 72 h of
incubation, SMMC-7721 cells stably expressing ADM-shRNA were
stained with annexin V-fluorescein isothiocyanate and PI using an
Apoptosis Detection kit (Sigma-Aldrich) according to the
manufacturer’s instructions. SMMC-7721 cells transfected with
non-specific vector-shRNA were used as a vehicle control. Apoptotic
cells were quantified using a FACSCalibur™ flow cytometer (BD
Biosciences, Franklin Lakes, NJ, USA).
Animal studies
All animal experiments in the present study were
performed in accordance with a protocol approved by the
Institutional Animal Care and Use Committee. A total of 48
six-week-old male BALB/c mice were randomly divided into six groups
[cisplatin plus ADM-shRNA, cisplatin plus vector, cisplatin plus
phosphate-buffered saline (PBS), saline plus ADM-shRNA, saline plus
vector and saline plus PBS; n=8 for each group] to receive
subcutaneous injections of 0.1 ml SMMC-7721 tumor cells into the
right flank. The first three groups received intratumoral
administrations of 10 mg/kg body weight of either ADM-shRNA,
NC-shRNA or an equal volume of PBS following intraperitoneal
injections of cisplatin (5 mg/kg body weight). The remaining three
groups were treated with saline rather than cisplatin as controls.
Either shRNA, cisplatin, saline or PBS was injected every three
days for 28 days. The mean tumor volumes of the primary tumors were
measured in three dimensions (a, b and c)
every three days with a caliper and calculated according to the
following formula: a × b × c ×0.52. At the end
of the experiment, the mice were sacrificed, tumors were harvested
and tumor protein levels were assessed using western blot
analysis.
Statistical analysis
All of the in vitro experimental data
represent at least three independent experiments and are expressed
as the mean ± standard error of the mean. Significant differences
were evaluated using a one-way analysis of variance (ANOVA)
followed by Bonferroni multiple comparisons tests using the
commercially available SPSS software package (SPSS, Inc., Chicago,
IL, USA). The degrees of freedoms of the ‘Treatment’ and
‘Residue’ for the F-value were also reported. Differences
were considered to be statistically significant when the P-value
was <0.05.
Results
ADM expression in human HCC tissue
samples
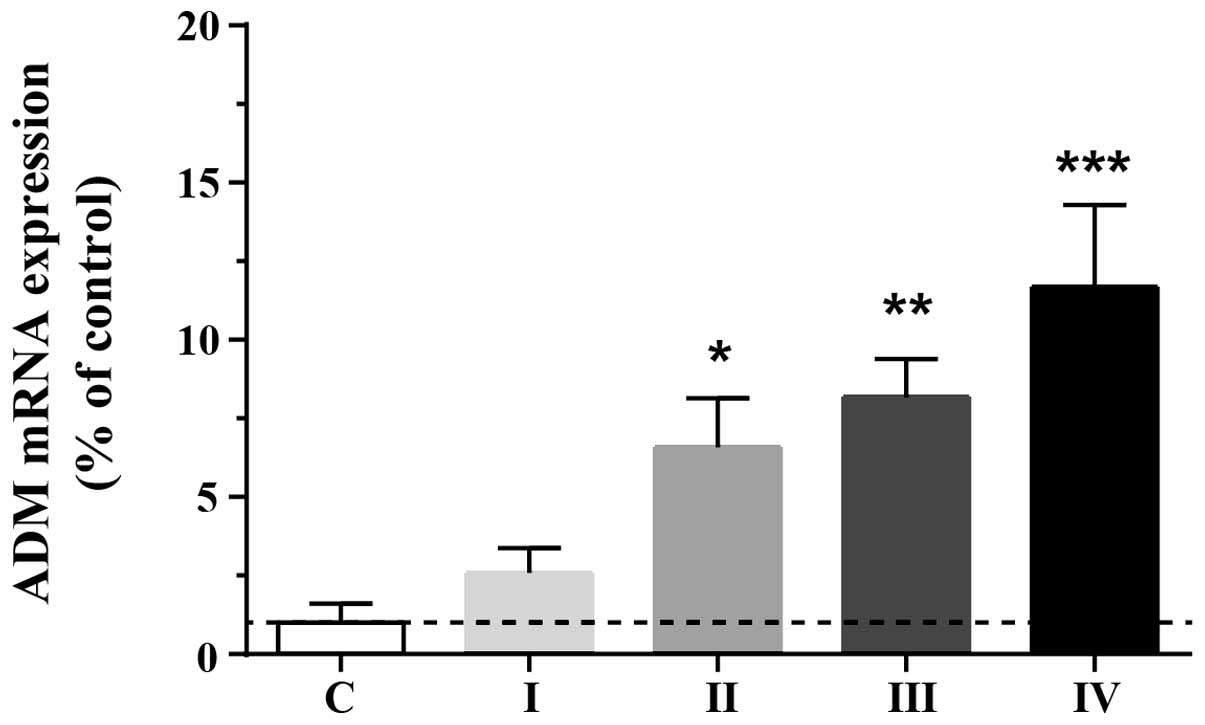
To investigate the adaptive upregulation of ADM in
HCC cells, endogenous expression levels of ADM were assessed in
normal and tumorous (stage I, II, III and IV) tissues. ADM protein
levels were markedly elevated in tumorous tissue compared with
normal liver tissue (F4,77=6.85; P<0.01). ADM levels
in stage II, III and IV cancer tissue were significantly higher
than those in the normal controls (Fig. 1), suggesting that the expression of
ADM is upregulated in human HCC tissue and that this may promote
tumor growth.
