Introduction
Clinical studies have demonstrated that in patients
with traumatic brain injury and fractures, excessive formation of
callus or a variant of heterotopic ossification is commonly
observed, which heals faster than fractures in patients with no
brain injury (1). Therefore, it is
possible that brain injury may be associated with accelerated
fracture healing. However, the mechanism underlying this is
unclear. Several studies have demonstrated that increased levels of
circulating growth factors or cytokines stimulate the local release
of growth factors, which in turn mediates increased osteogenesis
(2). Platelet-derived growth
factor (PDGF) is one of the most important growth factors and it
has been demonstrated that ischemic insult and penetrating injury
enhanced the expression of PDGF-β (3,4).
The PDGF gene was identified to be expressed by
numerous cell types during fracture healing in the human body, and
appears to function to promote bone formation and reconstruction
(5). As an important mitogen, PDGF
is highly expressed in bone tissue trauma, and has an important
role in the proliferation and differentiation of osteoblasts. PDGF
promotes DNA synthesis in bone cells in early cell cycle stages,
that stimulate the osteoblast entering from the static state G0/G1
to replicative S phase, increase secretory phase cells, enhance
monocyte and fibroblast migration and induce local fibroblast
proliferation and differentiation, thereby promoting bone formation
(6). PDGF also promotes fibroblast
growth in vitro and has a mitogenic effect on cells that
originated from mesenchymal tissues, including osteoblasts, which
facilitate bone formation and stimulate bone resorption (7). Therefore, PDGF is an important factor
in accelerating fracture healing in traumatic brain injury and
fracture.
The specific mechanism of PDGF in promoting fracture
healing remains unclear. Previously, Ren et al (8) found that phosphorylation of the
G-protein-coupled receptor kinase interacting protein 1 (GIT1)
tyrosine 321 is required for focal adhesions and for PDGF-activated
migration of osteoblast association with FAK. GIT1 widely exists in
mammals and has an important role in cell growth and migration
(9). This demonstrates that PDGF
is able to promote osteoblast migration through activating GIT1;
however, there have been no studies, to the best of our knowledge,
investigating the proliferation and apoptosis of osteoblasts.
Therefore, the present study aimed to determine the effect of PDGF
and GIT1 on osteoblast proliferation and apoptosis.
Materials and methods
Cell culture and transfection
Primary osteoblastic cells were isolated from
newborn CD1 mice (Institute of Laboratory Animal Sciences, Chinese
Academy of Medical Sciences and Peking Union Medical College,
Beijing, China) calvaria by digestion with 0.1% collagenase type IA
and 0.2% dispase as previously described (10). The cells were cultured in
Dulbecco’s modified Eagle’s medium (Invitrogen Life Technologies,
Carlsbad, CA, USA) with 10% fetal bovine serum (FBS; Invitrogen
Life Technologies), 100 U/l penicillin and 100 μg/l streptomycin,
in a humidified atmosphere with 5% CO2 at 37°C. The
cells were passaged four times and used in the experiments. For
cell transfection, 1×105 cells were seeded in a 6-well
plate. GIT1 small interfering (si)RNA (specific for rat
GIT1;AAGCTGCCAAGAAGAAGCTAC) and negative control siRNA
(AATTCTCCGACACGTGTCACT) were designed as previously described
(11) and synthesized by Ambion
Life Technologies (Guangzhou, Guangdong, China). GIT1 siRNA was
prepared and transfected at 100 nM for 24 h as previously described
(12). Osteoblastic cells were
serum-deprived for 24 h prior to the experimental treatments, then
stimulated for 24 h with PDGF (10 ng/ml; Sigma-Aldrich, St. Louis,
MO, USA).
Western blot
The cultured cells were washed with
phosphate-buffered saline (PBS) and lysed on ice for 20 min in RIPA
buffer. Next, the lysates were centrifuged at 10,000 × g for 10 min
at 4°C. The supernatants were collected and frozen at −80°C or used
immediately. The protein concentrations were determined by a
bicinchoninic acid protein assay (Pierce Biotechnology, Inc.,
Rockford, IL, USA). A total of 40 μg protein of each sample was
heated for 10 min at 100°C. Next, the samples were analyzed by 12%
SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and
electroblotted onto nitrocellulose membranes. The membranes were
blocked in 5% non-fat milk for 1 h and then incubated with GIT1
antibody (a mouse monoclonal IgG1; Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA) overnight at 4°C. GIT1 antibody-treated
membranes were washed and incubated in the appropriate secondary
antibody (1:7,000; Santa Cruz Biotechnology, Inc.) at 37°C for 1 h.
