Introduction
Laparoscopic surgery provides multiple clinical
benefits compared with open surgery, including decreased
postoperative pain, shorter hospital stays, a more rapid return to
preoperative activity, decreased postoperative ileus, preserved
immune function and improved cosmetic results (1,2).
Furthermore, fewer inflammatory mediators are released following
laparoscopic surgery than following conventional open surgery
(3,4). Abdominal laparoscopic surgery
requires the intentional establishment of a pneumoperitoneum in
order to provide adequate surgical exposure and operative freedom.
Carbon dioxide (CO2) is commonly used to induce the
pneumoperitoneum as it is a colorless, stable gas that is, buffered
in the blood, eliminated by the lung, is nonflammable and poses a
low risk of venous gas embolism (5). CO2-pneumoperitoneum has
been demonstrated to inhibit inflammatory cytokine release and
neutrophil accumulation (6–8).
However, the precise mechanisms underlying this effect are not
fully understood.
Ablation of sensory neuronal fibers has been
demonstrated to markedly increase inflammation severity suggesting
that sensory neurons are important in maintaining tissue integrity
by attenuating inflammatory responses (9). Capsaicin-sensitive sensory neurons
are nociceptive neurons located around vessels and within the
lining epithelia of numerous tissues, and are associated with
nonvascular smooth muscle (10).
Following stimulation of transient receptor potential vanilloid 1
(TRPV1) by a wide variety of noxious physical and chemical stimuli,
including heat (>42°C), low pH, certain lipids and exogenous
vanilloid derivatives, these sensory neurons release calcitonin
gene-related peptide (CGRP) which is involved in the aggravation of
inflammation such as tissue hyperemia and edema (9–11).
Our previous study demonstrated that stimulation of
sensory neurons inhibits hepatic apoptosis and inflammatory
responses in rats subjected to hepatic ischemia/reperfusion (I/R)
(12). I/R-induced apoptosis
increases neutrophil accumulation in damaged tissues (13) and activated neutrophils release
various inflammatory mediators that are capable of damaging
endothelial cells (14,15). These observations suggest that
excessive I/R-induced apoptosis permits a large number of
neutrophils to accumulate at sites of damaged tissue and contribute
to tissue injury by releasing inflammatory mediators that further
damage endothelial and parenchymal cells. Consistent with this
theory, our previous study demonstrated that reducing apoptosis in
animal models of hepatic I/R or water-immersion restraint stress
inhibited neutrophil accumulation-reduced tissue injury (12).
Acidic environments (low pH) are known to stimulate
sensory neurons through activation of TRPV1 (16). Abdominal insufflation with
CO2 causes a marked and rapid reduction in local tissue
pH (17). In addition,
CO2 has been demonstrated to stimulate sensory neurons
through intracellular acidification by carbonic anhydrase (18,19).
These observations suggest that abdominal insufflation with
CO2 may stimulate sensory neurons, thus inhibiting
apoptosis and subsequent neutrophil accumulation.
Based on these observations, it was hypothesized
that CO2-pneumoperitoneum may prevent I/R-induced
inflammatory responses and hepatic apoptosis through stimulation of
sensory neurons. In the present study, a rat model of I/R-induced
liver injury was used to examine this hypothesis and to investigate
the mechanism(s) by which laparoscopic surgery induces milder
inflammatory responses than open surgery.
Materials and methods
Animals and reagents
Pathogen-free male Wistar rats, weighing 200–250 g,
were obtained from Nihon SLC (Hamamatsu, Japan). Care and handling
of the animals were conducted in accordance with the National
Institutes of Health guidelines (Bethesda, MA, USA). All
experimental procedures were approved by the Nagoya City University
Animal Care Center (Nagoya, Aichi, Japan). SB366791, a specific
TRPV1 antagonist (20), was
purchased from Sigma-Aldrich (St. Louis, MO, USA). All reagents
were of analytic grade.
Experimental model
The rats were randomly divided into the following
five groups: sham-surgery, hepatic I/R, hepatic I/R with
CO2-pneumoperitoneum pretreatment, hepatic I/R with
air-pneumoperitoneum pretreatment, hepatic I/R with
CO2-pneumoperitoneum and SB366791 pretreatment. All rats
were deprived of food, but not of water, for 24 h prior to each
experiment. The liver was exposed by a midline laparotomy after the
induction of anesthesia. The ligation by silk was placed around the
right and left branches of the portal vein, the hepatic artery and
bile duct. The induction of ischemia of the median and left lobes
of the liver was performed completely by clamping the left branches
of the portal vein and hepatic artery for 60 min. The right lobe of
the liver was perfused to prevent the congestion of the intestine.
