Introduction
Femoral head necrosis or osteonecrosis of femoral
head (ONFH), also known as avascular necrosis, is jointly caused by
a variety of disorders of the femoral head blood supply, causing
subchondral bone degeneration and necrosis, thus leading to femoral
head collapse and eventually, hip joint degeneration (1). The disease occurs mainly in young
adults aged 35–55. If untreated, 70% of the patients suffer from
femoral head collapse and 3–4 years later total hip replacement is
required (2). Therefore, early
diagnosis and treatment are key elements in ONFH treatment. In
recent years, a variety of osteonecrotic disorders have been
associated with genetic polymorphisms, and a high correlation
between the incidence of ONFH and these polymorphisms was revealed
(3,4). Identification of these polymorphisms
by genetic screening before the appearance of clinical symptoms
would allow early prevention, diagnosis and treatment of ONFH in
vulnerable populations.
The COL2A1 gene encodes collagen type II α1,
a major extracellular matrix component. Type II collagen, also
known as cartilage collagen, is the major collagen of cartilage
cells. The first step in the synthesis of type II collagen involves
three pro-α1 (II) chains twisting together to form a
triple-stranded, rope-like procollagen molecule; this procollagen
chain contains the N- and C-terminal propeptide of the mature amino
acid sequence. Then, the peptide is secreted into the extracellular
matrix, and cleaved to form the mature type II collagen molecule
(5,6). According to previous reports,
mutations in the COL2A1 gene contribute to numerous
diseases, including spinal epiphyseal dysplasia (7), type 2 achondroplasia (8) and Stickler syndrome (9). However, whether pedigree mutations in
the COL2A1 gene may cause disease remains to be studied.
We studied one osteonecrosis pedigree from the
province of Anhui, China, and focused on microsatellite markers up-
and downstream of the COL2A1 gene for linkage analysis. We
designed primers for PCR amplification of the COL2A1 gene
exons and directly sequenced the products to identify
disease-causing mutations. This analysis revealed a mutation
potentially causing femoral head necrosis in the studied pedigree,
which provides a molecular basis for the diagnosis and prenatal
counseling in additional members of the family.
Materials and methods
Subjects
An osteonecrosis pedigree from the Wuwei County in
Anhui province, China, was used in this study. The pedigree
comprised three generations of a total of 18 Han individuals, 9 of
which showed brachydactyly symptoms. The pedigree relationships
were confirmed. We obtained written informed consent from all
subjects, and collected peripheral blood from 16 individuals, 8 of
which were healthy. Representative pictures of bilateral hip X-ray
analysis, performed in the 8 patients and the 8 healthy subjects,
are shown in Fig. 1. Peripheral
blood from 50 Han healthy individuals was collected at the
Department of Physiology, Medical College of Shantou University,
China. These individuals had no symptoms of osteonecrosis and no
kinship to the patients.
Nucleic acids isolation
Genomic DNA was extracted from 5 ml of peripheral
blood using EDTA as an anticoagulant according to a method
previously described with little modification (10). The concentration and purity of the
genomic DNA were assessed in a UV spectrophotometer (Tecan, San
Diego, CA, USA) at 260/280 nm.
Linkage analysis
Based on the chromosomal location and sequence of
the COL2A1 gene, two polymorphic microsatellite markers were
selected, D12S1663 (UniSTS:48691, 12q13.11) and D12S368
(UniSTS:9082, 12q13.13) up- and downstream of the gene for linkage
analysis. D12S1663 and D12S368 were amplified using ABI
PRISM® Linkage Mapping Set (Applied Biosystems, Foster
City, CA, USA). Following PCR, Prism-3100 sequencer (Applied
Biosystems) was used to inspect the fragment length for
microsatellite genotyping. These two markers were amplified from
DNA of the pedigree members, using PCR.
PCR primer synthesis
Primers for the amplification of the two
microsatellites were designed based on the sequence of the
COL2A1 gene (Entrez gene id, 1280; OMIM id, 120140), and
were previously described in Liu et al (11). The primers were synthesized by the
Shanghai Sangon Biological Engineering Technology and Services Co.,
Ltd. (Shanghai, China).