Following this, immune complexes were detected using the enhanced
chemiluminescence kit (AppliChem, Darmstadt, Germany) and
immunoreactive proteins were visualized by the Odyssey infrared
imaging system (LI-COR Biosciences, Lincoln, NE, USA). The values
were corrected with the absorbency of the internal control
(GAPDH).
Cell proliferation analysis
The osteoblastic proliferation was measured by
performing a 5-ethynyl-20-deoxyuridine (EdU) incorporation assay
using an EdU Apollo DNA in vitro kit (Guangzhou RiboBio Co.,
Ltd., Guangzhou, China) according to the manufacturer’s
instructions. EdU (100 μl) was added to the cultured cells and the
cells were cultured for an additional 4 h at 37°C. The cells were
fixed with 4% formaldehyde for 15 min and treated with 0.5% Triton
X-100 for 20 min at room temperature for permeabilization.
Following washing with PBS three times, 100 μl of 1X ApolloH
reaction cocktail (Guangzhou RiboBio Co., Ltd.) was added to each
well and the cells were incubated for 30 min at room temperature.
Next, the cells were stained with 100 μl Hoechst33342 for 30 min
and visualized under a fluorescent microscope (Olympus Corporation,
Tokyo, Japan). The EdU positive cells were counted using Image-Pro
Plus 6.0 software (Media Cybernetics, Bethesda, MD, USA). All
experiments were conducted in triplicate and three independent
experiments were performed.
Flow cytometric analysis of the cell
cycle
Cell cycle analysis was performed by flow cytometry.
Briefly, the cultured cells were trypsinized into single cell
suspensions and fixed with 70% ethanol for 30 min on ice. The cells
were then stained with propidium iodide (Sigma-Aldrich). The
labeled cells were washed with PBS and then analyzed by FACS
Calibur flow cytometry (Becton-Dickinson, Franklin Lakes, NJ, USA)
equipped with the ModiFit LT v2.0 software (Phoenix Flow Systems,
San Diego, CA, USA). The proportions of cells in G0/G1 and S phases
were represented as DNA histograms. For each experiment, 10,000
events/sample were recorded.
Cell apoptosis analysis
For apoptosis analysis, terminal deoxynucleotidyl
transferase-mediated dUTP nick end labeling (TUNEL) assay was
performed on the cultured cells. The cells from each sample were
processed using the In situ cell Death Detection kit (Roche
Diagnostics, Hong Kong, China) according to the manufacturer’s
instructions. The adherent cells were grown on the slides, fixed
with 4% formaldehyde for 25 min at 4°C and treated with 0.2% Triton
X-100 for 5 min at room temperature for permeabilization. The cells
were equilibrated in the equilibration buffer for 10 min, then 50
μl TDT buffer was added and incubated at 37°C for 1 h in a
humidified chamber. The cells were washed with 2X SSC three times
for 10 min each and incubated with 0.3% H2O2
at room temperature for 10 min. Streptavidin was then used to
incubate the cells at room temperature for 30 min, stained with 0.5
μg/ml DAPI for 5 min at room temperature in the dark and visualized
under a fluorescent microscope (Olympus Corporation).
Statistical Analysis
All of the experiments were repeated three times.
The results are expressed as the mean ± standard deviation. The
data were analyzed using SPSS 17.0 software (SPSS, Inc., Chicago,
IL, USA). Comparisons between groups were analyzed using Student’s
t-test. P<0.05 was considered to indicate a statistically
significant difference.
Results
PDGF stimulates GIT1 expression in
osteoblasts
To determine the effects of PDGF on GIT1 expression,
GIT1 expression in rat osteoblasts in response to PDGF was
examined. Western blot analysis demonstrated that GIT1 expression
significantly increased in osteoblasts following 24 h stimulation
with PDGF compared with the non-PDGF group (control group; Fig. 1). In addition, GIT1-specific siRNA
(GIT1-siRNA) was designed and transferred into osteoblasts. In the
cells transfected with GIT1-siRNA, there was no significant
difference in the GIT1 expression in the PDGF-stimulated cells
compared with the control group.
