Introduction
Gliomas are the most common primary tumor type of
the central nervous system and have a poor prognosis (1). In a meta-analysis of 12 randomized
clinical trials, the overall 1-year survival rate of high-grade
gliomas, such as glioblastomas and anaplastic astrocytomas, was
40%, and only slightly higher (46%) following combined radiotherapy
and chemotherapy (2). Current
clinical treatment of glioma includes radiotherapy, chemotherapy
and surgical operation (3).
However, an increasing number of studies have shown that the
resistance of glioma cells to conventional drugs is becoming a
challenging issue. Therefore, it is urgent to develop new and
effective therapeutic methods for the treatment of gliomas
(4).
The histone deacetylase (HDAC) family contains a
total of 18 proteins, grouped into classes I–IV based on their
homology and structure. Classes I, II and IV contain 11 family
members, which are called classical HDACs, whereas the 7 members of
the class III are referred to as sirtuins (5,6).
HDAC5 belongs to the class II, and has been shown to constitute an
important regulator of cell proliferation, cell-cycle progression
and apoptosis (7,8). Additional members of the HDAC family
were demonstrated to play critical roles in tumor initiation,
proliferation and metastasis, such as SIRT6 (9). A number of HDAC inhibitors possess
the ability to promote apoptosis and inhibit proliferation in tumor
cells and animal models (10). In
addition, a clinical study previously highlighted the potential of
HDAC inhibitors to be used as anticancer agents (11). However, the biological function of
HDAC5 in human glioma has never been investigated to date.
Therefore, in the present study, we examined the expression of
HDAC5 in human glioma samples and further investigated its
biological function.
Materials and methods
Tissue samples and ethics
Glioma and distant healthy tissues were collected
from therapeutic surgery at the Department of Neurosurgery, the
Fourth Affiliated Hospital of Guangxi Medical University (Guangxi,
China). All samples were obtained with informed consent from the
participants, and this study was approved by the Institutional
Review Board of the Fourth Affiliated Hospital of Guangxi Medical
University.
Cell culture
The human glioma cell lines U87 and LN-229 were
obtained from the Shanghai Institute of Cell Biology, Chinese
Academy of Sciences (Shanghai, China). Cells were cultured in
Dulbecco’s modified Eagle’s medium supplemented with 10% fetal
bovine serum, streptomycin (100 mg/ml) and penicillin (100 U/ml).
Cultured cells were maintained at 37°C and 5% CO2 in a
humid environment, and were passaged when the confluency had
reached 80%. Healthy human astrocytes were cultured in astrocyte
medium (Dulbecco’s modified Eagle’s medium/F12 supplemented with
10% fetal bovine serum, Gibco Laboratories, Grand Island, NY).
Plasmid construction, small interfering
RNA (siRNA) and transfection
The complementary DNA (cDNA) fragment encoding HDAC5
was obtained by reverse transcription (RT) performed on total RNA
extracted from the glioma cell line U87, using the Takara RNA PCR
kit reagents (Takara Bio Inc., Tokyo, Japan). RNA extraction was
performed using the TRIzol reagent (Invitrogen Life Technologies,
Carlsbad, CA, USA) following the manufacturer’s instructions. The
cDNA was then amplified by polymerase chain reaction (PCR) using
the following primers: forward, 5′-GGA ATT CAT GAA GTT GGA GGT GTT
CGT C-3′, and reverse, 5′-CCT CGA GCG CTA CTC AGG CTA GGA GCG TCT
CCA C-3′.
The PCR product was cloned into the mammalian
expression vector pcDNA3.1 (+)-flag (Invitrogen Life Technologies).
Two independent oligos targeting HDAC5 (siRNA-HDAC5), siRNA Notch 1
and scrambled control siRNA were purchased from Invitrogen Life
Technologies. Cells were transfected with lipofectamine 2000
(Invitrogen Life Technologies) according to the manufacturer’s
instructions.
Quantitative (q)PCR
In order to quantify the transcripts of the genes of
interest, qPCR was performed using the SYBR® Premix Ex
Taq™ kit (Takara Bio Inc.) on an Applied Biosystems® ABI
7500 system (Thermo Fisher Scientific). The cycling parameters were
as follows: an initial stage of 95°C for 30 sec, followed by a two
step program of 95°C for 5 sec and 60°C for 31 sec over 40 cycles,
performed in triplicate. The qPCR data were analyzed using the
2−ΔΔCt method (12).