PCR amplification and sequencing
The PCR reaction was performed in a final volume of
25 μl, comprising 2.5 μl of 10× PCR buffer (Takara Bio, Inc.,
Shiga, Japan), 2 μl of dNTPs (2.5 mmol/l), 0.1 μl of Taq polymerase
(5 U/μl), 0.5 μl of the forward and reverse primer, (50 pmol), 18.4
μl of double distilled water (ddH2O) and 1 μl of genomic
DNA (100–200 ng). The PCR was conducted on a GeneAmp PCR System
9700 (Applied Biosystems) with the following cycling conditions:
initial denaturation at 97°C for 5 min, followed by 35 cycles of
denaturation at 95°C for 45 sec, annealing at 52°C or 55°C (for
D12S1663 and D12S368, respectively) for 30 sec, extension at 72°C
for 45 sec, and a final extension at 72°C for 10 min. The PCR
products were subjected to denaturing polyacrylamide gel
electrophoresis to inspect the fragment length (expected sizes, 223
bp and 115 bp for D12S1663 and D12S368, respectively) for
microsatellite genotyping. They were next sequenced by the Shanghai
Sangon Biological Engineering Technology and Services Co., Ltd.
with a dideoxy-based sequencing protocol.
Results
Symptoms of ONFH in the pedigree
The proband II6 was female, 48 years old and of Han
ethnicity. The proband exhibited hip pain upon physical
examination, while imaging with X-ray (Fig. 1A) revealed femoral head collapse
with a narrow acetabulum gap and a clear hardened zone. However, in
the normal control groups (Fig.
1B) the femoral head was normal, the acetabulum gap was clear
and there was no hardened zone. The ONFH pedigree comprised three
generations (I–III) of a total of 18 people, 9 of which showed
brachydactyly symptoms. In all generations, both men and women had
the disease, inherited by one of the patient’s parents who was also
a patient, while healthy offspring was also observed (Fig. 2); this pattern is consistent with
an autosomal dominant genetic disease. Physical examination and
imaging studies on additional members of the family confirmed that
the patients have similar symptoms of ONFH to the proband.
Linkage and COL2A1 gene mutation
analysis
Linkage analysis of the COL2A1 gene in the
studied family with the D12S1663 and D12S368STR microsatellites is
shown in Fig. 2, and suggested
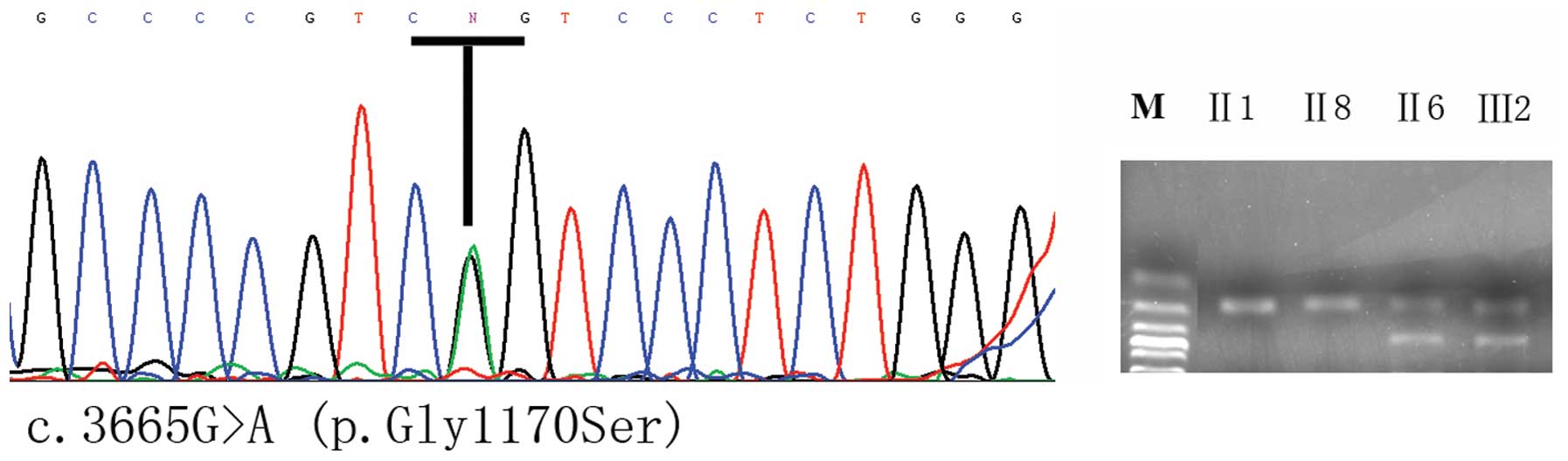
that COL2A1 may cause ONFH in this family. Sequencing of the
PCR-amplified COL2A1 exon regions in the proband and 8
additional family members revealed that a c.3665G>A heterozygous
mutation (Fig. 3) is present in
the first exon in seven patients, while this mutation was not
detected in 6 normal subjects of the pedigree, indicating that this
mutation may associate with the disease. An additional 50 Han
individuals not belonging to the pedigree were screened by PCR and
sequencing analysis, and the mutation was not identified in any of
them. The c.3665G>A mutation causes a glycine-to-serine
substitution on codon 1170, which is part of the sequence encoding
a GXY repeat in the collagen II protein (Fig. 3).