The abdomen was covered with plastic wrap to prevent desiccation
during the period of hepatic ischemia. During the period of hepatic
ischemia, the abdomen was covered with plastic wrap to prevent
desiccation. At the end of the period of ischemia, the ligatures
around the left branches of the portal vein and hepatic artery were
removed and the right branches of the portal vein, the hepatic
artery and the bile duct were ligated to prevent a shunt-like
effect to the right lobe following reperfusion (21,22).
This procedure directed all subsequent portal and hepatic blood
flow, with the exception of a small amount of flow to the caudal
hepatic lobe, through the previously ischemic liver lobes. The
wound was closed with 3-0 silk. Sham-surgery animals were similarly
handled, however, no ligature was placed to obstruct the blood flow
to the left and median hepatic lobes. Blood flow to the right lobe
of the liver was occluded in sham-surgery animals as in animals
subjected to hepatic I/R. The rats assigned to the pneumoperitoneum
group underwent either CO2 or air insufflation of their
abdomen using a single 23-gauge needle for 30 min prior to hepatic
ischemia. SB366791 was dissolved in normal saline with 1% dimethyl
sulfoxide and 500 μg/kg was intraperitoneally administered 60 min
prior to ischemia as described previously (23). The median lobe of the liver was
removed following the indicated period of reperfusion for
histological analysis as described below.
Histology and immunohistochemistry
Following 6 h of hepatic I/R, the rats were perfused
with 4% paraformaldehyde in 0.1 M phosphate-buffered saline. The
median lobe of the liver was removed and embedded in paraffin.
Paraffin-embedded samples were sectioned every 3 μm and then
deparaffinized. Apoptosis was assessed with hematoxylin and eosin
(H&E) and the terminal deoxynucleotidyl transferase dUTP nick
end-labeling (TUNEL) staining methods. In H&E stained sections,
cells with morphological features of apoptosis, including cell
shrinkage, retraction of cell borders and chromatin condensation
and margination were counted (24,25).
TUNEL staining was performed with the MEBSTAIN Apoptosis kit direct
(MBL Co., Nagoya, Japan) as described previously (26,27).
The number of apoptotic cells within each of five randomly selected
high-power microscopy fields (HPF) at ×400 magnification (BZ9000;
Keyence, Osaka, Japan) was determined and the data are expressed as
the average number of TUNEL-positive cells per HPF.
Immunofluorescence staining of endothelial
monocyte-activated polypeptide-II (EMAP-II) was performed by
pretreating the sections with proteinase K (1:40 dilution) and then
blocking with Tris/NaCl/blocking reagent buffer (TNB; Perkin Elmer
Life Sciences, Boston, MA, USA) for 1 h at room temperature. The
sections were then incubated with the primary antibody (monoclonal
mouse anti-EMAP-II; Abcam, Cambridge, UK) diluted 1:100 in TNB
overnight at 4°C, and then washed and incubated with Alexa
Fluorophore 568 nm polyclonal donkey anti-mouse antibody
(Invitrogen, Mount Waverley, Australia) at 1:500 dilution in TNB
for 1 h at room temperature. The sections were counterstained with
4′-6-diamidino-2-phenylindole (Sigma-Aldrich). In the control
experiments, primary antibodies were omitted to verify the absence
of non-specific binding of secondary antibodies. Staining intensity
was semi-quantitatively scored as 0 (negative), 1 (weak), 2
(moderate) and 3 (strong), and the proportion of cells staining
positively at any intensity was scored as 0 (<5% of cells), 1
(5–30% of cells), 2 (31–60% of cells) and 3 (>60% of cells), as
described previously (28,29). The intensity and proportion scores
were added for each sample to give the immunohistochemistry scores,
which ranged between 0 and 6.
Statistical analysis
Data are expressed as the mean ± standard deviation.