MTT assay
Proliferation of cells was determined using an MTT
assay (Sigma, St. Louis, MO, USA). Briefly, the glioma cells
transfected with indicated oligonucleotides were seeded into
96-well plates at a density of 3×104 (cells/well).
Subsequently, 10 ml of 5 mg/ml MTT was added and incubated in the
dark at 37°C for 2 h. The absorbance was determined at a wavelength
of 490 nm using a BioRad microplate reader (FluoDia T70; Photon
Technology International, Inc., Lawrenceville, NJ, USA).
Western blotting
Cells were harvested by trypsinization and lysed in
buffer (Beyotime Institute of Biotechnology, Shanghai, China). The
proteins were then subjected to sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and transferred onto a
polyvinylidene fluoride membrane (Millipore, Billerica, MA, USA).
The membranes were blocked in phosphate-buffered saline (PBS)/0.1%
Tween-20 with 5% nonfat dry milk and then incubated with primary
antibodies in PBS/0.1% Tween-20 with 0.1–5% nonfat dry milk.
Antibodies directed against HDAC5 (rabbit polyclonal, dilution
1:2,000), Notch 1 (rabbit polyclonal, dilution 1:2,000) and
glyceraldehyde 3-phosphate dehydrogenase (GAPDH; mouse monoclonal,
dilution 1:5,000) were purchased from Santa Cruz Biotechnology,
Inc. (Santa Cruz, CA, USA) with GAPDH used as a loading
control.
Statistical analysis
All data were expressed as mean ± standard error of
the mean, and were analyzed using the SPSS software (IBM, Armonk,
NY, USA). P-values (P) for the comparisons between groups were
determined by analysis of variance (ANOVA), with P<0.05