Discussion
In this study, we identified, by linkage analysis, a
mutation in the first exon of the COL2A1 gene that is
potentially associated with necrosis of the femoral head in Anhui
pedigrees. Specifically, we found that the genetic basis of the
disease in the members of the studied family may be a c.3665G>A
mutation (p.G1170S). This mutation, present on the codon 1170 of
the human COL2A1 gene, causes a glycine-to-serine amino acid
change in the collagen type II GXY repeat region. The mutation
co-segregated with the disease in the pedigree, and was not
detected in 50 healthy Han subjects that did not belong to the
pedigree, which further supports that the mutation may be
pathogenic.
COL2A1 gene mutations can cause a range of
serious changes in the encoded product, the collagen type II α1
protein. Chan et al found that another COL2A1
mutation can lead to spinal epiphyseal dysplasia (7). The study of Godfrey and Hollister
(12) confirmed one case of
perinatal death in the presence of dwarfism caused by a
heterozygous mutation in COL2A1, resulting in abnormal
assembly and folding of type II collagen. Francomano et al
(13) found, using linkage
analysis, that COL2A1 and the Stickler syndrome are
associated. Subsequently in 1990, Ahmad et al (9) identified a COL2A1 mutation in
a family with the Stickler syndrome. Knowlton et al
(14) reported genetic linkage of
COL2A1 to osteoarthritis associated with achondroplasia in a
pedigree, followed by Ala-Kokko et al (15). Wilkin et al (16) identified a COL2A1 gene
mutation in patients with Hackney Manchester hypoplasia; the
mutation caused type II collagen defects, rendering the protein
shorter. Otospondylomegaepiphyseal dysplasia (OSMED) is a skeletal
disorder, associated with severe sensorineural hearing loss,
enlarged epiphysis and the early onset of osteoarthritis. Miyamoto
et al (17) identified a
COL2A1 mutation at a splice-acceptor site within intron 10
in an OSMED patient. Liu et al identified three families in
which there was autosomal dominant inheritance of the avascular
necrosis of the femoral head (ANFH), and mapped the chromosomal
position of the gene to 12q13. Haplotype analysis and sequencing of
ANFH patients of two pedigrees allowed the authors to identify a
G-to-A mutation in exon 50 of COL2A1. The mutation was
reported to lie on the codon 1170 and to cause a glycine-to-serine
replacement in the collagen II GXY repeat region. A G-to-A mutation
in exon 33 (codon 717) of the COL2A1 gene was further
identified in a third pedigree, also resulting in a
glycine-to-serine replacement. These mutations may lead to an
alteration in the structure and function of type II collagen
(11). Su et al (18) performed mutation screening in a
Chinese Han pedigree with osteoarthritis, avascular necrosis, and
Legg-Calvé-Perthes disease, and also found a G-to-A mutation in
exon 50 of the COL2A1 gene. In 40-year-old patients with
femoral head necrosis, Kannu et al (19) identified a novel heterozygous
mutation in the COL2A1 sequence encoding the C-propeptide.
Previous studies have shown that mutations in this position
generally cause serious limb deformities and even death (20), but the identified mutation in this
patient did not cause severe limb deformities.
In conclusion, the present study reports the
identification of a COL2A1 mutation in a Chinese Han
pedigree. Although the functions of the COL2A1 protein are
understood to a certain degree, the exact mechanisms by which
mutations in the gene cause osteonecrosis remain largely unknown.
Additional studies on bigger samples and including distinct ethnic
backgrounds are required to investigate in depth the pathogenesis
of ONFH.
References
|
1
|
Zhao HY, Counterattack O and Kang PD:
Osteonecrosis etiology and pathogenesis research progress. Chin J
Orthopaedic Surg. 20:32–40. 2009.
|
|
2
|
Ficat RP: Idiopathic bone necrosis of the
femoral head. Early diagnosis and treatment. J Bone Joint Surg Br.