Hypoxia-induced ADM, VEGF and HIF-1α
expression in HCC cells
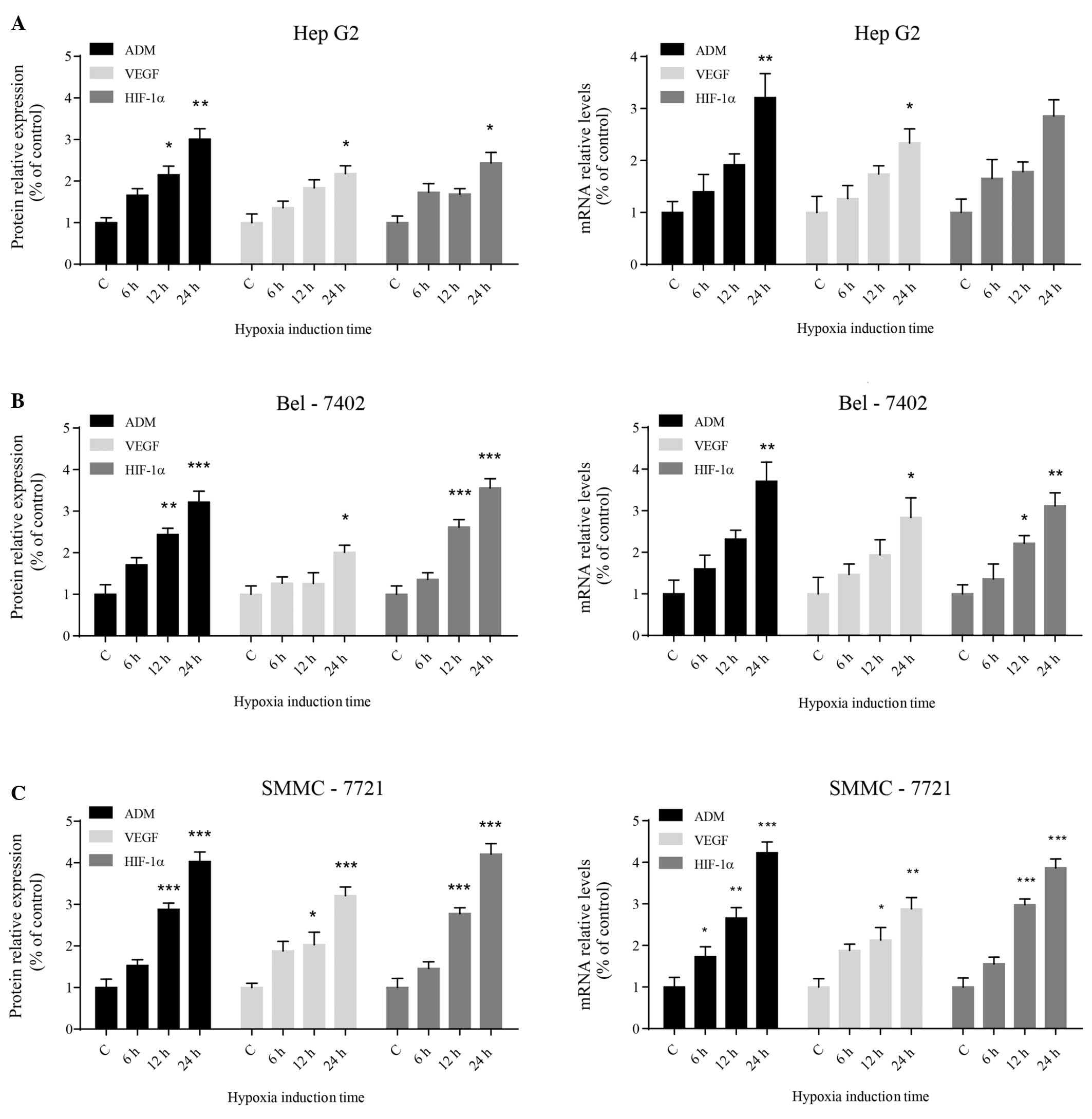
To investigate whether hypoxia activates ADM
expression in HCC cells, the expression of ADM and other cancer
promoting genes, such as VEGF and HIF-1α, was assessed following
exposure to hypoxic conditions. Western blot analysis showed that
the protein expression levels of ADM, VEGF and HIF-1α increased
significantly in the three different HCC cell lines with increasing
time under hypoxic conditions (Fig.