GIT1 is required for PDGF-stimulated
osteoblast proliferation
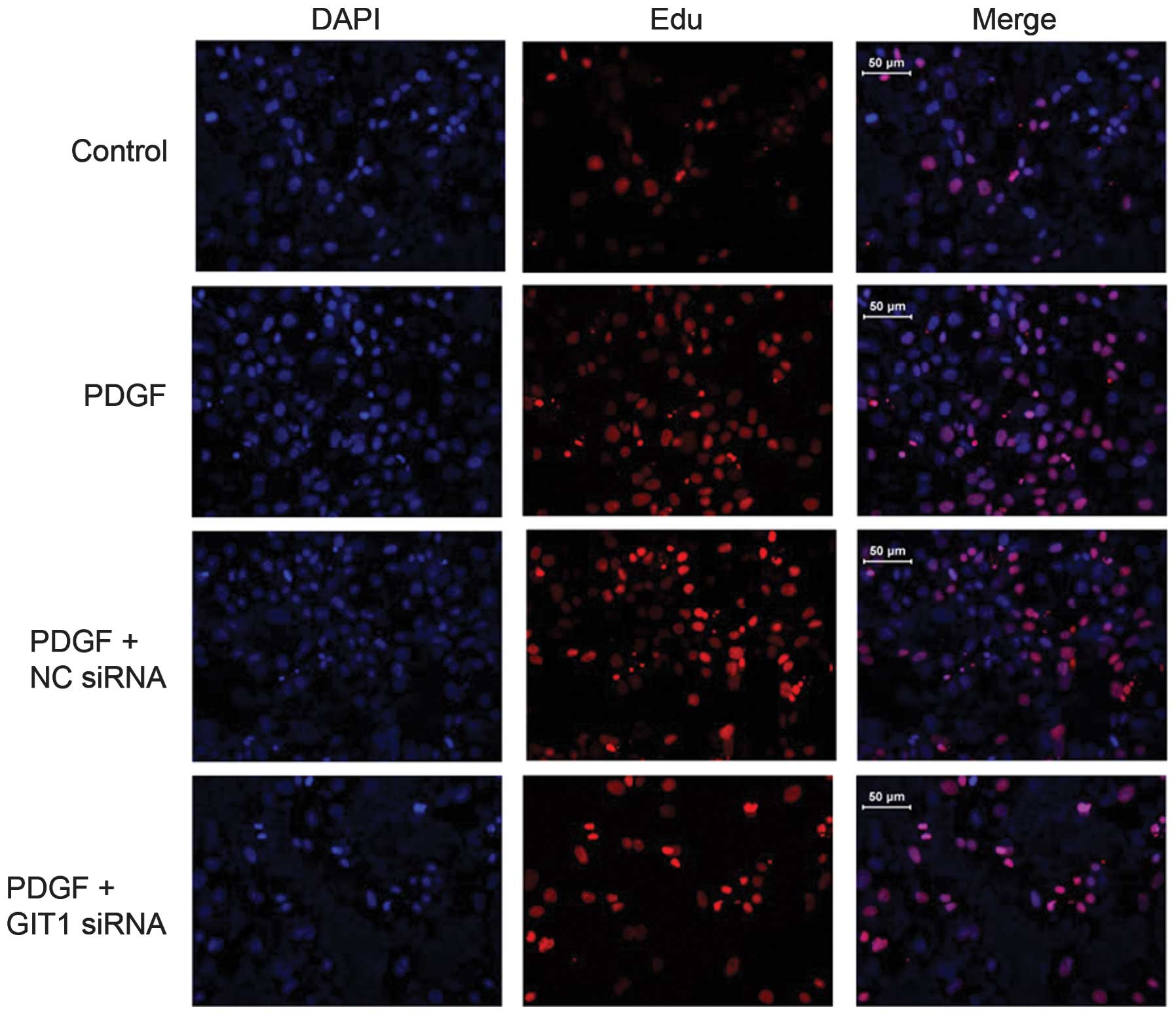
It is suggested that PDGF is an important cytokine
for fracture healing. To determine the function of PDGF, PDGF was
added in cultured osteoblasts from rats and the growth rate of
osteoblasts was examined using the EdU incorporation assay. The
growth rate of osteoblasts was significantly increased following
stimulation with PDGF compared with the control group (Fig. 2). The results suggested that PDGF
promoted osteoblast proliferation. As GIT1 expression was
significantly increased in osteoblasts following PDGF stimulation,
it was hypothesized that PDGF-mediated osteoblast proliferation
occurs via GIT1 activation. To provide evidence for the function of
GIT1 in osteoblast proliferation, the expression of GIT1 in
osteoblasts was examined using specific siRNAs. The results
demonstrated that cells transfected with GIT1-siRNA significantly
reduced the osteoblasts growth induced by PDGF, which suggested
that GIT1 is critical for PDFG-induced osteoblast proliferation.