The results were compared by analysis of variance followed by
Bonferroni’s multiple comparison test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effects of CO2- and
air-pneumoperitoneum on hepatic apoptosis in rats subjected to
hepatic I/R
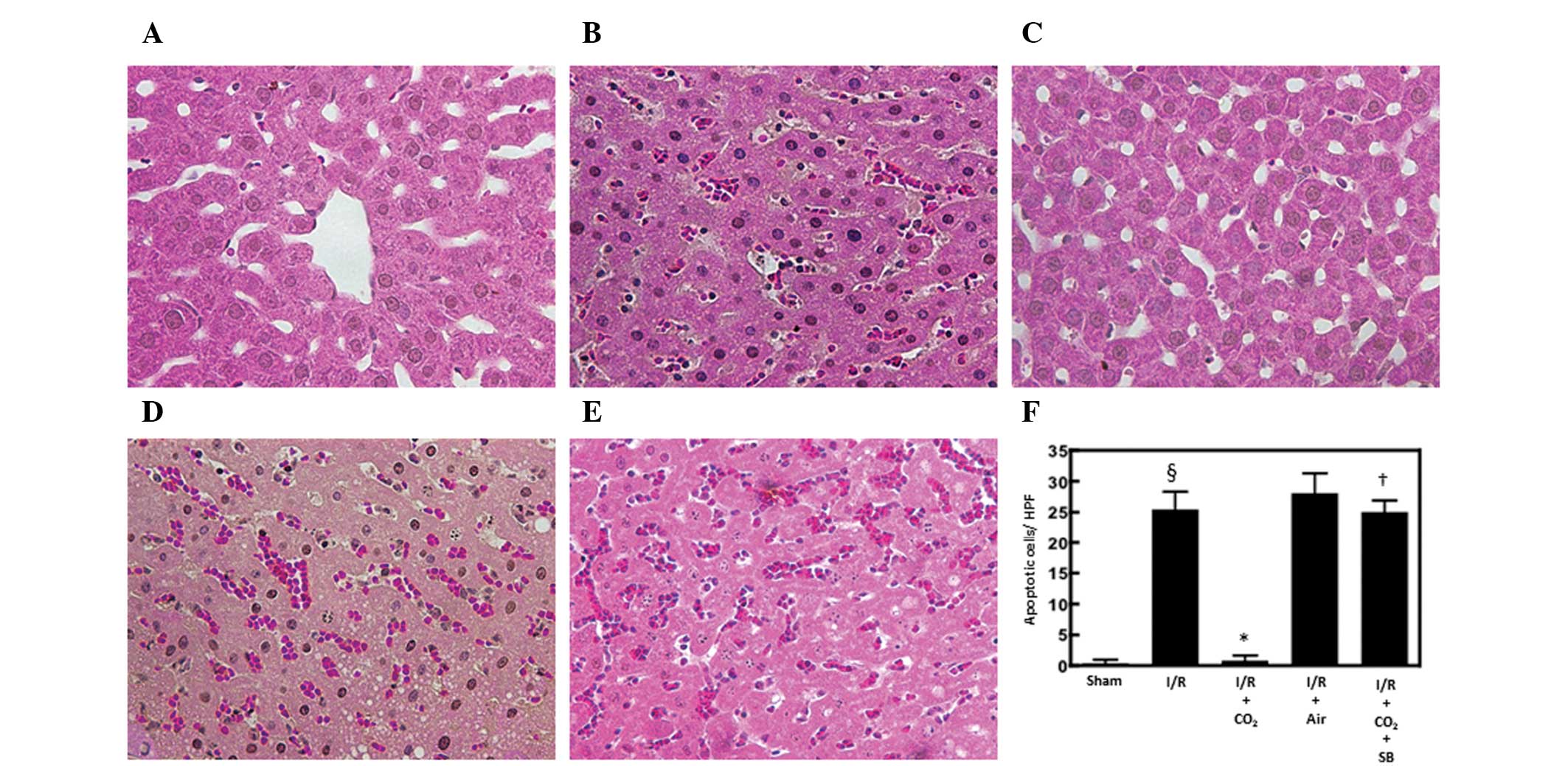
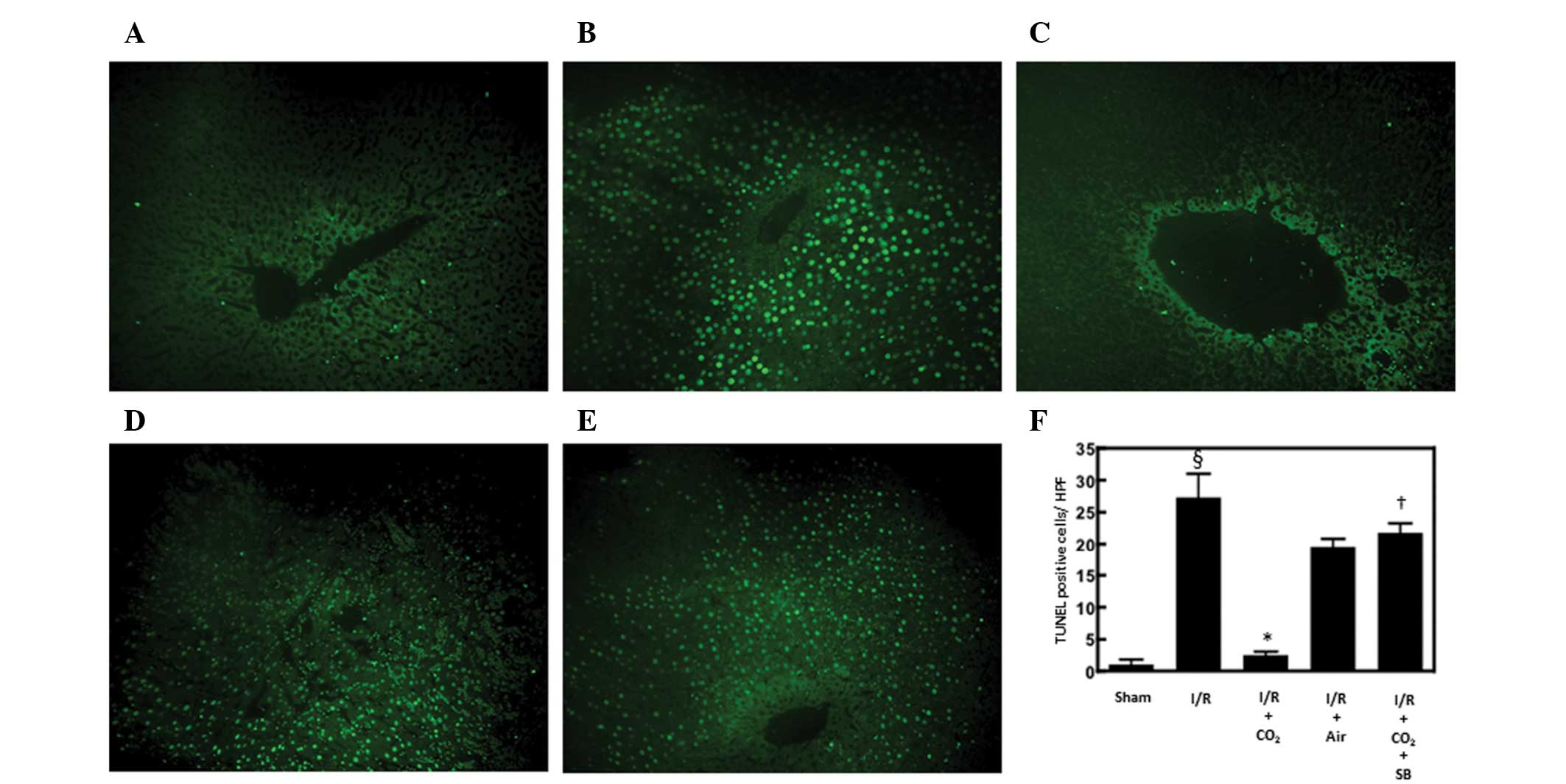
Hepatic apoptosis has been demonstrated to be
critical in the development of I/R-induced tissue injury by
inducing inflammatory responses (13). The effects of CO2- and
air-pneumoperitoneum on the number of apoptotic cells in the livers
of rats subjected to hepatic I/R were examined using H&E and
TUNEL staining methods. Compared with sham surgery, hepatic I/R
increased the number of apoptotic cells observed following 6 h of
reperfusion (P<0.01). CO2-pneumoperitoneum inhibited
this increase (P<0.01), however, air-pneumoperitoneum did not
(Figs. 1 and 2).