2A). The levels of ADM, VEGF and HIF-1α transcripts correlated
with the protein expression levels (Fig. 2B). Among the three types of human
HCC cell lines that were tested, SMMC-7721 cells appeared to be the
most sensitive to hypoxic stress. The statistical results of these
data are summarized in Table
II.
 | Table IIHypoxia-induced ADM, VEGF and HIF-1
expression in hepatocellular carcinoma cells. |
Table II
Hypoxia-induced ADM, VEGF and HIF-1
expression in hepatocellular carcinoma cells.
| Protein | mRNA |
|---|
|
|
|
|---|
| Gene | F-value (3,8) | P-value | F-value (3,8) | P-value |
|---|
| HepG2 |
| ADM | 19.56 | 0.0005a | 9.054 | 0.0060a |
| VEGF | 14.09 | 0.0015a | 5.370 | 0.0256b |
| HIF-1α | 9.280 | 0.0055a | 7.288 | 0.0112b |
| Bel-7402 |
| ADM | 21.19 | 0.0004a | 11.51 | 0.0028a |
| VEGF | 4.761 | 0.0345b | 4.304 | 0.0439b |
| HIF-1α | 37.84 | <0.0001c | 11.70 | 0.0027a |
| SMMC-7721 |
| ADM | 55.29 | <0.0001c | 26.01 | 0.0002a |
| VEGF | 16.76 | 0.0008a | 10.66 | 0.0036a |
| HIF-1α | 53.46 | <0.0001c | 49.90 | <0.0001c |
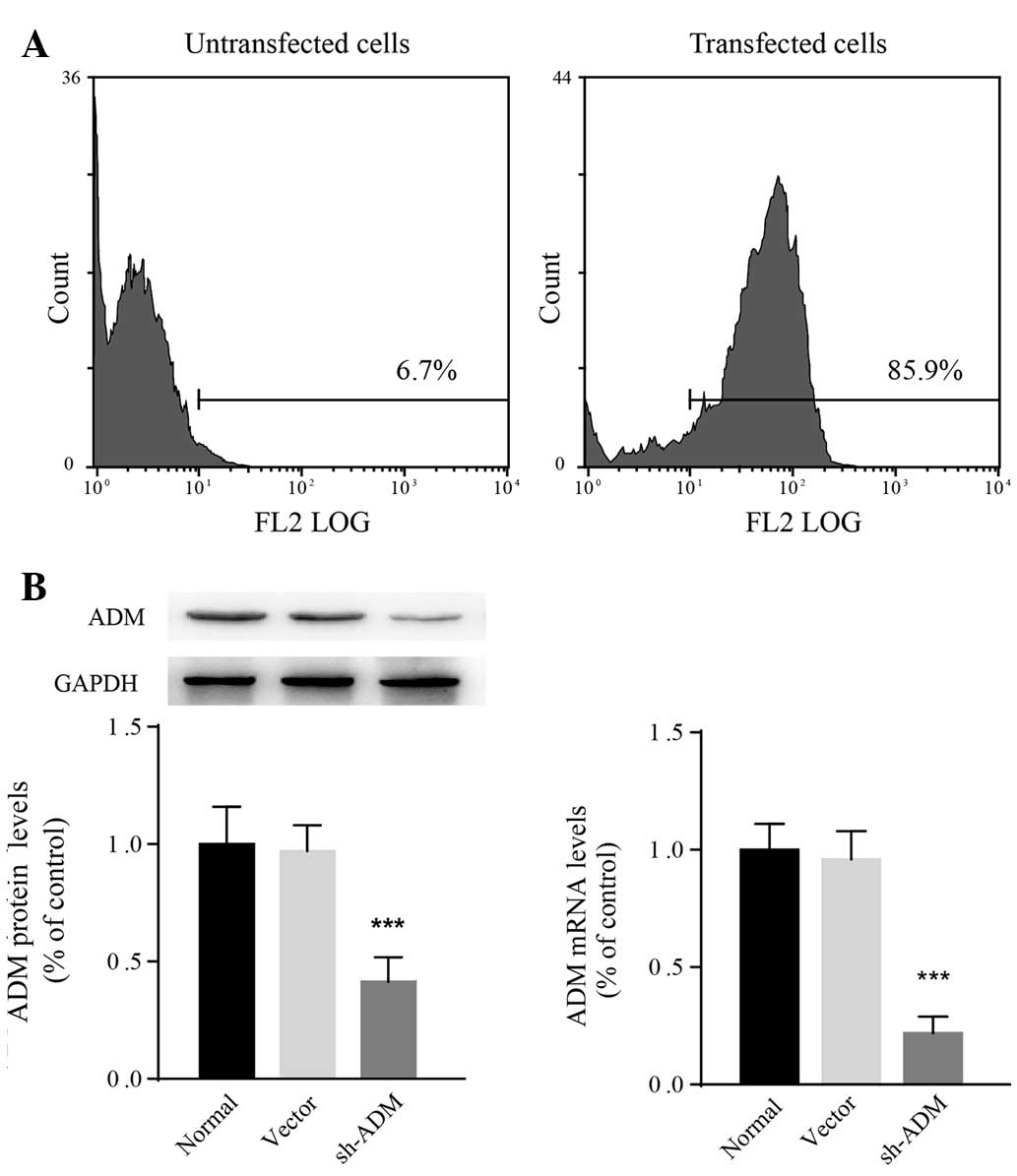
Generation of SMMC-7721 cells that stably
express ADM-shRNA
Accumulating evidence suggests that HCC development
is closely associated with constitutive upregulation of ADM
(12,14,15).