considered to indicate statistically significant differences.
Results
HDAC5 expression is increased in human
glioma tissues and cells
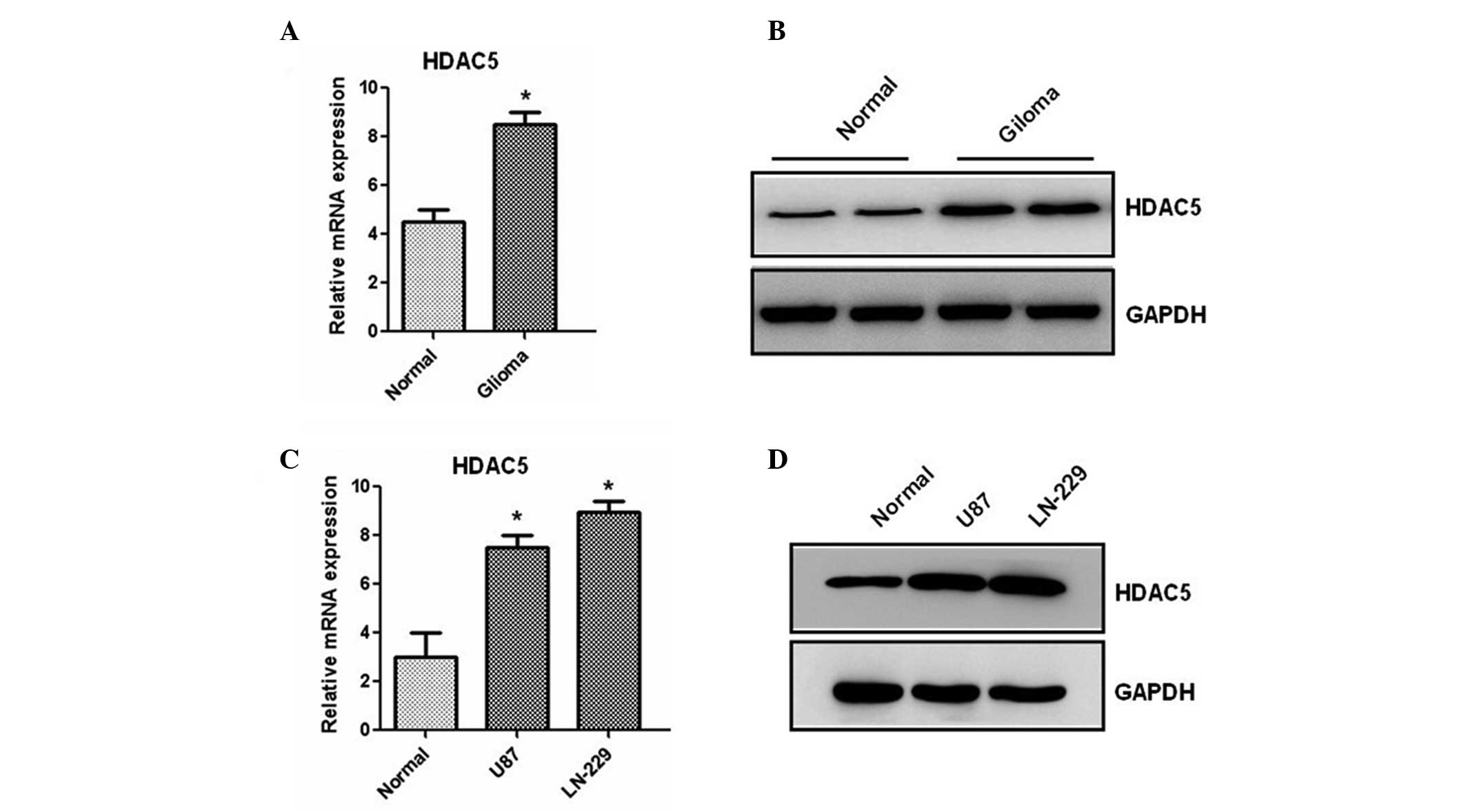
We first analyzed the expression of the HDAC5
gene in 20 paired glioma and adjacent non-tumor healthy tissues by
RT-qPCR. Results showed that the mRNA level of HDAC5 is
significantly increased in human cancer compared to healthy tissues
(Fig. 1A). Then, western blotting
was used to determine the protein expression of HDAC5. We found
that the protein level of HDAC5 is also increased in glioma samples
(Fig. 1B). Furthermore, two glioma
cell lines were analyzed by RT-qPCR and western blotting. As shown
in Fig. 1C and D, higher
expression of HDAC5, at both the mRNA and the protein level, was
observed in the two lines compared to healthy human astrocytes,
suggesting that HDAC5 expression is upregulated in glioma tissues
and cell lines.
HDAC5 overexpression promotes
proliferation of glioma cells
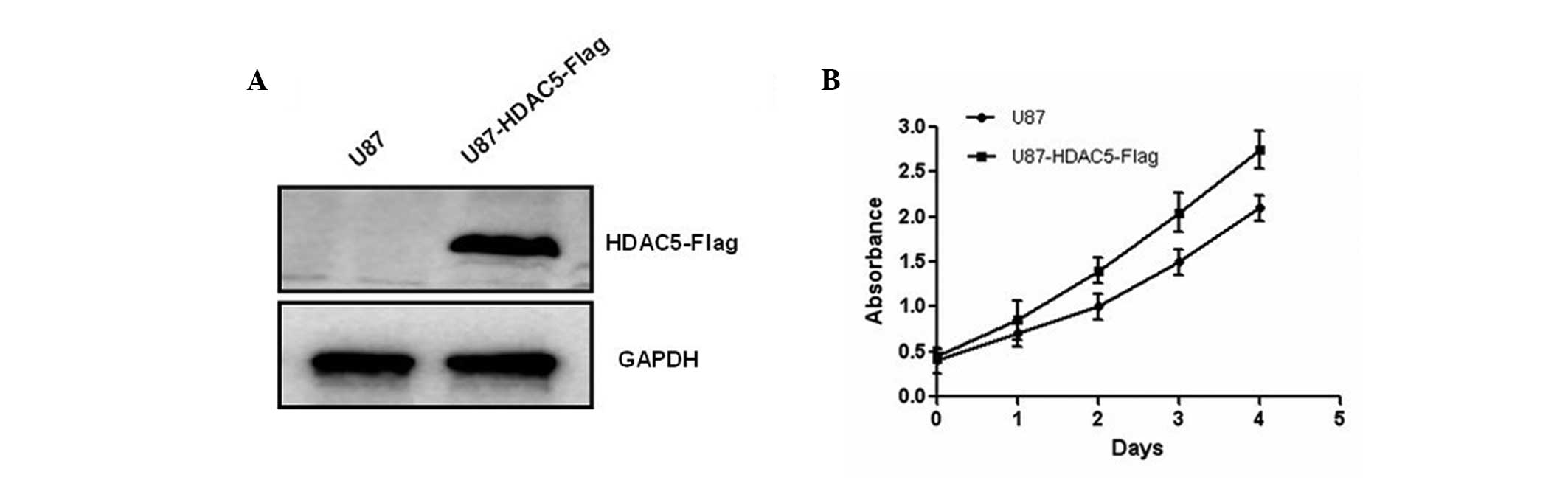
To further investigate the biological role of HDAC5
in human glioma, U87 cells were transfected with a plasmid
containing the HDAC5 gene, which allowed its overexpression
(Fig. 2A). Results of the MTT
assay further showed that HDAC5 overexpression promotes glioma cell
proliferation (Fig. 2B). In
addition, U87 cells were transfected with siRNA targeting
HDAC5. Efficient silencing of HDAC5 was achieved with
two independent siRNA oligos compared to scramble siRNA-transfected
U87 cells (Fig. 3A and B).