Effects of CO2- and
air-pneumoperitoneum on hepatic EMAP-II expression in rats
subjected to hepatic I/R
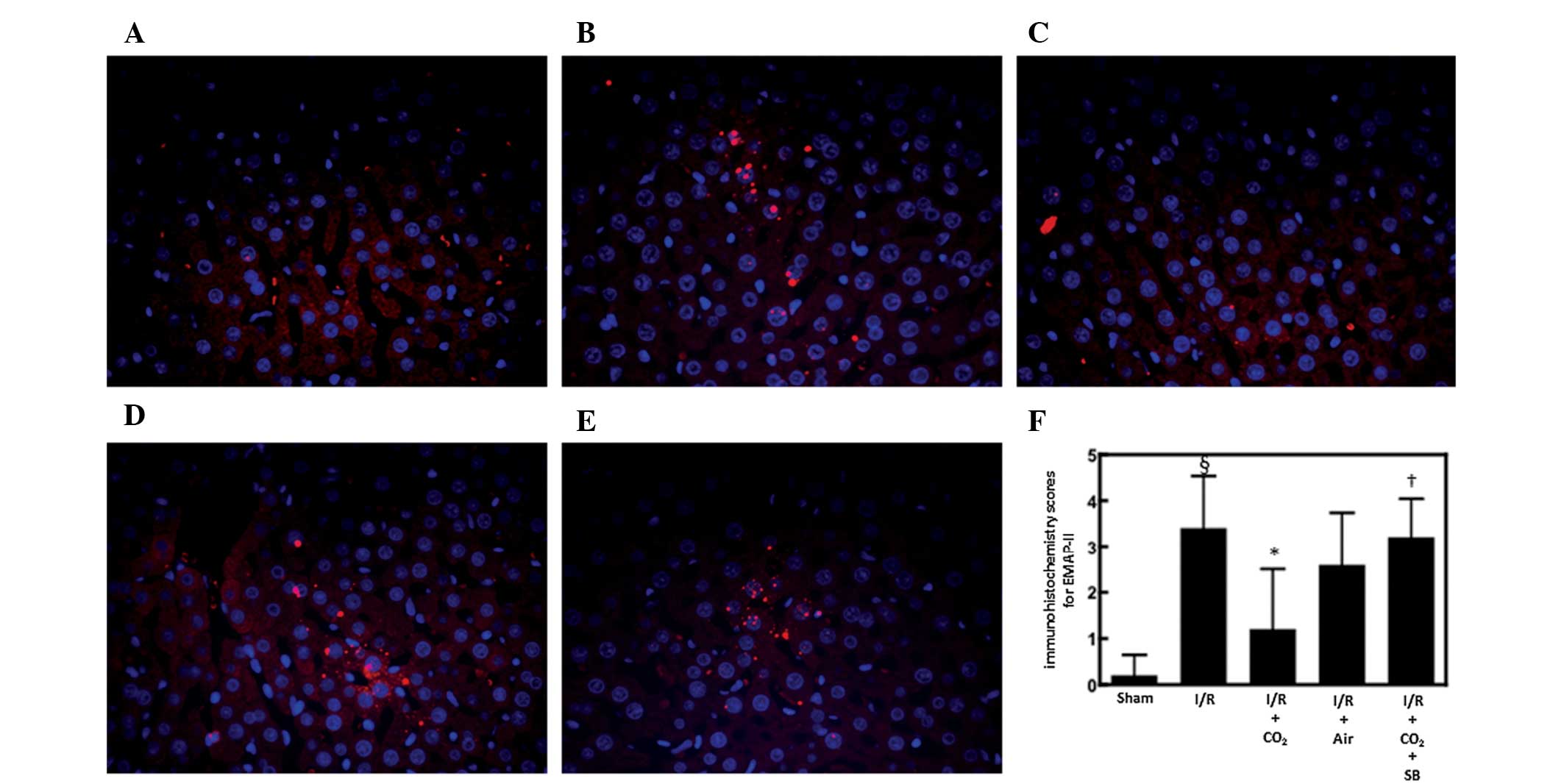
EMAP-II is a chemoattractant for monocytes and
neutrophils (30) and is produced
by apoptotic cells by cleavage of the precursor protein proEMAP-II
(31). It was postulated that
CO2-pneumoperitoneum inhibited I/R-induced hepatic
apoptosis by inhibiting the hepatic activation of EMAP-II during
I/R. To examine this possibility, the effects of CO2-
and air-pneumoperitoneum on EMAP-II expression were analyzed in the
livers of rats subjected to hepatic I/R. Immunofluorescence
staining for EMAP-II in the livers of animals subjected to hepatic
I/R was increased 6 h after reperfusion compared with the
sham-surgery animals (P<0.01; Fig.
3). CO2-pneumoperitoneum reduced this increase more
strongly compared with that of air pneumoperitoneum (Fig. 3).
Effect of SB366791 on alterations induced
by CO2-pneumoperitoneum in rats subjected to hepatic
I/R
To determine whether CO2-pneumoperitoneum
reduces I/R-induced hepatic apoptosis and inflammatory responses by
activating TRPV1 in sensory neurons, the effects of the specific
TRPV1 antagonist SB366791 on alterations induced by
CO2-pneumoperitoneum were analyzed in rats subjected to
hepatic I/R. Administration of SB366791 completely reversed the
ability of CO2-pneumoperitoneum to inhibit I/R-induced
apoptosis and hepatic EMAP-II expression (P<0.05; Figs. 1–3).