As SMMC-7721 cells are sensitive to hypoxic stress, a lentiviral
ADM-shRNA was transfected into SMMC-7721 cells. The transfection
efficiency following puromycin selection was determined to be 85.9%
using flow cytometry (Fig. 3A).
According to qPCR and western blot analysis, both ADM mRNA
(F2,15=12.94; P=0.0005) and protein
(F2,15=18.43; P<0.0001) levels in SMMC-7721 cells
decreased significantly following transfection with ADM-shRNA
(Fig. 3B). The relative ADM mRNA
expression in cells transfected with ADM-shRNA was 0.23±0.08-fold
lower than that in normal SMMC-7721 cells (P<0.0001). The
expression of ADM was not observed to be different between
vector-control-transfected and untransfected cells. These results
revealed that the ADM-shRNA used in the present study successfully
knocked down ADM in SMMC-7721 cells.
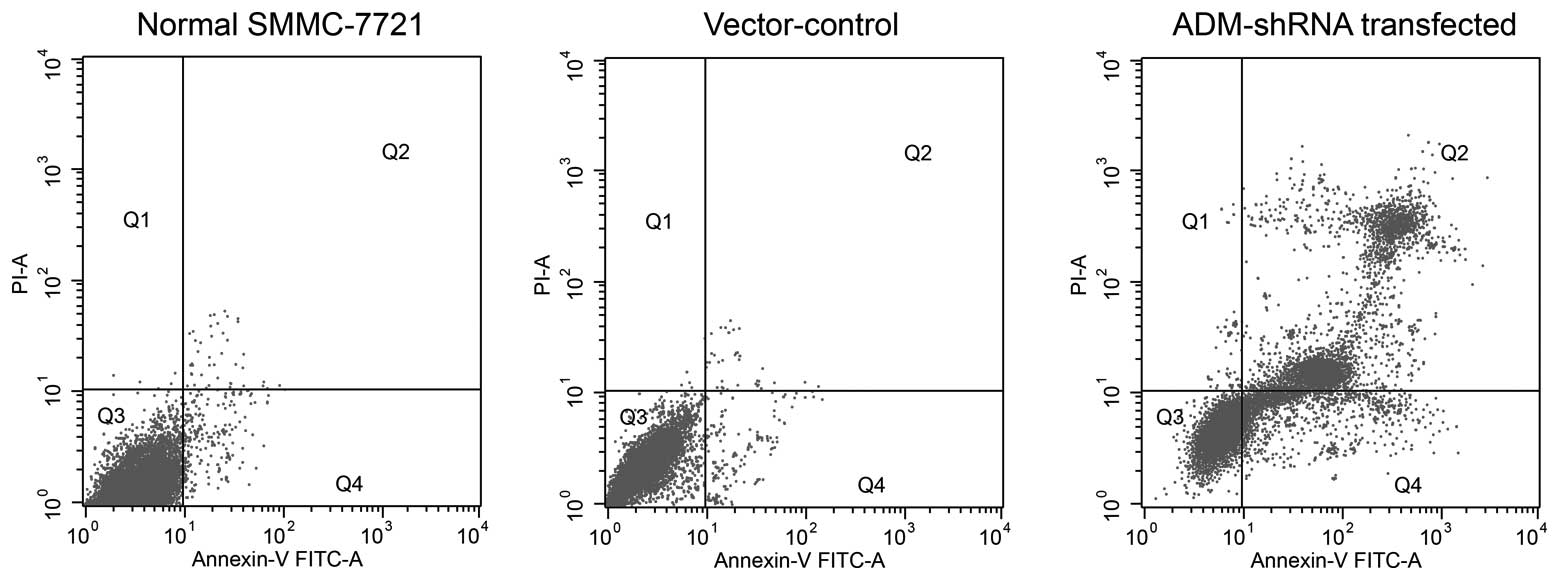
ADM-knockdown promotes apoptosis in
SMMC-7721 cells
After 72 h of cell growth, significantly increased
levels of cell death were observed in SMMC-7721 cells stably
expressing ADM-shRNA (mean, 37.34±0.71% apoptotic cells) as
compared with the untransfected cells (mean, 5.44±0.31% apoptotic
cells) (F2,14=12.13; P<0.0035; post-hoc, P=0.0021)
(Fig. 4). The apoptosis observed
in the vector-control-transfected group (mean, 9.66±0.40% apoptotic
cells) was slightly increased compared with that observed in the
untransfected SMMC-7721 cells; however, this difference failed to
reach statistical significance (P=0.0642).
