Introduction
Spinal cord injury (SCI) is characterized by an
immediate, irreversible destruction of tissue at the lesion site
with secondary expansion of damage over time, resulting in
irreversible loss of sensation and movement. The consequences of
SCI are lifetime debilitation, the extent of which increases with
more rostral injury site (1).
Global estimates of the number of new cases annually range from
15~40/million (2). There is
currently no effective treatment for acute SCI, except for
high-dose methylprednisolone (MP) therapy, the efficacy of which
has been challenged by numerous studies, due to the marginal
therapeutic effects and high incidence of pulmonary complications
(3,4). An ideal post-SCI pharmacotherapy
would suppress the pathogenic processes leading to secondary
injury, including inflammation and apoptosis, and promote nerve
regeneration (5,6).
Numerous studies have demonstrated a significant
decrease in neuronal cyclic adenosine monophosphate (cAMP)
following SCI, which may exacerbate secondary neuronal injury and
inhibit neural regeneration (7,8).
Therefore, elevation of cAMP in neurons may facilitate recovery or
reduce secondary pathology following SCI. Indeed, PDE4 inhibitors
have been implicated as possible therapeutics to reduce secondary
pathological responses and activate regenerative mechanisms in
animal models of SCI, by maintaining cAMP levels (9,10).
The mechanisms for the post-SCI decrease in cAMP are elusive, and a
lack of selective agents with tolerable side effects has hampered
the clinical application of this treatment strategy. cAMP is a
ubiquitous secondary messenger in all life forms. In mammals,
intracellular levels are under the dual regulation of adenylate
cyclases (ACs), which are activated by numerous different receptors
and by phosphodiesterases (PDEs), and are also under complex
upstream regulation (11). There
are ten known AC isoforms (AC1-10), each with distinct modes of
regulation and expression patterns (12) and four different cAMP-specific PDE4
isozymes (PDE4A, PDE4B, PDE4C and PDE4D) (13). Several studies have indicated that
PDE4D is expressed by spinal cord oligodendrocytes (14). The AC3 isoform is distributed
throughout the brain, spinal cord and olfactory epithelium
(15,16). The present study examined the
possibility that SCI triggers a post-traumatic inhibition of AC and
(or) activation of PDE, thereby causing a decline in cellular cAMP
concentration, and whether agents that maintain post-injury
intracellular cAMP concentration may limit secondary damage and
improve functional outcome. Meglumine cyclic adenylate (MCA) is a
cAMP analog currently available for cardiovascular treatments in
China. This compound of cAMP and meglumine acts both as a cAMP
analogue and a PDE inhibitor, and may therefore be a particularly
efficient cAMP upregulator. In the present study, the post-SCI
changes in AC3 and PDE4D activities, tissue cAMP concentrations and
the recovery of motoric function were examined in a rat model of
SCI.