Discussion
The present study demonstrated that
CO2-pneumoperitoneum prevented I/R-induced hepatic
apoptosis, while air-pneumoperitoneum did not. Pretreatment with
the specific TRPV1 antagonist SB366791 abrogates this effect. Our
previous study demonstrated that stimulation of sensory neurons in
animals subjected to hepatic I/R inhibits hepatic apoptosis by
inducing CGRP (12). These
observations suggest that CO2-pneumoperitoneum
stimulates sensory neurons through TRPV1 activation, thereby
inhibiting hepatic apoptosis in rats following hepatic I/R. Since
hepatic apoptosis was not inhibited by air-pneumoperitoneum, it is
likely that abdominal distension alone does not induce this
effect.
The precise mechanisms by which
CO2-pneumoperitoneum activates sensory neurons are not
clear. Acidic environments (low pH) are known to stimulate sensory
neurons through activation of TRPV1 (16), and abdominal insufflation with
CO2 causes a marked and rapid reduction in local tissue
pH (17). Furthermore,
CO2 has been demonstrated to stimulate sensory neurons
through intracellular acidification by carbonic anhydrase (18,19)
and to stimulate CGRP release through a decrease in intracellular
pH in sensory neurons in vitro (32). These observations suggest that
abdominal insufflation with CO2 may stimulate sensory
neurons by decreasing intracellular pH.
CO2-pneumoperitoneum strongly inhibited
I/R-induced increases in the hepatic expression of EMAP-II compared
with that of air-pneumoperitoneum. Pretreatment with SB366791
completely reversed this effect. These observations suggest that
CO2-pneumoperitoneum, and not abdominal distension
alone, may inhibit I/R-induced increases in EMAP-II expression by
activating TRPV1. EMAP-II is a chemoattractant for neutrophils
(30). Its precursor protein,
proEMAP-II, is cleaved by caspase-3 to the biologically active form
(33). Caspases are activated in
apoptotic cells. Following renal I/R, caspase activation increases
neutrophil accumulation in the kidney by increasing EMAP-II
expression (13). These
observations suggest that, in addition to inducing apoptosis,
caspases contribute to tissue inflammation and injury by increasing
neutrophil infiltration at damaged sites through activation of
EMAP-II. Our previous study demonstrated that stimulation of
sensory neurons inhibits I/R-induced increases of caspase-3 and
apoptosis in hepatic tissue (12).
Thus, it is possible that CO2-pneumoperitoneum prior to
hepatic I/R inhibits caspase activation through stimulation of
sensory neurons, thereby attenuating hepatic apoptosis and the
expression of EMAP-II.
Based on our results, it was postulated that
CO2-pneumoperitoneum stimulates sensory neurons in the
liver, thereby inhibiting I/R-induced inflammatory responses and
hepatic apoptosis. Our results further suggest that the effects of
CO2-pneumoperitoneum are unlikely to be associated with
abdominal distension alone. Inflammatory responses are critical in
the development of post-operative complications (34), and the present study indicates that
CO2-pneumoperitoneum may contribute to the clinical
benefits of laparoscopic surgery by attenuating inflammatory
responses via stimulation of sensory neurons.
Ischemic preconditioning, the most effective
gastroprotective intervention, involves sensory neurons (35,36).
The mechanism underlying the beneficial effects of laparoscopic
surgery may be similar to those involved in ischemic
preconditioning. It is thus intriguing to consider the possibility
that CO2-pneumoperitoneum may be beneficial when
performed as a preconditioning stimulus, that is prior to open
surgery. This possibility should be examined in the clinical
setting.
References
|
1
|
Mealy K, Gallagher H, Barry M, Lennon F,
Traynor O and Hyland J: Physiological and metabolic responses to
open and laparoscopic cholecystectomy. Br J Surg. 79:1061–1064.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zmora O, Hashavia E, Munz Y, Khaikin M,
Shabtai M, Ayalon A, Dinur L and Rosin D: Laparoscopic colectomy is
associated with decreased postoperative gastrointestinal
dysfunction. Surg Endosc. 23:87–89. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Redmond HP, Watson RW, Houghton T, Condron
C, Watson RG and Bouchier-Hayes D: Immune function in patients
undergoing open vs laparoscopic cholecystectomy. Arch Surg.
129:1240–1246. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vittimberga FJ Jr, Foley DP, Meyers WC and
Callery MP: Laparoscopic surgery and the systemic immune response.