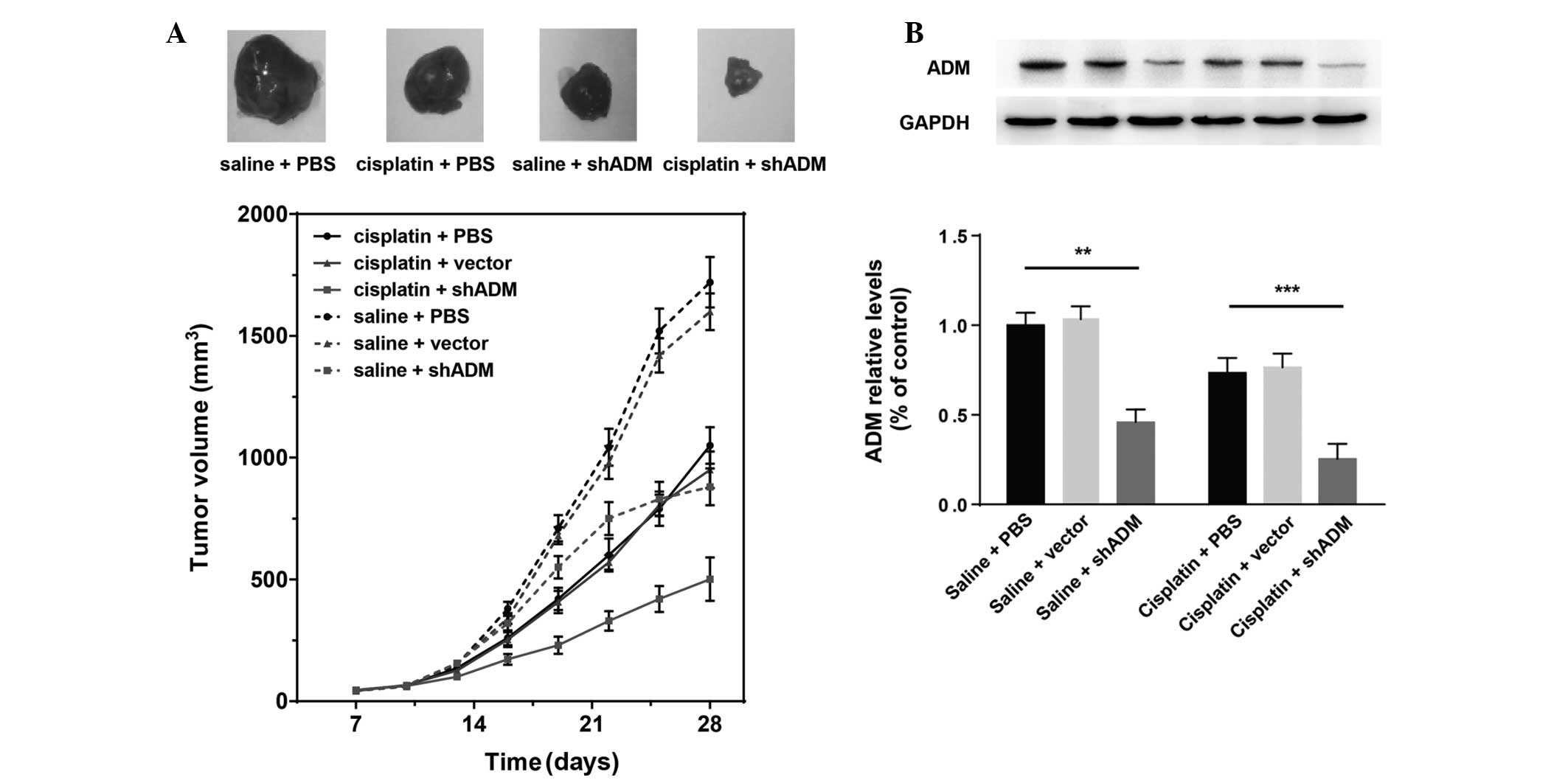
Therapeutic efficacy of the combination
of cisplatin and ADM-knockdown on tumor growth in vivo
To elucidate whether ADM-shRNA and cisplatin are
able to act synergistically in vivo, SMMC-7721 cells were
transplanted into mice to generate subcutaneous tumors. In
conjunction, a subset of the mice was treated with cisplatin. Tumor
growth was significantly inhibited in mice injected with cisplatin
(Fig. 5A). When cisplatin
treatment was combined with ADM-shRNA delivery, tumor growth
inhibition was significantly higher than that following treatment
with cisplatin alone (71.39 versus 39.21%; Bonferroni post-hoc,
P<0.05). Tumor inhibition rates are shown in Table III. Injection of the ADM-shRNA
plasmid alone also suppressed tumor growth (49.45%). The tumor
volume did not differ significantly between controls treated with
vector-shRNA and control mice injected with PBS. Analysis of the
proteins expressed in the tumors showed a marked decrease in ADM
(Fig. 5B). Two-way ANOVA revealed
that cisplatin (F1,30=8.37; P<0.01) and ADM-shRNA
(F2,30=27.59; P<0.0001) exerted significant effects
on tumor ADM expression, with a significant interaction between
these two factors (F2,30=0.66; P<0.05) (Fig. 5B). Consistent with the results
regarding tumor growth, injection of ADM-shRNA plasmid
significantly suppressed ADM expression in tumor tissue (saline
plus shADM versus saline plus PBS, Bonferroni post-hoc, P<0.01).
Cisplatin combined with ADM-shRNA significantly inhibited tumor ADM
expression, as compared with treatment with cisplatin alone
(cisplatin plus shADM versus cisplatin plus PBS, Bonferroni
post-hoc, P<0.0001). These findings support the hypothesis that
the combination of cisplatin and ADM-shRNA can significantly
suppress liver cancer tumor growth in vivo.
 | Table IIIRate of tumor inhibition 28 days
after SMMC-7721 cell implantation. |
Table III
Rate of tumor inhibition 28 days
after SMMC-7721 cell implantation.
| Group (n=8) | Tumor volume
(mm3) | Inhibition rate
(%) |
|---|
| Saline |
| PBS | 1720±38.33 | - |
| Vector | 1599±36.98 | 9.20 |
| ADM-shRNA | 884±11.15 | 49.45 |
| Cisplatin |
| PBS | 1050±75.30 | 39.21 |
| Vector | 955±25.37 | 45.70 |
| ADM-shRNA | 501±16.60 | 71.39a |
Discussion
The present study demonstrated that the expression
of ADM in human HCC tissue was associated with the degree of
malignancy and clinical prognosis. ADM levels were elevated under