Materials and methods
Animals and surgical methods
A total of 48 healthy adult Sprague-Dawley (SD) rats
of (age, 6–8 months) both sexes (Laboratory Animal Center of
Sichuan University, Chengdu, China) weighing 250–310 g (mean, 280
g) were used. The animals were maintained in standard housing
conditions (22±2°C, 55% humidity, light from 6:00 am to 8:00 pm)
and fed a standard dry diet and water ad libitum. All of the
experimental procedures were approved by the Institutional Animal
Care and Use Committee of Sichuan University. Following
experimental SCI, the rats were randomly divided into three equal
treatment groups of 16: A (methylprednisolone group), B (MCA group)
and C (control group). An acute T11 SCI was induced by Allen’s
method under aseptic conditions (17). Briefly, under 1% pentobarbital
sodium (30 mg/kg-ip) anesthesia, the rats were fixed in a prone
position on a small arched table, and the hair at the operation
site (around T11) was shaved and treated with povidone iodine
solution. One to three minutes prior to the surgical procedure, the
rats were administered 2.5 ml of warm saline solution containing
penicillin G sodium (10,000 units/kg-bw) by subcutaneous injection
in the abdominal wall as an antimicrobial agent. A 3-cm skin
incision was made posterior to the midline and continued down to
the lumbosacral fascia, which was incised to expose the tips of the
spinous processes. With blunt dissection, the paraspinal
musculature was subperiosteally dissected and the lumbar vertebral
segments exposed. A T10–T12 total laminectomy was performed to
expose the spinal dura mater and nerve roots. A small, curved, hard
and sterilized plastic plate was placed on the surface of the T11
dura mater, and an SCI was induced by dropping a 7-g weight from 3
cm onto the plate. Upon completion of the surgical procedure, the
lumbosacral fascia and other layers were sutured layer by layer. A
total of 30 min following the injury, group A was intraperitoneally
injected with a single 30 mg/kg bw dose of methylprednisolone
(Pfizer/Pharmacia and Upjohn, Kalamazoo, MI, USA), group B with 2
mg/kg MCA (Wang Bang Biochemical Pharmaceutical Co., Ltd., China)
once daily for seven days, and group C with an equal volume of
saline. Following the surgical procedure, the animals also received
2.5 ml warm saline solution containing penicillin G sodium (10,000
units/kg bw). The animals were positioned over a heating pad to
maintain their body temperature and administered a light
concentration of diethyl ether (1 ml diethyl ether in a 250-ml
wide-mouthed bottle for 0.5–1.0 min) for pain relief following
waking. Following SCI, the rats were housed one per cage. Each
underwent manual bladder evacuation three times daily and wound
dressings were changed daily. Eight randomly selected animals from
each group were examined for six weeks on the inclined plane and
Gale scale tests. Seven days following SCI, the spinal cord samples
were obtained from the other eight rats in each group for
pathological examination and to determine the cAMP levels and both
AC3 and PDE4D activities by a specific ELISA and quantitative
immunohistochemistry.
Behavioral testing
Inclined plane
The inclined plane test assesses the ability of an
animal to maintain position on a slope. The highest angle of
inclination maintained for 5 sec was determined as a measure of
stability. The test was conducted from week one to week six
post-SCI (18). Functional motor
deficits and recovery are expressed by the inclined plane (IP)
maintenance ratio (post-injury/pre-injury).
Gale scale
The animals’ motor functions were also observed and
scored into six levels by the Gale scale (Table I) (19).
 | Table IEffects of MP (group A) and MCA (group
B) on motor function six weeks following spinal cord injury,
compared with the saline-treated controls (group C). |
Table I
Effects of MP (group A) and MCA (group
B) on motor function six weeks following spinal cord injury,
compared with the saline-treated controls (group C).
| Groups | N | Gale scale
(point) | IP maintaining ratio
(%) |
|---|
| A (MP) | 8 | 3.56±0.26 | 0.39±0.023 |
| B (MCA) | 8 | 3.67±0.29 | 0.42±0.021 |
| C (saline) | 8 | 2.68±0.21a | 0.28±0.012a |
| P-value | | <0.05 | <0.05 |
Pathological examination
Tissue sample preparation
Seven days following injury, half of the animals in
groups A-C were anesthetized by ether inhalation and the injured
spinal cord was re-exposed. The entire section of injured spinal
column was excised by breaking the upper, lower, left and right
vertebrae connected to the ribs. A 1.5-cm section of the vertebral
column containing the lesion site was cut into two equal portions
through the center of the lesion site. The upper-half portion
(rostral side, from the injury epicenter tissue up to 6 mm rostral
side) was fixed in 10% neutral formalin for 12 h, and then the
spinal cord was isolated and further fixed for another 12 h for
pathological examination. The remaining caudal portion (caudal
side, from the injury epicenter tissue up to 9 mm caudal side) was
further cut into three equal portions, which were immediately
wrapped in aluminum foil and immersed in liquid nitrogen for later
cAMP assay and estimation of AC3 and PDE4D activities. The upper
portion of the injured spinal cord sample (rostral side) was
assessed for cAMP by ELISA, the middle portion was used to detect
AC3, and the lower portion (caudal side) was used to detect PDE4D
by immunohistochemistry.