Ann Surg. 227:326–334. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mann C, Boccara G, Grevy V, Navarro F,
Fabre JM and Colson P: Argon pneumoperitoneum is more dangerous
than CO2 pneumoperitoneum during venous gas embolism.
Anesth Analg. 85:1367–1371. 1997.PubMed/NCBI
|
|
6
|
Araújo Filho I, Honorato Sobrinho AA, Rego
AC, Garcia AC, Fernandes DP, Cruz TM, Costa TC and Medeiros AC:
Influence of laparoscopy and laparotomy on gasometry, leukocytes
and cytokines in a rat abdominal sepsis model. Acta Cir Bras.
21:74–79. 2006.PubMed/NCBI
|
|
7
|
Hanly EJ, Fuentes JM, Aurora AR, Bachman
SL, De Maio A, Marohn MR and Talamini MA: Carbon dioxide
pneumoperitoneum prevents mortality from sepsis. Surg Endosc.
20:1482–1487. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pitombo MB, Lupi OH, Gomes RN, Amâncio R,
Refinetti RA, Bozza PT and Castro-Faria-Neto HC: Inflammatory
response and bacterial dissemination after laparotomy and abdominal
CO2 insufflation in a murine model of peritonitis. Surg
Endosc. 20:1440–1447. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Okajima K and Harada N: Regulation of
inflammatory responses by sensory neurons: molecular mechanism(s)
and possible therapeutic applications. Curr Med Chem. 13:2241–2251.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Maggi CA and Meli A: The sensory-efferent
function of capsaicin-sensitive sensory neurons. Gen Pharmacol.
19:1–43. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Caterina MJ, Schumacher MA, Tominaga M,
Rosen TA, Levine JD and Julius D: The capsaicin receptor: a
heat-activated ion channel in the pain pathway. Nature.
389:816–824. 1997. View
Article : Google Scholar
|
|
12
|
Kawai M, Harada N, Takeyama H and Okajima
K: Neutrophil elastase contributes to the development of
ischemia/reperfusion-induced liver injury by decreasing the
production of insulin-like growth factor-I in rats. Transl Res.
155:294–304. 2010. View Article : Google Scholar
|
|
13
|
Daemen MA, van ‘t Veer C, Denecker G,
Heemskerk VH, Wolfs TG, Clauss M, Vandenabeele P and Buurman WA:
Inhibition of apoptosis induced by ischemia-reperfusion prevents
inflammation. J Clin Invest. 104:541–549. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jaeschke H: Molecular mechanisms of
hepatic ischemia-reperfusion injury and preconditioning. Am J
Physiol Gastrointest Liver Physiol. 284:G15–G26. 2003.PubMed/NCBI
|
|
15
|
Massip-Salcedo M, Roselló-Catafau J,
Prieto J, Avila MA and Peralta C: The response of the hepatocyte to
ischemia. Liver Int. 27:6–16. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Holzer P: The pharmacological challenge to
tame the transient receptor potential vanilloid-1 (TRPV1)
nocisensor. Br J Pharmacol. 155:1145–1162. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
West MA, Hackam DJ, Baker J, Rodriguez JL,
Bellingham J and Rotstein OD: Mechanism of decreased in vitro
murine macrophage cytokine release after exposure to carbon
dioxide: relevance to laparoscopic surgery. Ann Surg. 226:179–190.
1997. View Article : Google Scholar
|
|
18
|
Akiba Y, Ghayouri S, Takeuchi T, Mizumori
M, Guth PH, Engel E, Swenson ER and Kaunitz JD: Carbonic anhydrases
and mucosal vanilloid receptors help mediate the hyperemic response
to luminal CO2 in rat duodenum. Gastroenterology.
131:142–152. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Prabhakar E and Lawson SN: The
electrophysiological properties of rat primary afferent neurones
with carbonic anhydrase activity. J Physiol. 482:609–622. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Varga A, Németh J, Szabó A, McDougall JJ,
Zhang C, Elekes K, Pintér E, Szolcsányi J and Helyes Z: Effects of
the novel TRPV1 receptor antagonist SB366791 in vitro and in vivo
in the rat. Neurosci Lett. 385:137–142. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hayashi H, Chaudry IH, Clemens MG and Baue
AE: Hepatic ischemia models for determining the effects of
ATP-MgCl2 treatment. J Surg Res. 40:167–175. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jaeschke H, Farhood A, Bautista AP,
Spolarics Z, Spitzer JJ and Smith CW: Functional inactivation of
neutrophils with a Mac-1 (CD11b/CD18) monoclonal antibody protects
against ischemia-reperfusion injury in rat liver. Hepatology.