Silencing of HDAC5 in these cells inhibited cell
proliferation (Fig. 3C). Similar
results were also observed in LN-229 cells (data not shown). Taken
together, our results demonstrated that HDAC5 promotes the
proliferation of glioma cells.
HDAC5 promotes glioma cell proliferation
via Notch 1 upregulation
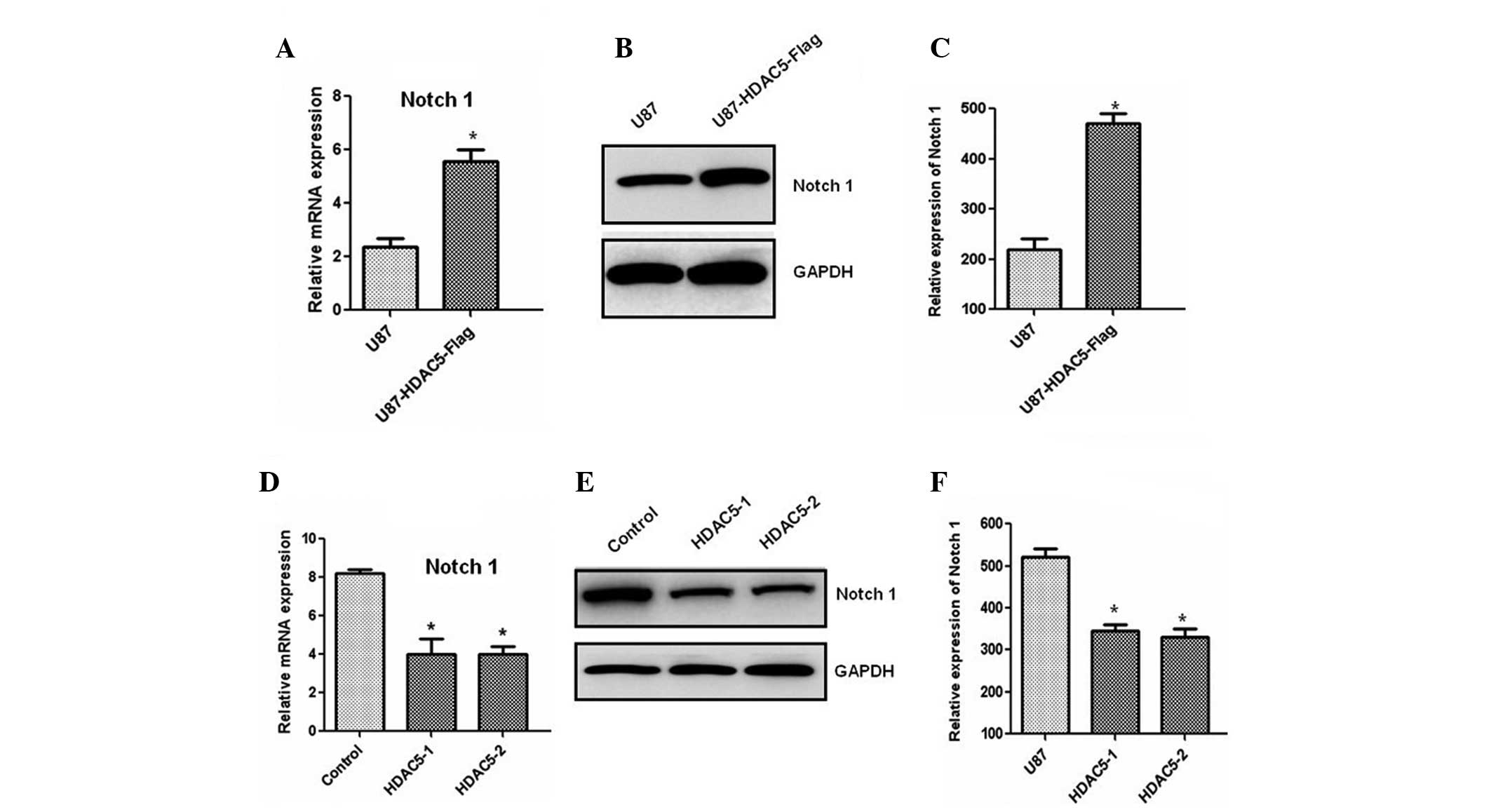
Next, we investigated the molecular mechanism
underlying the effect of HDAC5 on cell proliferation. The results
demonstrated that the Notch 1 mRNA level was significantly
increased in the cells overexpressing HDAC5 (Fig. 4A). An increase in the Notch 1