Hematoxylin and eosin (H&E)
staining
The tissue samples were fixed in 10% neutral
formalin for 24 h, dehydrated, embedded in paraffin and sectioned
into 5-μm slices. The middle sections were treated with H&E. An
Olympus optical microscope CX21 was used (Olympus, Tokyo,
Japan).
Measurement of cAMP
The cAMP content was measured by ELISA (2nd
Generation, DE0450; R&D Inc., R&D Systems, Inc.,
Minneapolis, MN, USA). Spinal cord samples were removed from the
liquid nitrogen, ground, weighed and dissolved in ten volumes of 5%
trichloroacetic acid (TCA). After the raw lysate was centrifuged at
40 g for 10 min, the supernatant was drawn and mixed with three
volumes of hydrated ether. Following dehydration, the ED2 detection
buffer was added. A 200-μl sample was mixed with 10 μl acetic
anhydride reagent. Similarly, 1 ml cAMP standard in ED2 detection
phosphate-buffered saline was mixed with 50 μl acetylation reagent.
The cAMP conjugate, cAMP antibody solution, p-nitrophenyl
phosphatase substrate and 50 μl of the reaction termination
solution were added to each micropore. An ELISA reader (detection
wavelength, 405 nm) was used to rapidly determine the optical
density (OD) of each micropore. A standard curve was plotted using
the logarithmic concentration of acetylated standard cAMP (x-axis)
vs. the OD value (y-axis). The cAMP concentrations of the samples
were estimated from the standard curve.
Estimation of AC3 and PDE4D activity
by immunohistochemistry
Cryostat sections were cut at 4–8 mm, warmed to room
temperature (30 min) and fixed in acetone for 10 min at 4°C. Fixed
slices were washed three times in phosphate-buffered saline (PBS; 5
min/wash), soaked in 3% hydrogen peroxide for 5–10 min to quench
endogenous peroxidase activity, and then washed again in PBS (5 min
× 3). To determine the number of AC3- and PDE4D-positive cells, the
slices were incubated in rabbit polyclonal Santa’s AC3
immunoglobulin (Ig)G or PDE4D IgG (200 μg/ml), followed by a
secondary antibody (Beijing Zhongshan Golden Bridge Biotechnology
Co., Ltd., Beijing, China) and visualization using a
peroxidase-labeled streptavidin-avidin kit (Beijing Zhongshan
Golden Bridge Biotechnology Co., Ltd.) and diaminobenzidine. Six
arbitrary fields of view were chosen at high magnification (x400)
for each AC3- or PDE4D-stained slice and six arbitrary neurons in
each field were used to determine the average density of AC3 or
PDE4D immunostaining in the cytoplasm. The mean density was used as
an estimate of the AC3 or PDE4D activity.
Statistical analysis
The SPSS 12.0 software program (SPSS, Inc., Chicago,
IL, USA) was used for statistical analyses. Values are presented as
the mean ± standard deviation. Group mean values were compared by
one-way analysis of variance with post-hoc least-significant
difference pair-wise comparisons (q-test).
Results
Behavioral assessments
Six weeks following SCI, the IP maintenance ratio
and the Gale scale were significantly higher in treatment groups A
(methylprednisolone) and B (MCA) compared with the vehicle-treated
group C (P<0.05), indicating improved recovery of motor
function. Methylprednisolone and MCA were equally effective, as
there was no significant difference in the behavioral assessments
between groups A and B (P>0.05; Table I).