However, the cells transfected with GIT1-siRNA that were stimulated
with PDGF proliferated faster than those in the control group,
which implied other downstream targets may be involved in
PDGF-stimulated osteoblast proliferation.
Effects of PDGF and GIT1 on osteoblasts
cell cycle
To investigate the mechanism of PDGF on the
proliferation of osteoblasts, the stages of the cell cycle that
were regulated by PDGF in osteoblasts were examined. As expected,
PDGF markedly increased the proportion of cells in S phase, with a
corresponding decrease in the number of cells in the G0/G1 phase
(Fig. 3) compared with the control
group, while PDGF-induced cells transfected with GIT1-siRNA had no
significant change in cell cycle stage. These results indicate that
the knockdown of GIT1 inhibited osteoblast transition from the
stationary phase (G0/G1 phase) to the replicative phase (S phase).
Therefore, PDGF promotes osteoblast proliferation possibly through
the activation of GIT1.
PDGF inhibits osteoblast apoptosis
through upregulating GIT1 expression
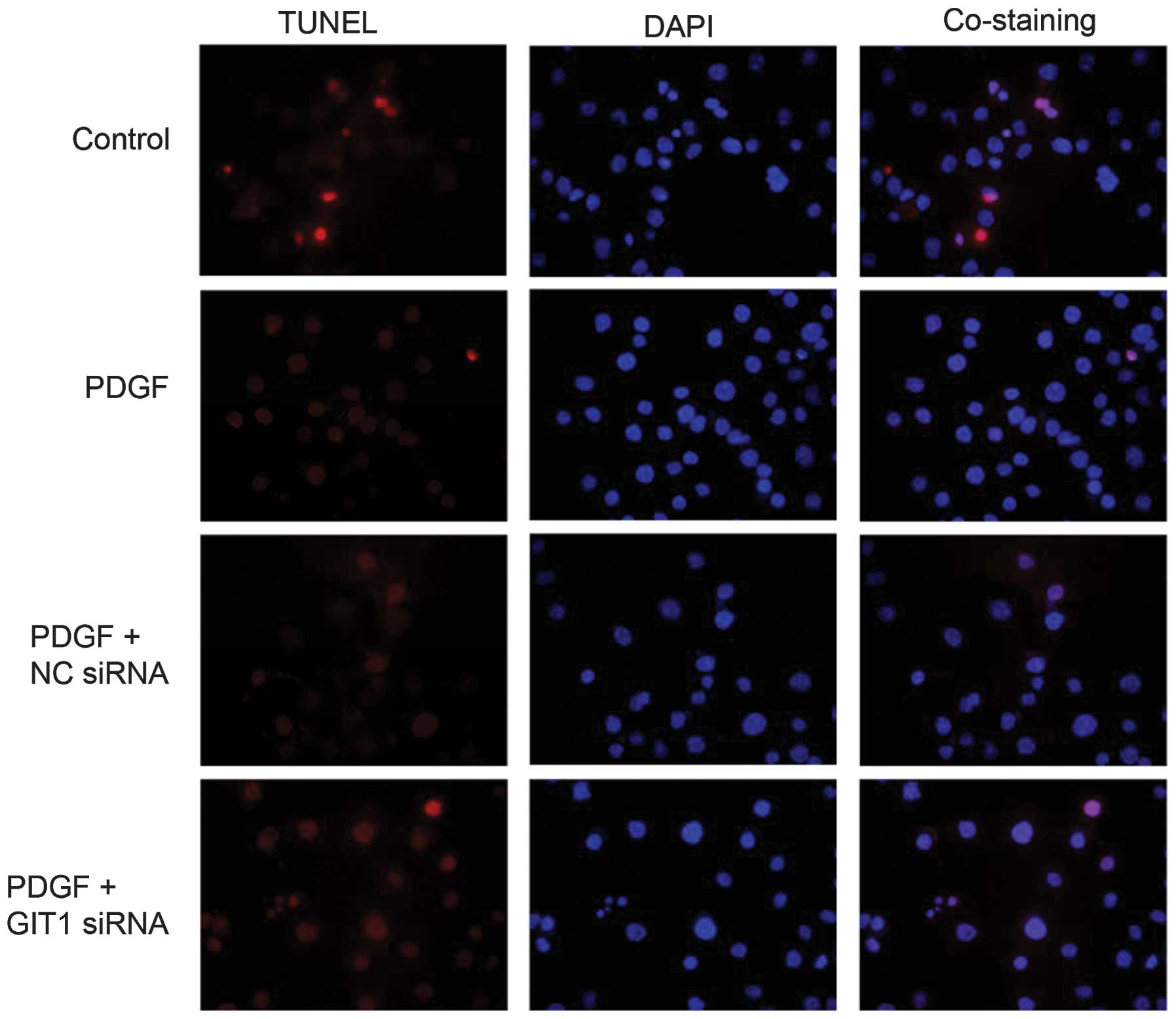
To elucidate the role of PDGF and GIT1 in osteoblast
apoptosis, a TUNEL assay was utilized to examine the effect of PDGF
for osteoblast apoptosis. PDGF-induced osteoblast apoptosis
significantly decreased compared with the non-PDGF cells (Fig. 4). These data demonstrated that PDGF
was able to inhibit osteoblast apoptosis. No significant decrease
in apoptosis was identified in the cells transfected with
GIT1-siRNA when stimulated by PDGF. These results suggest that
knockdown of GIT1 expression inhibited the anti-apoptotic function
of PDGF in osteoblasts.
Discussion
PDGF is secreted by platelets during the early
phases of fracture-healing and has been identified to have pivotal
roles in the fracture healing process. Andrew et al
(5) and Horner et al
(7) demonstrated the widespread
expression of PDGF-A chain and PDGF-α receptor in human bone
formation, using in situ hybridization and
immunohistochemistry, and indicated that this growth factor may
exert autocrine and paracrine effects to regulate osteogenesis
during skeletal development. PDGF was able to stimulate osteoblast
and chondrocyte proliferation, promoting cartilage formation and