protein level was also observed by western blotting (Fig. 4B and C). Consistent with these
results, silencing of the HDAC5 gene markedly reduced Notch
1 expression at the mRNA (Fig. 4D)
and the protein level (Fig. 4E and
F). These results indicated that HDAC5 may act as an upstream
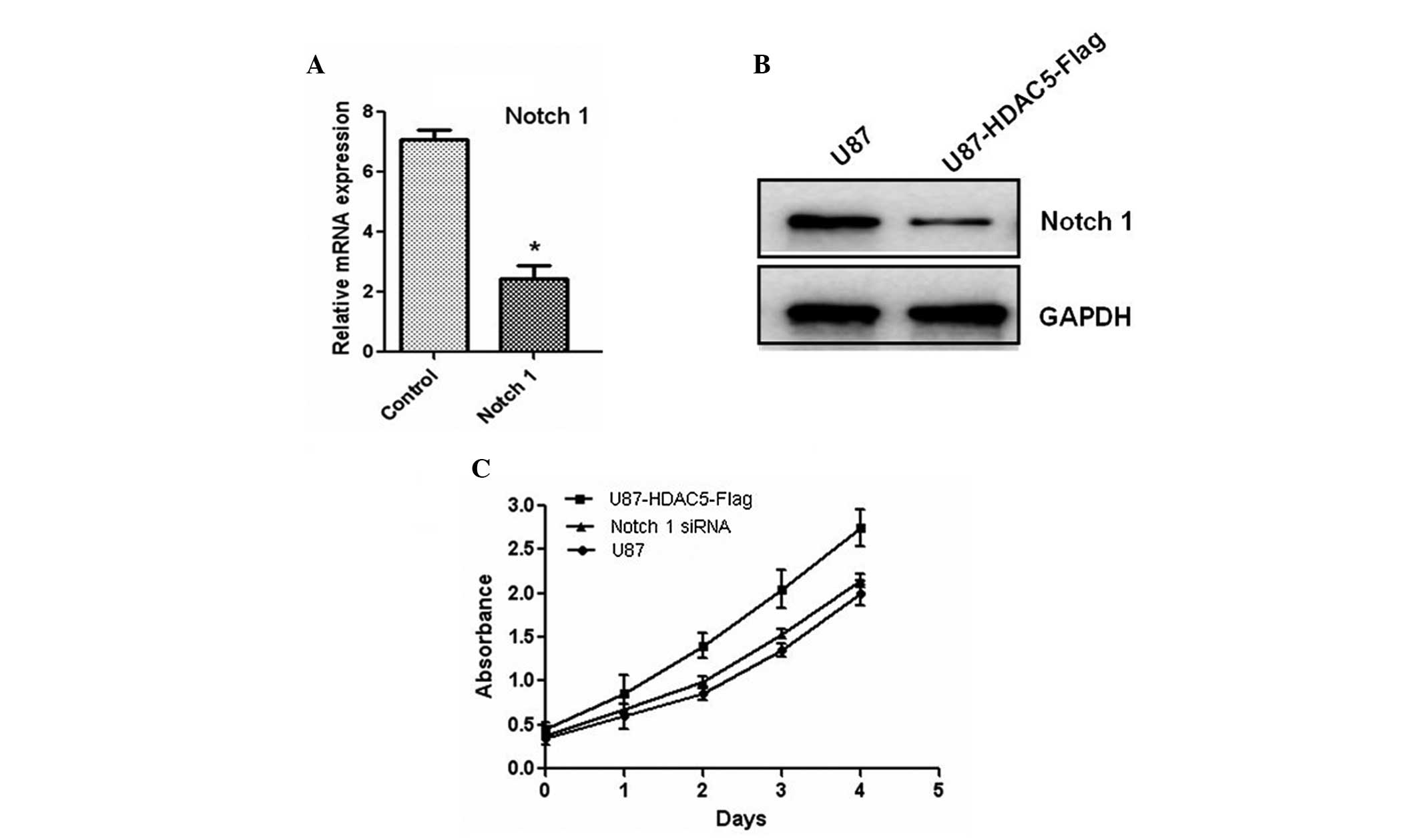
regulator of Notch 1. Furthermore, the Notch 1 gene was
silenced using siRNA oligos, and the protein levels of Notch 1 were
also reduced (Fig. 5A and B). The
decreased expression of Notch 1 attenuated the effect of HDAC5 on
glioma cell proliferation (Fig.