hypoxic conditions. SMMC-7721 cells showed an increased rate of
cell death following inhibition of the expression of ADM using RNA
interference. Furthermore, the inhibitory effect of cisplatin on
tumor growth in vivo was significantly enhanced by combined
treatment with ADM-shRNA. These results suggest that ADM may be a
novel diagnostic and therapeutic target in HCC.
It was found in the present study that ADM mRNA
expression in human HCC tissue is higher than that in adjacent
normal liver tissue. This may result from hypoxia in situ,
which has been shown to increase ADM mRNA expression in a variety
of tumor cell types, including lung, breast, ovarian, colon and
prostate cancer (16).
Furthermore, it has been observed that hypoxia increases expression
of ADM mRNA and secreted protein levels in a variety of tissues
(17). These increases in ADM are
at least partially mediated by the activation of HIF-1 and VEGF
(18,19). The increased expression of ADM mRNA
may result in increased angiogenic activity in tumor tissue
(20). There is increasing
evidence suggesting an important role for angiogenesis in HCC
invasion and metastasis (21).
Hypoxia is a key microenvironmental regulatory
factor in tumor growth that influences the progression of HCC by
enhancing proliferation, angiogenesis and metastasis as well as
chemo- and radio-resistance (15,22).
Evidence suggests that hypoxia affects the development and
evolution of the tumor microenvironment by regulating the
differentiation of tumor and stromal cells; thus, hypoxia plays a
direct role in the maintenance of cancer stem cells and inhibition
of differentiation of mesenchymal stem cells (23). Cancer cells adapt to hypoxic
environments via the transcriptional activation of HIF-1 and VEGF,
which activate the expression of genes involved in the glycolytic
system, angiogenesis and cell survival (24). Consistent with previous results
from Park et al (12), data
in the present study showed that hypoxic conditions induced HIF-1,
VEGF and ADM expression in three types of human HCC cell lines and
that SMMC-7721 cells were the most sensitive to hypoxia. In human
cancer cells, hypoxia-induced ADM signaling is enhanced
predominantly via HIF-1, which is an important transcription factor
produced in response to hypoxia. This transcription factor
stimulates the synthesis of ADM and VEGF via hypoxic response
elements located on their respective genes (9,25).