intramembranous ossification in callus, the mRNA expression of
which was highest in callus in the late stages of fracture
(11). Therefore, it is important
to investigate the specific mechanism of action of PDGF on
osteoblast proliferation and understand the molecular mechanisms
involved in fracture healing.
In the present study, it was demonstrated that GIT1
expression was significantly increased in osteoblasts following
stimulation with PDGF, suggesting that PDGF was able to promote
GIT1 expression. GIT1 is a multidomain scaffold protein expressed
in various cell types, including neurons, endothelial cells and
vascular smooth muscle cells. GIT1 is rapidly emerging as a
scaffolding protein that has multiple binding domains for several
different molecules, including ARF, Rac1, Cdc42, GTPases, PAK, PIX,
MEK1 and PLC (12). In this
manner, GIT1, through its function as an adaptor protein, is able
to regulate protrusive activity and cell migration (13). A study demonstrated that GIT1 was
expressed in osteoblasts and osteoclasts (14), and may participate in bone
metabolism. GIT1 knockdown in mice resulted in increased bone mass
due to disorganized podosome belt formation in osteoclasts
resulting in impaired bone resorption, by affecting RANK signaling
pathways (15). It has been
demonstrated that the GIT1Y321F mutation inhibited PDGF-induced
osteoblastic cell migration. Phosphorylation of tyrosine 321 of
GIT1 is required for PDGF-induced association with FAK activation
in focal adhesions for osteoblastic cell migration (8). The aforementioned studies demonstrate
that the mechanism underlying GIT protein regulation of bone
metabolism involves adjusting the dynamic changes of the
cytoskeleton and acting as a scaffold protein involved in signal
transduction.
In the present study, the effect of PDGF on rat
primary osteoblasts was examined and it was demonstrated that PDGF
induced a marked increase in cell growth and a decrease in the
apoptosis of osteoblasts. Lee et al (15) revealed that the PDGF-BB releasing
molded PLLA-TCP (porous poly (L-lactide)-tricalcium phosphate)
membrane was demonstrated to be effective for continuous
stimulation of bone regeneration when injected into rabbits.