17:915–923. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nakayama T, Harada N, Asano M, Nomura N,
Saito T, Mishima A and Okajima K: Atrial natriuretic peptide
reduces ischemia/reperfusion-induced spinal cord injury in rats by
enhancing sensory neuron activation. J Pharmacol Exp Ther.
322:582–590. 2007. View Article : Google Scholar
|
|
24
|
Soeda J, Miyagawa S, Sano K, Masumoto J,
Taniguchi S and Kawasaki S: Cytochrome c release into cytosol with
subsequent caspase activation during warm ischemia in rat liver. Am
J Physiol Gastrointest Liver Physiol. 281:G1115–G1123.
2001.PubMed/NCBI
|
|
25
|
Martin EJ and Forkert PG: Evidence that
1,1-dichloroethylene induces apoptotic cell death in murine liver.
J Pharmacol Exp Ther. 310:33–42. 2004. View Article : Google Scholar
|
|
26
|
Kelly KJ, Sandoval RM, Dunn KW, Molitoris
BA and Dagher PC: A novel method to determine specificity and
sensitivity of the TUNEL reaction in the quantitation of apoptosis.
Am J Physiol Cell Physiol. 284:C1309–C1318. 2003. View Article : Google Scholar
|
|
27
|
Gavrieli Y, Sherman Y and Ben-Sasson SA:
Identification of programmed cell death in situ via specific
labeling of nuclear DNA fragmentation. J Cell Biol. 119:493–501.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chaerkady R, Harsha HC, Nalli A, Gucek M,
Vivekanandan P, Akhtar J, Cole RN, Simmers J, Schulick RD, Singh S,
Torbenson M, Pandey A and Thuluvath PJ: A quantitative proteomic
approach for identification of potential biomarkers in
hepatocellular carcinoma. J Proteome Res. 7:4289–4298. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Barnes DM, Harris WH, Smith P, Millis RR
and Rubens RD: Immunohistochemical determination of oestrogen
receptor: comparison of different methods of assessment of staining
and correlation with clinical outcome of breast cancer patients. Br
J Cancer. 74:1445–1451. 1996. View Article : Google Scholar
|
|
30
|
Kao J, Fan YG, Haehnel I, Brett J,
Greenberg S, Clauss M, Kayton M, Houck K, Kisiel W, Seljelid R, et
al: A peptide derived from the amino terminus of
endothelial-monocyte-activating polypeptide II modulates
mononuclear and polymorphonuclear leukocyte functions, defines an
apparently novel cellular interaction site, and induces an acute
inflammatory response. J Biol Chem. 269:9774–9782. 1994.
|
|
31
|
Knies UE, Behrensdorf HA, Mitchell CA,
Deutsch U, Risau W, Drexler HC and Clauss M: Regulation of
endothelial monocyte-activating polypeptide II release by
apoptosis. Proc Natl Acad Sci USA. 95:12322–12327. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vause C, Bowen E, Spierings E and Durham
P: Effect of carbon dioxide on calcitonin gene-related peptide
secretion from trigeminal neurons. Headache. 47:1385–1397.
2007.PubMed/NCBI
|
|
33
|
van Horssen R, Eggermont AM and ten Hagen
TL: Endothelial monocyte-activating polypeptide-II and its
functions in (patho)physiological processes. Cytokine Growth Factor
Rev. 17:339–348. 2006.PubMed/NCBI
|
|
34
|
Jacobi CA, Wenger F, Opitz I and Müller
JM: Immunologic changes during minimally invasive surgery. Dig
Surg. 19:459–463. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pawlik M, Ptak A, Pajdo R, Konturek PC,
Brzozowski T and Konturek SJ: Sensory nerves and calcitonin gene
related peptide in the effect of ischemic preconditioning on acute
and chronic gastric lesions induced by ischemia-reperfusion. J
Physiol Pharmacol. 52:569–581. 2001.
|
|
36
|
Pajdo R, Brzozowski T, Konturek PC,
Kwiecien S, Konturek SJ, Sliwowski Z, Pawlik M, Ptak A, Drozdowicz
D and Hahn EG: Ischemic preconditioning, the most effective
gastroprotective intervention: involvement of prostaglandins,
nitric oxide, adenosine and sensory nerves. Eur J Pharmacol.
427:263–276. 2001. View Article : Google Scholar
|