The increased ADM signaling has been demonstrated to enhance
vascular smooth muscle cell maturation and thereby to promote tumor
vessel growth (26). The growth of
the tumor blood supply is essential in enabling tumor progression.
The production and secretion of substances that affect vasodilation
and angiogenesis may be necessary for tumor survival. Angiogenesis
is closely associated with tumor prognosis, and, in clinical
practice, cases with a high density of blood vessels have poor
prognoses. Furthermore, ADM levels have been found to be correlated
with the expression of N-cadherin, which is an adhesion molecule
that is involved in cell migration, invasion and metastasis of
cancer cells (27). High levels of
N-cadherin expression predict low recurrence-free and overall
survival rates in patients with HCC (28), suggesting that the abnormal
activation of ADM signaling in HCC tissue may also predict an
unfavorable prognosis.
Evidence suggests that ADM exerts a wide range of
effects on cell growth and apoptosis. These effects have been shown
to be dependent on the cell type and experimental conditions
(29). In tumor cells, ADM was
found to inhibit apoptosis by upregulating B-cell lymphoma 2 and to
increase cell growth and survival by activating oncogenic proteins,
including Ras, Raf and protein kinase C (9). In HCC cells, signaling pathways
independent of cyclic adenosine monophosphate and mitogen-activated
protein kinases, including phosphinositide 3 kinase/Akt signaling,
may have key roles in the regulation of cell proliferation and
apoptosis induced by ADM (12).
Given that ADM signaling was shown to accelerate HCC cell growth,
an shRNA against ADM was used in the present study to investigate
whether the inhibition of ADM expression in HCC cells suppresses
hypoxia-induced tumor growth. Knockdown of ADM significantly
inhibited cell proliferation and increased apoptosis under hypoxic
stress conditions in SMMC-7721 cells. A previous study demonstrated
that exogenous ADM antagonists or RNA interference specific for ADM
expression effectively reduced ovarian cancer cell migration and
osteosarcoma cell proliferation (30). Owing to the strong effect of ADM on
angiogenesis and tumor growth in multiple tumor types, shRNA
interference targeting ADM may be a powerful tool against oncogene
expression and may be used therapeutically against several types of
human cancer. In the present study, SMMC-7721 cells were
transplanted into mice to generate subcutaneous tumors, and it was
found that ADM-shRNA and cisplatin can act synergistically in
vivo. These results further suggest the effectiveness of ADM
shRNA in reducing HCC tumor growth.
In conclusion, the present study demonstrated that
ADM is overexpressed in human HCC tissue compared with the tissue
adjacent to the tumors. HCC cell proliferation was inhibited and
HCC cell apoptosis was promoted by knocking down ADM using RNA
interference. Treating tumor cells with ADM-shRNA combined with
cisplatin inhibited HCC tumor growth to a greater extent than
treatment with cisplatin alone. Therefore, the selective
interruption of ADM signaling by shRNA may be an effective
interventional therapeutic strategy in HCC.
References
|
1
|
Moradpour D and Blum HE: Pathogenesis of
hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 17:477–483.
2005. View Article : Google Scholar
|
|
2
|
Tanaka S and Arii S: Current status and
perspective of antiangiogenic therapy for cancer: hepatocellular
carcinoma. Int J Clin Oncol. 11:82–89. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gwak GY, Yoon JH, Kim KM, Lee HS, Chung JW
and Gores GJ: Hypoxia stimulates proliferation of human hepatoma
cells through the induction of hexokinase II expression. J Hepatol.
42:358–364. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liotta LA, Steeg PS and Stetler-Stevenson
WG: Cancer metastasis and angiogenesis: an imbalance of positive
and negative regulation. Cell. 64:327–336. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hanahan D and Folkman J: Patterns and
emerging mechanisms of the angiogenic switch during tumorigenesis.
Cell. 86:353–364. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Carmeliet P and Jain RK: Angiogenesis in
cancer and other diseases. Nature. 407:249–257. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dvorak HF: Vascular permeability
factor/vascular endothelial growth factor: a critical cytokine in
tumor angiogenesis and a potential target for diagnosis and
therapy. J Clin Oncol. 20:4368–4380. 2002. View Article : Google Scholar
|
|
8
|
Kitamura K, Kangawa K, Kawamoto M, et al:
Adrenomedullin: a novel hypotensive peptide isolated from human
pheochromocytoma. Biochem Biophys Res Commun. 192:553–560. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nikitenko LL, Fox SB, Kehoe S, Rees MC and
Bicknell R: Adrenomedullin and tumour angiogenesis. Br J Cancer.