PDGF-BB may induce bone regeneration at early stages, in
combination with a space making PLLA-TCP guide membrane. Knockdown
of GIT1 expression resulted in no evident changes in the cell
proliferation and apoptosis following PDGF stimulation, indicating
that knockdown of GIT1 inhibited the cell proliferation of PDGF
stimulated osteoblasts. This is consistent with an earlier study,
which demonstrated that GIT1 may promote the cell proliferation and
inhibit cell apoptosis (16).
Thus, the results implicated that PDGF may efficiently promote the
cell proliferation of osteoblasts through activating GIT1. However,
the cells transfected with GIT1-siRNA that were stimulated with
PDGF grew faster than the control group, which implied other
downstream targets were involved in PDGF-stimulated osteoblasts
proliferation as well as GIT1.
An analysis of the cell cycle distribution in
PDGF-treated osteoblasts using flow cytometry revealed that PDGF
treatment increased the number of cells in the S phase and
decreased the cell numbers in the G0/G1 phase. This indicated that
PDGF promoted cell entry into the S phase in osteoblasts and that
the cyclin D1 protein level was increased following treatment with
PDGF. However, effects of PDGF on the cell cycle and the cyclin D1
protein level exhibited no significant difference compared with
that of the cells transfected with GIT1-siRNA. Yang et al
(6) demonstrated that in
osteoblasts pretreated by PDGF-AA for 24 h, G0/G1 phase was
decreased and the number of cells in S and G2-M phases increased.
PDGF-A mRNA may be expressed by human fetal osteoblasts and the
level of its expression is enhanced by PDGF-AA and PDGF-BB. It is
suggested PDGF-AA enhances cell replication by accelerating the
cell cycle and inducing the quiescent cells into the proliferation
phase of the cell cycle to modulate the fetal bone formation.
Cyclin D1, which belongs to the D group of cyclins,
is expressed in all mammalian cells, except lymphocytes and myeloid
cells. It varies in abundance with the cell cycle and peaks mid-G1
phase (17). In a number of cell
types, cyclin D1 is rate-limiting for cell cycle progression and
its abundance varies with the cell cycle (18,19).
In the present study it was demonstrated that activation of GIT1
was required for PDGF to upregulate cyclin D1 and stimulated cell
cycle progression. A study demonstrated that GIT1 has an important
role in cell proliferation and apoptosis, which is essential for
maintaining normal postnatal mitochondrial integrity and cardiac
function (20). GIT1 and GIT2,
binds to the CTT (COOH-terminal tail) of IP3R and inhibits
apoptosis by regulation of the IP3R-mediated Ca2+ signal
(21). Furthermore, PDGF increased
GIT1 tyrosine phosphorylation in osteoblasts and this response was
observed for PDGF treatments up to 6 h after treatment and began to
decline there after. GIT1 is the key phosphorylation site mediating
PDGF-induced ERK1/2 activity, ERK1/2-GIT1 interactions and VEGF
mRNA expression in osteoblasts (22). This implied that GIT1 may be a more
potent downstream factor to mediate the PDGF proliferation effects
in osteoblasts.
In conclusion, these findings extend the
understanding of the crucial role of PDGF in osteoblast
proliferation and provide insights into the novel regulatory
relationship between PDGF and GIT1.
References
|
1
|
Huang W, Li Z, Li Z and Yang R: Does
traumatic brain injury result in accelerated mandibular fracture
healing? J Oral Maxillofac Surg. 70:2135–2142. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Onodera S, Nishihira J, Yamazaki M,
Ishibashi T and Minami A: Increased expression of macrophage
migration inhibitory factor during fracture healing in rats.
Histochem Cell Biol. 121:209–217. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Iihara K, Sasahara M, Hashimoto N, Uemura
Y, Kikuchi H and Hazama F: Ischemia induces the expression of the
platelet-derived growth factor-B chain in neurons and brain
macrophages in vivo. J Cereb Blood Flow Metab. 14:818–824. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Takayama S, Sasahara M, Iihara K, Handa J
and Hazama F: Platelet-derived growth factor B-chain-like
immunoreactivity in injured rat brain. Brain Res. 653:131–140.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Andrew JG, Hoyland JA, Freemont AJ and
Marsh DR: Platelet-derived growth factor expression in normally
healing human fractures. Bone. 16:455–460. 1995.PubMed/NCBI
|
|
6
|
Yang D, Chen J, Jing Z and Jin D:
Platelet-derived growth factor (PDGF)-AA: a self-imposed cytokine
in the proliferation of human fetal osteoblasts. Cytokine.