Pathological examination
H&E staining
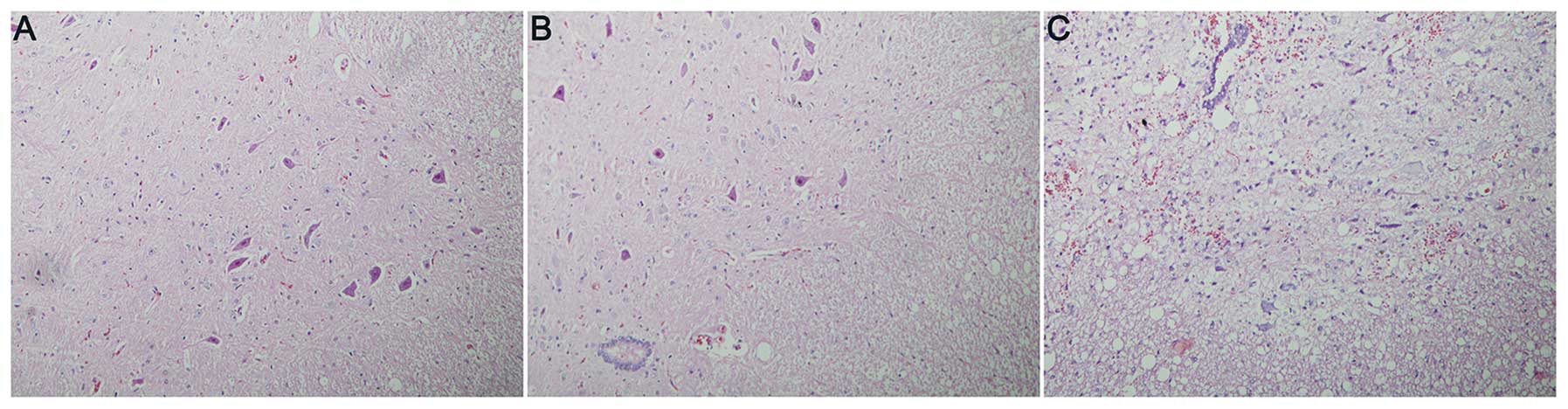
Higher densities of intact neurons were observed in
groups A and B compared with those in group C. In spinal cord
slices from drug-treated rats, the intercellular matrix was
uniform, the majority of the neurons were morphologically normal,
large and irregular, the membrane was intact, the cytoplasm was
uniformly and lightly stained, and the nuclei and nucleoli were
darkly stained and clear. Large numbers of small glial cells were
observed among the neurons and there was little hemorrhage or edema
(Fig. 1A and B). In the control
group (group C), however, the spinal cord tissues were sparse and
significantly swollen with evident hemorrhage, few neurons were
observed, and those remaining had swollen cell bodies, with uneven
cytoplasmic staining. Numerous nuclei were contracted and
degenerated (Fig. 1C).
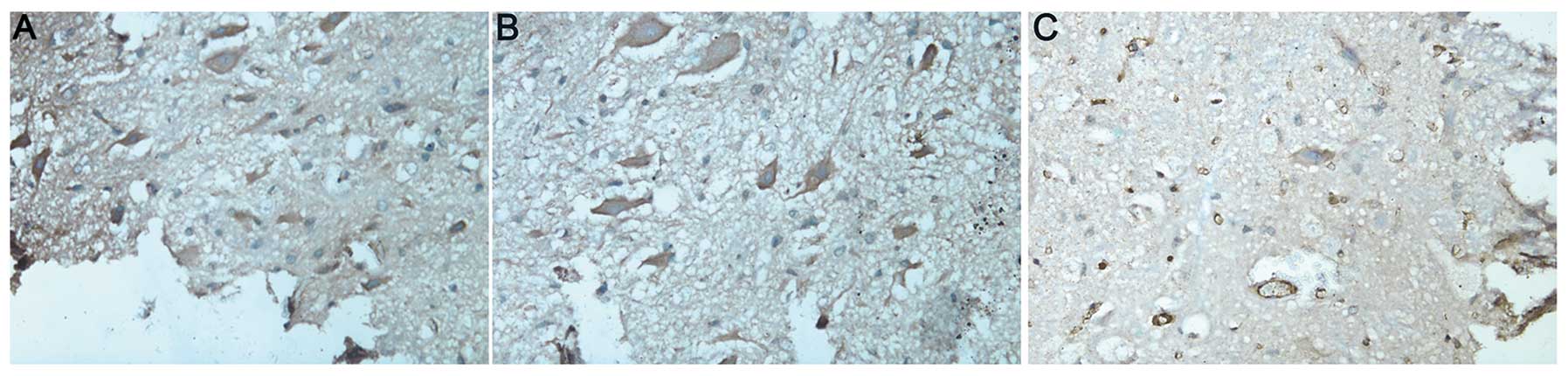
AC3 activity
In groups A and B, the neurons in the spinal cord
were large and irregular with intact membranes. The nuclei and
nucleoli were evident. Brown-yellow, dense and uniform cytoplasm
immunostained for AC3 was visualized surrounding the nuclei
(Fig. 2A and B). In the control
group (group C), there were fewer neurons and those remaining had
inhomogeneous cytoplasm with evident vacuolization and pale
amber-yellow staining (Fig. 2C).