5C). Taken together, these results indicated that HDAC5
promotes cell proliferation by upregulating Notch 1.
Discussion
It has been reported that certain members of the
HDAC family play critical roles in promoting carcinogenesis
(9). However, the biological
function of HDAC5 in glioma cells remains poorly understood. In the
current study, the biological function of HDAC5 was investigated in
glioma tissues and cell lines for the first time, to the best of
our knowledge.
HDACs remove the acetyl groups from the
N-acetylly-sines on a histone and modify the chromatin structure,
therefore modulating the expression levels of numerous genes. Their
aberrant expression is closely related to tumor initiation and
development (13). In our study,
we found that both the mRNA and protein expression of HDAC5 were
significantly increased in glioma tissues and cell lines.
The class II HDAC family member HDAC5 has been shown
to be an important regulator of cell-cycle progression,
proliferation and apoptosis in numerous cancer cell lines and
animal models (14). For example,
HDAC5 was shown to translocate from the nucleus to the cytoplasm
during myoblast differentiation and suppress the expression of the
cell-cycle activator cyclin D3, confirming its involvement in cell
differentiation and proliferation (15,16).
A recent study demonstrated that HDAC5 and HDAC9 are significantly
upregulated in high-risk medulloblastoma in comparison to low-risk
medulloblastoma samples, and that their expression is associated
with poor survival, indicating that HDAC5 and HDAC9 may be valuable
markers for risk stratification (17). Another study reported that HDAC5
upregulates Twist 1, and revealed a so-far unknown link between
HDAC5 and osteosarcoma progression (18).
In our study, overexpression of HDAC5 enhanced
glioma cell proliferation. In addition, HDAC5 silencing
using siRNA inhibited glioma cell proliferation, indicating that
HDAC5 may be a positive regulator of glioma cell proliferation.
The Notch family of proteins plays an important role
in cell proliferation, differentiation and apoptosis. The Notch 1
protein shares structural features with other members of this
family, such as the presence of an extracellular domain consisting
of multiple epidermal growth factor-like repeats and an
intracellular domain consisting of multiple, different domain types
(19). A number of studies have
shown that Notch 1 is overexpressed in cancer cell lines, including
gliomas (20). Downregulation of
Notch 1 and its ligands by RNA interference induces apoptosis and
inhibits proliferation in multiple glioma cell lines. Therefore,
Notch 1 has been proposed to constitute a promising therapeutic
target in the treatment of glioma (21,22).
Our study found that HDAC5 significantly increases the expression
of Notch 1. Furthermore, Notch 1 silencing attenuated the
proliferative effect of HDAC5 on glioma cells.
In conclusion, our study demonstrated that HDAC5
promotes the proliferation of glioma cells via upregulation of
Notch 1 and may provide novel therapeutic targets in the treatment
of gliomas.
References
|
1
|
Medina Villaamil V, Alvarez García A,
Aparicio Gallego G, et al: Tissue array analysis for the
differentiation of gliosis from gliomas. Mol Med Rep. 4:451–457.
2011.PubMed/NCBI
|
|
2
|
Stewart LA: Chemotherapy in adult
high-grade glioma: a systematic review and meta-analysis of
individual patient data from 12 randomised trials. Lancet.