12:1271–1274. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Horner A, Bord S, Kemp P, Grainger D and
Compston JE: Distribution of platelet-derived growth factor (PDGF)
A chain mRNA, protein, and PDGF-alpha receptor in rapidly forming
human bone. Bone. 19:353–362. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ren Y, Yu L, Fan J, et al: Phosphorylation
of GIT1 tyrosine 321 is required for association with FAK at focal
adhesions and for PDGF-activated migration of osteoblasts. Mol Cell
Biochem. 365:109–118. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Manabe R, Kovalenko M, Webb DJ and Horwitz
AR: GIT1 functions in a motile, multi-molecular signaling complex
that regulates protrusive activity and cell migration. J Cell Sci.
115:1497–1510. 2002.
|
|
10
|
Inoue D, Santiago P, Horne WC and Baron R:
Identification of an osteoclast transcription factor that binds to
the human T cell leukemia virus type I-long terminal repeat
enhancer element. J Biol Chem. 272:25386–25393. 1997. View Article : Google Scholar
|
|
11
|
Park YJ, Ku Y, Chung CP and Lee SJ:
Controlled release of platelet-derived growth factor from porous
poly(L-lactide) membranes for guided tissue regeneration. J Control
Release. 51:201–211. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hsu RM, Tsai MH, Hsieh YJ, Lyu PC and Yu
JS: Identification of MYO18A as a novel interacting partner of the
PAK2/betaPIX/GIT1 complex and its potential function in modulating
epithelial cell migration. Mol Biol Cell. 21:287–301. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gavina M, Za L, Molteni R, Pardi R and de
Curtis I: The GIT-PIX complexes regulate the chemotactic response
of rat basophilic leukaemia cells. Biol Cell. 102:231–244. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Menon P, Yin G, Smolock EM, Zuscik MJ, Yan
C and Berk BC: GPCR kinase 2 interacting protein 1 (GIT1) regulates
osteoclast function and bone mass. J Cell Physiol. 225:777–785.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee SJ, Park YJ, Park SN, et al: Molded
porous poly (L-lactide) membranes for guided bone regeneration with
enhanced effects by controlled growth factor release. J Biomed
Mater Res. 55:295–303. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pang J, Xu X, Wang X, et al:
G-protein-coupled receptor kinase interacting protein-1 mediates
intima formation by regulating vascular smooth muscle
proliferation, apoptosis, and migration. Arterioscler Thromb Vasc
Biol. 33:999–1005. 2013. View Article : Google Scholar
|
|
17
|
Baldin V, Lukas J, Marcote MJ, Pagano M
and Draetta G: Cyclin D1 is a nuclear protein required for cell
cycle progression in G1. Genes Dev. 7:812–821. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xiong W, Pestell RG, Watanabe G, Li J,
Rosner MR and Hershenson MB: Cyclin D1 is required for S phase
traversal in bovine tracheal myocytes. Am J Physiol.
272:L1205–L1210. 1997.PubMed/NCBI
|
|
19
|
Beier F, Lee RJ, Taylor AC, Pestell RG and
LuValle P: Identification of the cyclin D1 gene as a target of
activating transcription factor 2 in chondrocytes. Proc Natl Acad
Sci USA. 96:1433–1438. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pang J, Xu X, Getman MR, et al: G protein
coupled receptor kinase 2 interacting protein 1 (GIT1) is a novel
regulator of mitochondrial biogenesis in heart. J Mol Cell Cardiol.
51:769–776. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang S, Hisatsune C, Matsu-Ura T and
Mikoshiba K: G-protein-coupled receptor kinase-interacting proteins
inhibit apoptosis by inositol 1,4,5-triphosphate receptor-mediated
Ca2+ signal regulation. J Biol Chem. 284:29158–29169.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rui Z, Li X, Fan J, et al: GIT1Y321
phosphorylation is required for ERK1/2- and PDGF-dependent VEGF
secretion from osteoblasts to promote angiogenesis and bone
healing. Int J Mol Med. 30:819–825. 2012.PubMed/NCBI
|