The mean density of AC3-positive cells was lower than that in
groups A and B (P<0.05; Table
II).
 | Table IIMean density of AC3- and
PDE4D-positive spinal neurons at the lesion site one week following
spinal cord injury (mean ± standard deviation). |
Table II
Mean density of AC3- and
PDE4D-positive spinal neurons at the lesion site one week following
spinal cord injury (mean ± standard deviation).
| Groups | N | AC3-positive cell
count | PDE4D-positive cell
count |
|---|
| A (MP) | 8 | 636.3±28.6 | 239.3±21.7 |
| B (MCA) | 8 | 622.2±36.1 | 251.2±32.3 |
| C (control
group) | 8 | 372.8±33.2a | 568.1±39.2a |
| P-value | | <0.05 | <0.05 |
PDE4D activity
In groups A and B, the spinal neurons were large,
irregularly shaped and exhibited intact membranes with clearly
visible nuclei and nucleoli. These neurons were stained light
yellow (Figs. 3A and B). In the
control group (group C), there were fewer neurons, with
inhomogeneous cytoplasm and evident vacuolations. These neurons
were stained an amber color (Fig.
3C). The number of PDE-4D-positive cells (calculated as the
mean density) was significantly higher than that in groups A and B
(P<0.05; Table II).
cAMP concentration
Spinal cAMP levels at seven days following injury
was higher in group A and B than those in group C (P<0.05).
Furthermore, cAMP levels in group B were higher those that in group
A (P<0.05; Table III).
 | Table IIIcAMP concentration at the lesion site
seven days folllowing spinal cord injury. |
Table III
cAMP concentration at the lesion site
seven days folllowing spinal cord injury.
| Groups | N | cAMP concentration
(pmol/ml, mean ± standard deviation) |
|---|
| A (MP) | 8 | 70.0±3.6 |
| B (MCA) | 8 | 112.5±4.5 |
| C (control
group) | 8 | 35.0±1.8a |
| P-value | | <0.05 |
Discussion
cAMP and SPI
Cyclic adenosine monophosphate (cAMP) was first
isolated from liver homogenates by Sutherland in 1957, and is now
known to be distributed in all mammalian tissues except red blood
cells (20). The concentration of
cAMP is regulated by ACs and PDEs. The synthesis and degradation of
cAMP is notably faster in nerve tissue than in other tissues,
suggesting it has specific functions in rapid localized
intracellular signaling (21).
Recent studies have demonstrated that cAMP levels in the spinal
cord decrease following SCI, while the elevation of intracellular
cAMP may reduce secondary SCI and promote regeneration (9,22).