359:1011–1018. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang Y, Zhou Z, Luo H, et al: Combination
of tamoxifen and antisense human telomerase RNA inhibits glioma
cell proliferation and anti-apoptosis via suppression of telomerase
activity. Mol Med Rep. 3:935–940. 2010.PubMed/NCBI
|
|
4
|
Lefranc F, Facchini V and Kiss R:
Proautophagic drugs: a novel means to combat apoptosis-resistant
cancers, with a special emphasis on glioblastomas. Oncologist.
12:1395–1403. 2007. View Article : Google Scholar
|
|
5
|
Witt O, Deubzer HE, Milde T and Oehme I:
HDAC family: What are the cancer relevant targets? Cancer Lett.
277:8–21. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
de Ruijter AJ, van Gennip AH, Caron HN,
Kemp S and van Kuilenburg AB: Histone deacetylases (HDACs):
characterization of the classical HDAC family. Biochem J.
370:737–749. 2003.PubMed/NCBI
|
|
7
|
Voelter-Mahlknecht S, Ho AD and Mahlknecht
U: Chromosomal organization and localization of the novel class IV
human histone deacetylase 11 gene. Int J Mol Med. 16:589–598.
2005.PubMed/NCBI
|
|
8
|
Sen N, Kumari R, Singh MI and Das S:
HDAC5, a key component in temporal regulation of p53-mediated
transactivation in response to genotoxic stress. Mol Cell.
52:406–420. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Witt O, Deubzer HE, Milde T and Oehme I:
HDAC family: What are the cancer relevant targets? Cancer Lett.
277:8–21. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wiegmans AP, Alsop AE, Bots M, et al:
Deciphering the molecular events necessary for synergistic tumor
cell apoptosis mediated by the histone deacetylase inhibitor
vorinostat and the BH3 mimetic ABT-737. Cancer Res. 71:3603–3615.
2011. View Article : Google Scholar
|
|
11
|
Munster P, Marchion D, Bicaku E, et al:
Clinical and biological effects of valproic acid as a histone
deacetylase inhibitor on tumor and surrogate tissues: phase I/II
trial of valproic acid and epirubicin/FEC. Clin Cancer Res.
15:2488–2496. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
|
|
13
|
Ronsch K, Jager M, Schopflin A, Danciu M,
Lassmann S and Hecht A: Class I and III HDACs and loss of active
chromatin features contribute to epigenetic silencing of CDX1 and
EPHB tumor suppressor genes in colorectal cancer. Epigenetics.
6:610–622. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Khan O and La Thangue NB: HDAC inhibitors
in cancer biology: emerging mechanisms and clinical applications.
Immunol Cell Biol. 90:85–94. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Roy S, Shor AC, Bagui TK, Seto E and
Pledger WJ: Histone deacetylase 5 represses the transcription of
cyclin D3. J Cell Biochem. 104:2143–2154. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
McKinsey TA, Zhang CL, Lu J and Olson EN:
Signal-dependent nuclear export of a histone deacetylase regulates
muscle differentiation. Nature. 408:106–111. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Milde T, Oehme I, Korshunov A, et al:
HDAC5 and HDAC9 in medulloblastoma: novel markers for risk
stratification and role in tumor cell growth. Clin Cancer Res.
16:3240–3252. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen J, Xia J, Yu YL, et al: HDAC5
promotes osteosarcoma progression by upregulation of Twist 1
expression. Tumour Biol. 35:1383–1387. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rana NA and Haltiwanger RS: Fringe
benefits: functional and structural impacts of O-glycosylation on
the extracellular domain of Notch receptors. Curr Opin Struct Biol.
21:583–589. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ayukawa T, Matsumoto K, Ishikawa HO, et
al: Rescue of Notch signaling in cells incapable of GDP-L-fucose
synthesis by gap junction transfer of GDP-L-fucose in
Drosophila. Proc Natl Acad Sci USA. 109:15318–15323. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hu B, Nandhu MS, Sim H, et al: Fibulin-3
promotes glioma growth and resistance through a novel paracrine
regulation of Notch signaling. Cancer Res. 72:3873–3885. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Berry N, Gursel DB and Boockvar JA: Notch
inhibition via micro-RNA blocks glioma development. Neurosurgery.
70:N20–N22. 2012. View Article : Google Scholar : PubMed/NCBI
|