94:1–7. 2006. View Article : Google Scholar
|
|
10
|
Jiménez N, Abasolo I, Jongsma J, et al:
Androgen-independent expression of adrenomedullin and
peptidylglycine alpha-amidating monooxygenase in human prostatic
carcinoma. Mol Carcinog. 38:14–24. 2003.PubMed/NCBI
|
|
11
|
Li Z, Takeuchi S, Otani T and Maruo T:
Implications of adrenomedullin expression in the invasion of
squamous cell carcinoma of the uterine cervix. Int J Clin Oncol.
6:263–270. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Park SC, Yoon JH, Lee JH, et al:
Hypoxia-inducible adrenomedullin accelerates hepatocellular
carcinoma cell growth. Cancer Lett. 271:314–322. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Edmondson HA and Steiner PE: Primary
carcinoma of the liver: a study of 100 cases among 48,900
necropsies. Cancer. 7:462–503. 1954. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Deville JL, Salas S, Figarella-Branger D,
et al: Adrenomedullin as a therapeutic target in angiogenesis.
Expert Opin Ther Targets. 14:1059–1072. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu SD, Ma YS, Fang Y, Liu LL, Fu D and
Shen XZ: Role of the microenvironment in hepatocellular carcinoma
development and progression. Cancer Treat Rev. 38:218–225. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zudaire E, Martínez A and Cuttitta F:
Adrenomedullin and cancer. Regul Pept. 112:175–183. 2003.
View Article : Google Scholar
|
|
17
|
Oehler MK, Norbury C, Hague S, Rees MC and
Bicknell R: Adrenomedullin inhibits hypoxic cell death by
upregulation of Bcl-2 in endometrial cancer cells: a possible
promotion mechanism for tumour growth. Oncogene. 20:2937–2945.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Oladipupo S, Hu S, Kovalski J, et al: VEGF
is essential for hypoxia-inducible factor-mediated
neovascularization but dispensable for endothelial sprouting. Proc
Natl Acad Sci USA. 108:13264–13269. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Frede S, Freitag P, Otto T, Heilmaier C
and Fandrey J: The proinflammatory cytokine interleukin 1beta and
hypoxia cooperatively induce the expression of adrenomedullin in
ovarian carcinoma cells through hypoxia inducible factor 1
activation. Cancer Res. 65:4690–4697. 2005. View Article : Google Scholar
|
|
20
|
Ribatti D, Nico B, Spinazzi R, Vacca A and
Nussdorfer GG: The role of adrenomedullin in angiogenesis.
Peptides. 26:1670–1675. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jung KH, Zheng HM, Jeong Y, et al:
Suppression of tumor proliferation and angiogenesis of
hepatocellular carcinoma by HS-104, a novel phosphoinositide
3-kinase inhibitor. Cancer Lett. 328:176–187. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Harris AL: Hypoxia - a key regulatory
factor in tumour growth. Nat Rev Cancer. 2:38–47. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim Y, Lin Q, Glazer PM and Yun Z: Hypoxic
tumor microenvironment and cancer cell differentiation. Curr Mol
Med. 9:425–434. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tang N, Wang L, Esko J, et al: Loss of
HIF-1alpha in endothelial cells disrupts a hypoxia-driven VEGF
autocrine loop necessary for tumorigenesis. Cancer Cell. 6:485–495.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Carmeliet P, Dor Y, Herbert JM, et al:
Role of HIF-1alpha in hypoxia-mediated apoptosis, cell
proliferation and tumour angiogenesis. Nature. 394:485–490. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Iwase T, Nagaya N, Fujii T, et al:
Adrenomedullin enhances angiogenic potency of bone marrow
transplantation in a rat model of hindlimb ischemia. Circulation.
111:356–362. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hazan RB, Phillips GR, Qiao RF, Norton L
and Aaronson SA: Exogenous expression of N-cadherin in breast
cancer cells induces cell migration, invasion, and metastasis. J
Cell Biol. 148:779–790. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gwak GY, Yoon JH, Yu SJ, et al:
Anti-apoptotic N-cadherin signaling and its prognostic implication
in human hepatocellular carcinomas. Oncol Rep. 15:1117–1123.
2006.PubMed/NCBI
|
|
29
|
Shichiri M and Hirata Y: Regulation of
cell growth and apoptosis by adrenomedullin. Hypertens Res.
26(Suppl): S9–S14. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pang X, Shang H, Deng B, Wen F and Zhang
Y: The interaction of adrenomedullin and macrophages induces
ovarian cancer cell migration via activation of RhoA signaling
pathway. Int J Mol Sci. 14:2774–2787. 2013. View Article : Google Scholar : PubMed/NCBI
|