Intracellular cAMP levels may be increased and maintained by a
peripheral conditioning lesion, administration of cell-permeant
cAMP analogs such as dbcAMP, or by treatment with PDE4 inhibitors,
including rolipram. While each of these methods has been
demonstrated to suppress secondary pathological responses, reduce
the inhibition of axonal regeneration, and activate regenerative
processes, each has specific disadvantages for routine clinical
use. Nikulina et al (23)
found that rolipram delivered for two weeks together with embryonic
spinal cord transplantation following C3/4 SCI promoted axonal
growth into the transplant, attenuated astrogliosis, a glial
proliferative and hypertrophic reaction considered to block axonal
elongation, and, most importantly, improved functional recovery in
Evans-Hooded rats. The authors suggested that rolipram promoted
regeneration of spinal axons by elevating neuronal cAMP
concentration (23). Pearse et
al (24) found that cAMP
levels decreased by ~70% following thoracic contusion SCI and
remained below baseline for at least two weeks post-SCI. Immediate
subcutaneous injection of rolipram prevented this decrease in cAMP
and the occurrence of secondary SCI damage. In addition, this
treatment promoted the growth of nerve fibers when combined with
Schwann cell transplantation and direct dbcAMP injection into the
injured site (24).
Consistent with other studies, the present study
demonstrated that cAMP levels in the spinal cord were significantly
below baseline seven days following SCI, while MCA and MP enhanced
cAMP levels post-SCI (MCA more than MP). The MCA and MP groups also
demonstrated improved maintenance of histological structure, with
significantly more intact neurons, less tissue hemorrhaging and
edema, and fewer regions of low cell density or necrosis, as
revealed by H&E staining. In addition, MCA- and MP-treated rats
demonstrated superior motor recovery as assessed by the IP
stability test and Gale scale test battery compared with the
control group. Administration of MCA and MP at the early stages of
injury reversed the decrease and sustained cAMP levels post-SCI,
possibly accounting for enhanced recovery of motor function.
However, MCA may present certain advantages over other analogues or
PDE4D inhibitors. Rolipram is also a monoamine oxidase inhibitor,
which, at doses causing PDE4 suppression, may induce nausea and
vomiting (25). By contrast, MCA
acts as a cAMP analogue and PDE4 antagonist, and may therefore be
more effective at lower doses.
ACs, PDEs and SCI
There are 11 classes of PDE (PDE1-11) and a total of
30 isozymes (26), of which PDE4,
7 and 8 are highly specific for the hydrolysis of cAMP, PDE5, 6 and
9 are highly specific for the hydrolysis of cGMP, and PDE1, 2, 3
are able to hydrolyze both cAMP and cGMP (but PDE3 has greater
activity on cAMP). These PDE isoenzymes are differentially
distributed in tissues and organs, with PDE4 broadly expressed in
the nervous system. There are four PDE4 isozymes, PDE4A, PDE4B,
PDE4C and PDE4D, mainly distributed within inflammatory and nerve
cells (27). Pérez-Torres et
al (28) detected abundant
PDE4 in human, monkey and rat brains by in situ
hybridization and immunohistochemistry. Similarly, Lamontagne et
al (29) found PDE4D was
widely distributed in the central nervous system (CNS), with
particularly strong activity in the nodose ganglion, area postrema,
solitary tract nucleus and locus coeruleus. There are ten adenylate
cyclase (AC) isozymes (AC1-10) (30), and several of them are involved in
signal transduction in the CNS. Abaffy et al (2003) found
strong AC1 and AC3 expression in the nuclei of rat sensory neurons
(30). In the present study, the
AC3 and PDE4D activities in the spinal cord following SCI were also
measured. Both MCA and MP treatments significantly increased the
post-SCI AC3-positive cell count and reduced the PDE4D-positive
cell count, thereby maintaining post-traumatic cAMP levels,
protecting spinal neurons and improving post-SCI recovery.