67:3–9. 1985.PubMed/NCBI
|
|
3
|
Shang XF, Su H, Chang WW, Wang CC, Han Q
and Xu ZW: Association between MTHFR C677T polymorphism and
osteonecrosis of the femoral head: a meta-analysis. Mol Biol Rep.
39:7089–7094. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim H, Cho C, Cho Y, Cho S, Yoon K and Kim
K: Significant associations of PAI-1 genetic polymorphisms with
osteonecrosis of the femoral head. BMC Musculoskelet Disord.
12:1602011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cheah KS, Stoker NG, Griffin JR, et al:
Identification and characterization of the human type II collagen
gene (COL2A1). Proc Natl Acad Sci USA. 82:2555–2559. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Strom CM and Upholt WB: Isolation and
characterization of genomic clones corresponding to the human type
II procollagen gene. Nucleic Acids Res. 12:1025–1038. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chan D, Taylor TK and Cole WG:
Characterization of an arginine 789 to cysteine substitution in
alpha 1 (II) collagen chains of a patient with spondyloepiphyseal
dysplasia. J Biol Chem. 268:15238–15245. 1993.PubMed/NCBI
|
|
8
|
Vissing H, D’Alessio M and Lee B: Glycine
to serine substitution in the triple helical domain of pro-alpha 1
(II) collagen results in a lethal perinatal form of short-limbed
dwarfism. J Biol Chem. 264:18265–18267. 1989.PubMed/NCBI
|
|
9
|
Ahmad NN, Ala-Kokko L, Knowlton RG, et al:
Stop codon in the procollagen II gene (COL2A1) in a family with the
Stickler syndrome (arthro-ophthalmopathy). Proc Natl Acad Sci USA.
88:6624–6627. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou X, He X, He B, et al: Etifoxine
promotes glial-derived neurotrophic factor-induced neurite
outgrowth in PC12 cells. Mol Med Rep. 8:75–80. 2013.PubMed/NCBI
|
|
11
|
Liu YF, Chen WM, Lin YF, et al: Type II
collagen gene variants and inherited osteonecrosis of the femoral
head. N Engl J Med. 352:2294–2301. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Godfrey M and Hollister DW: Type II
achondrogenesis-hypochondrogenesis: identification of abnormal type
II collagen. Am J Hum Genet. 43:904–913. 1988.PubMed/NCBI
|
|
13
|
Francomano CA, Liberfarb RM, Hirose T, et
al: The Stickler syndrome: evidence for close linkage to the
structural gene for type II collagen. Genomics. 1:293–296. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Knowlton RG, Katzenstein PL, Moskowitz RW,
et al: Genetic linkage of a polymorphism in the type II procollagen
gene (COL2A1) to primary osteoarthritis associated with mild
chondrodysplasia. N Engl J Med. 322:526–530. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ala-Kokko L, Baldwin CT, Moskowitz RW and
Prockop DJ: Single base mutation in the type II procollagen gene
(COL2A1) as a cause of primary osteoarthritis associated with a
mild chondrodysplasia. Proc Natl Acad Sci USA. 87:6565–6568. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wilkin DJ, Artz AS, South S, et al: Small
deletions in the type II collagen triple helix produce kniest
dysplasia. Am J Med Genet. 85:105–112. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Miyamoto Y, Nakashima E, Hiraoka H, et al:
A type II collagen mutation also results in
oto-spondylo-megaepiphyseal dysplasia. Hum Genet. 118:175–178.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Su P, Li R, Liu S, et al: Age at
onset-dependent presentations of premature hip osteoarthritis,
avascular necrosis of the femoral head, or Legg-Calvé-Perthes
disease in a single family, consequent upon a p. Gly1170Ser
mutation of COL2A1. Arthritis Rheum. 58:1701–1706. 2008.
|
|
19
|
Kannu P, O’Rielly DD, Hyland JC and Kokko
LA: Avascular necrosis of the femoral head due to a novel C
propeptide mutation in COL2A1. Am J Med Genet A. 155A:1759–1762.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kannu P, O’Rielly DD, Hyland JC and Kokko
LA: Avascular necrosis of the femoral head due to a novel C
propeptide mutation in COL2A1. Am J Med Genet A. 155A:1759–1762.
2011. View Article : Google Scholar : PubMed/NCBI
|