MCA treatment mechanism for acute
SCI
MCA is a compound of cAMP and meglumine with a mean
blood half-life of 60–150 min. It has been used in China for the
treatment of cardiovascular diseases with low toxicity and cost
(32). In addition, MCA has
physiological effects similar to cAMP and PDE inhibitors but with
enhanced liposolubility and membrane permeability. In clinical
cardiovascular applications, MCA serves as a cardiotonic agent to
treat bradyarrhythmias or heart failure with high stability, low
toxicity and low cost, but has not yet been used for studies on SCI
treatment. The typical MCA dosage in adults is 1–3 mg/kg/d, while a
dose of up to 2.5–5 mg/kg/d (iv.qd) in children has been reported
to be safe, as its LD50 is 1996±149 mg/kg-iv (33). In the present study, a dose of 2
mg/kg was used, based on other clinical applications and
preliminary experiments by our group.
In conclusion, cAMP levels in the spinal cord
decreased following SCI, and elevation of intracellular cAMP levels
reduced secondary SCI and increased the regenerative capacity.
Therefore, cAMP analogues or tolerable PDE inhibitors may benefit
patients in the early stages post-SCI. MCA is a dual cAMP analogue
and PDE4 inhibitor and may be a prototype for an alternative
treatment strategy. Further studies are required to examine its
pharmacokinetics and full spectrum of actions following SCI.
References
|
1
|
Wiedemann C: Repair: CSI in SCI. Nat Rev
Neurosci. 10:6212009. View
Article : Google Scholar
|
|
2
|
Cripps RA, Lee BB, Wing P, et al: A global
map for traumatic spinal cord injury epidemiology: towards a living
data repository for injury prevention. Spinal Cord. 49:493–501.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ito Y, Sugimoto Y, Tomioka M, Kai N and
Tanaka M: Does high dose methylprednisolone sodium succinate really
improve neurological status in patient with acute cervical cord
injury?: a prospective study about neurological recovery and early
complications. Spine (Phila Pa 1976). 34:2121–2124. 2009.
View Article : Google Scholar
|
|
4
|
Bracken M: Steroids for acute spinal cord
injury. Cochrane Database Syst Rev. 3:CD0010462002.
|
|
5
|
Baptiste DC and Fehlings MG: Emerging
drugs for spinal cord injury. Expert Opin Emerg Drugs. 13:63–80.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kwon BK, Hillyer J and Tetzlaff W:
Translational research in spinal cord injury: a survey of opinion
from the SCI community. J Neurotrauma. 27:21–33. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Abe N and Cavalli V: Nerve injury
signaling. Curr Opin Neurobiol. 18:276–283. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Udina E, Ladak A, Furey M, et al:
Rolipram-induced elevation of cAMP or chondroitinase ABC breakdown
of inhibitory proteoglycans in the extracellular matrix promotes
peripheral nerve regeneration. Exp Neurol. 223:143–152. 2010.
View Article : Google Scholar
|
|
9
|
Hannila SS and Filbin MT: The role of
cyclic AMP signaling in promoting axonal regeneration after spinal
cord injury. Exp Neurol. 209:321–332. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mekhail M, Almazan G and Tabrizian M:
Oligodendrocyte-protection and remyelination post-spinal cord
injuries: a review. Prog Neurobiol. 96:322–339. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gancedo JM: Biological roles of cAMP:
variations on a theme in the different kingdoms of life. Biol Rev
Camb Philos Soc. 88:645–668. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Seifert R, Lushington GH, Mou TC, Gille A
and Sprang SR: Inhibitors of membranous adenylyl cyclases. Trends
Pharmacol Sci. 33:64–78. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Michalski JM, Golden G, Ikari J and
Rennard SI: PDE4: a novel target in the treatment of chronic
obstructive pulmonary disease. Clin Pharmacol Ther. 91:134–142.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Whitaker CM, Beaumont E, Wells MJ, et al:
Rolipram attenuates acute oligodendrocyte death in the adult rat
ventrolateral funiculus following contusive cervical spinal cord
injury. Neurosci Lett. 438:200–204. 2008. View Article : Google Scholar
|
|
15
|
Pavan B, Paganetto G and Dalpiaz A:
Dopamine-sensitive adenylyl cyclases in neuronal development:
physiopathological and pharmacological implications. Drug Discov
Today. 16:520–529. 2011. View Article : Google Scholar
|
|
16
|
Col JA, Matsuo T, Storm DR and Rodriguez
I: Adenylyl cyclase-dependent axonal targeting in the olfactory
system. Development. 134:2481–2489. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Koozekanani SH, Vise WM, Hashemi RM and
McGhee RB: Possible mechanisms for observed pathophysiological
variability in experimental spinal cord injury by the method of
Allen. J Neurosurg. 44:429–434. 1976. View Article : Google Scholar
|
|
18
|
Rivlin AS and Tator CH: Objective clinical
assessment of motor function after experimental spinal cord injury
in the rat. J Neurosurg. 47:577–581. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gale K, Kerasidis H and Wrathall JR:
Spinal cord contusion in the rat: behavioral analysis of functional
neurologic impairment. Exp Neurol. 88:123–134. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gilman AG: Silver spoons and other
personal reflections. Annu Rev Pharmacol Toxicol. 52:1–19. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Song XJ, Wang ZB, Gan Q and Walters ET:
cAMP and cGMP contribute to sensory neuron hyperexcitability and
hyperalgesia in rats with dorsal root ganglia compression. J
Neurophysiol. 95:479–492. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bhatt DH, Otto SJ, Depoister B and Fetcho
JR: Cyclic AMP-induced repair of zebrafish spinal circuits.
Science. 305:254–258. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nikulina E, Tidwell JL, Dai HN, Bregman BS
and Filbin MT: The phosphodiesterase inhibitor rolipram delivered
after a spinal cord lesion promotes axonal regeneration and
functional recovery. Proc Natl Acad Sci USA. 101:8786–8790. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pearse DD, Pereira FC, Marcillo AE, et al:
cAMP and Schwann cells promote axonal growth and functional
recovery after spinal cord injury. Nat Med. 10:610–616. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Page CP and Spina D: Selective PDE
inhibitors as novel treatments for respiratory diseases. Curr Opin
Pharmacol. 12:275–286. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhu J, Yang Q, Dai D and Huang Q: X-ray
crystal structure of phosphodiesterase 2 in complex with a highly
selective, nanomolar inhibitor reveals a binding-induced pocket
important for selectivity. J Am Chem Soc. 135:11708–11711. 2013.
View Article : Google Scholar
|
|
27
|
Liu S, Li Y, Kim S, et al:
Phosphodiesterases coordinate cAMP propagation induced by two
stimulatory G protein-coupled receptors in hearts. Proc Natl Acad
Sci USA. 109:6578–6583. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Perez-Torres S, Miro X, Palacios JM, et
al: Phosphodiesterase type 4 isozymes expression in human brain
examined by in situ hybridization histochemistry and[3H]rolipram
binding autoradiography. Comparison with monkey and rat brain. J
Chem Neuroanat. 20:349–374. 2000.PubMed/NCBI
|
|
29
|
Lamontagne S, Meadows E, Luk P, et al:
Localization of phosphodiesterase-4 isoforms in the medulla and
nodose ganglion of the squirrel monkey. Brain Res. 920:84–96. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Abaffy T, Trubey KR and Chaudhari N:
Adenylyl cyclase expression and modulation of cAMP in rat taste
cells. Am J Physiol Cell Physiol. 284:C1420–C1428. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gavaldà A and Roberts RS:
Phosphodiesterase-4 inhibitors: a review of current developments
(2010–2012). Expert Opin Ther Pat. 23:997–1016. 2013.PubMed/NCBI
|
|
32
|
Wu D, Zhao Y, Yang Y, Wang W and Xu R: [A
multicenter clinical study on clinical effects of meglumine cyclic
adenylate in treating patients with chronic pulmonary heart
disease]. Zhonghua nei ke za zhi. 40:467–470. 2001.(In
Chinese).
|
|
33
|
Xing SH, Gu SL and Meng QC: Meglumine
cyclic adenylate pediatric dosage calculations. XuZhou Acta
Academiae Medicinae. 19:370–372. 1999.
|