Introduction
Currently, the therapies for nerve injury focus on
revealing the molecular mechanism of injured neuron death and
regeneration. It was reported that nerve injuries caused by several
factors could induce molecular apoptosis and regenerative events in
neurons, and functional recovery of the nervous system depended on
the interaction between apoptosis and regeneration signals
(1). Therefore, the objective of
studies switched to enhancing the activities of regenerative
signaling pathways and inhibiting the transduction of apoptotic
signals (2). Nitric oxide synthase
(NOS), which is able to generate nitric oxide (NO) by utilizing
L-arginine as a substrate and nicotinamide adenine dinucleotide
phosphate (NADPH) as the hydrogen delivery body, can be divided
into neuronal nitric oxide synthase (nNOS), endothelial nitric
oxide synthase (eNOS) and inducible nitric oxide synthase (iNOS).
According to their tissue sources, nNOS and eNOS are collectively
referred to as constitutive nitric oxide synthase (cNOS) (3). The majority of nNOS is located in the
neurons, only some of which is in the nerve fibers surrounded by
cerebrovascular fluid. In addition, it is generally expressed at a
low level and as a catalyst to generate a small quantity of NO.
Therefore, it is important in the control of cognitive function,
synaptic plasticity and the neurosecretory system (4). However, nNOS is a ‘double-edged
sword’ during injuries of the nervous system, in other words, it
has been demonstrated to have positive and negative roles during
neural regeneration in different disease models (5). It is well documented that nNOS is a
factor which is able to promote the survival of differentiated PC12
cells (6). In addition, several
studies have revealed that transcription factors also have critical
roles in the death and regeneration procedures of injury-induced
neurons in the peripheral and central nervous system (7). There were types of inducible
transcriptional factors closely associated with the injury of
neuron axons in the central nervous system, however, only the
proto-oncogene-coding c-jun protein and its family member JunD were
constantly expressed following axon injuries (8). The c-jun N-terminal kinase (JNK),
also termed the stress activated protein kinase, was one of the
downstream signaling molecules in the mitogen-activated protein
kinase pathway, which was activated via cytokines and growth
factors following stimulation by ultraviolet, ionizing radiation
and heat shock (9,10). In addition, c-jun was the main
transcriptional factor expressed by cerebral neurons during
degenerative procedures, including ischemic tolerance and dementia
(11). Furthermore, as a member of
the AP-1 family, c-jun may be a controller of target genes by
combining with certain other transcription factors, including Jun,
Fos and ATF (12). Similarly to
nNOS, c-jun was demonstrated to have a dual role in injuries to the
nervous system (13). According to
previous studies, the activation of c-jun mediated the apoptotic
process in differentiated PC12 cells and other neurons (14,15).
The expression level of c-jun has been demonstrated
to be upregulated when the expression level of nNOS is inhibited
and the survival rate of neurons enhanced by utilizing neurotrophic
factors or antioxidants, indicating a close association between
c-jun and nNOS (1,16,17).
Furthermore, the differentiation of PC12 cells induced the
upregulation of c-jun and nNOS expression in a study by Cheng et
al (18). The activation of
c-jun mediated the apoptosis of PC12 cells and the nNOS protein
maintained the survival of PC12 cells (6,14).
Finally, the application of c-jun siRNA for downregulating the
expression of c-jun was previously indicated to reduce the
expression level of nNOS in differentiated PC12 cells (18,19).
Therefore, in order to clarify whether the association between
c-jun and nNOS involved up and downregulation or crosstalk,
pheochromocytoma differentiated PC12 cells were selected as the
cell model and 7-nitroindazole (7-NI) as the inhibitor of nNOS to
downregulate the expression level of nNOS. Thus, the effects of the
changes in the nNOS gene on the expression level of c-jun and the
association between c-jun and nNOS in neurons were determined in
order to improve our understanding of the molecular mechanisms
underlying neural diseases.
Materials and methods
Cell culture
Differentiated PC12 cells, a rat neuronal cell line
derived from pheochromocytoma cells, were purchased from the Cell
Resource Center, Shanghai Institute for Biological Sciences, China
Scientific Academy (Shanghai, China). The culture of differentiated
PC12 cells was established by modification of a previously
described procedure (18,19). Cells were maintained in Dulbecco’s
modified Eagle’s medium (DMEM, high glucose; Gibco-BRL, Carlsbad,
CA, USA) supplemented with 10% fetal bovine serum and antibiotics
(100 U/ml penicillin A and 100 g/l streptomycin; Gibco-BRL) at 37°C
in a humidified 5% CO2 incubator. The monolayer cells
were subcultured once they reached ~80% confluence as previously
described (19). In brief, cells
were washed 2–3 times with D-Hanks’ solution following aspirating
the culture medium, and then 0.25% trypsin was added for digestion
at 37°C. Digestion was not terminated by appropriate high glucose
DMEM complete medium until the cells became rounded, the cell
processes retracted and the cell gap became large. Monolayer cells
were collected by centrifugation for 3 min at 90 × g and diluted
and plated on new flasks for subculture. Subsequent experiments
were performed following 3–4 passages when cells were in a stable
state.
The other three sets of PC12 cells were treated with
growth medium only (normal control), dimethyl sulfoxide (DMSO;
solvent control group) and 7-NI (nNOS inhibitor group; Cell
Signaling Technology, Danvers, MA, USA), respectively.
Immunofluorescence staining for nNOS and
c-jun expression in vitro
Differentiated PC12 cells were seeded onto
poly-lysine-coated 96-well plates at a density of 2×104
cells/ml for adherence overnight. Subsequently, the cultured cells
were fixed with 4% paraformaldehyde in phosphate-buffered saline
(PBS) at room temperature (RT) for 15 min. Following rinsing three
times with PBS, the cells were treated with 0.3% Triton X-100 and
0.1% bovine serum albumin (BSA) in PBS at RT for 30 min, and
incubated with either rabbit anti-nNOS primary antibody (1:2,000;
Cell Signaling Technology), rabbit anti-c-jun primary antibody
(1:400; Cell Signaling Technology) or serum at 4°C overnight. Then,
following washing three times with PBS, cells were incubated with
fluorescein isothiocyanate-conjugated anti-rat IgG (1:200; Cell
Signaling Technology) or rhodamine-conjugated anti-rabbit IgG
(1:800; Cell Signaling Technology) at RT for an additional 2 h in
the dark, respectively. Following rinsing the cells a further three
times with PBS, the nuclei were stained with Hoechst 33258 for 20
min prior to being washed briefly with PBS and then visualized
under a Leica DMI4000 B fluorescence microscope (Leica Microsystems
GmbH, Wetzlar, Germany). Cells stained without primary or secondary
antibodies served as negative controls.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT)
assay
The viability of differentiated PC12 cells was
assessed by measuring the dehydrogenase activity in the culture,
which was indicated by MTT tests (mitochondrial activity, as a
measure of cell death) as described previously (20). Briefly, following incubation with
DMSO as a solvent control or 10, 50, 100, 200 and 400 μmol/l 7-NI
in 96-well microtiter plates for 24 h, the medium was removed and
the cells were incubated in 150 μl culture medium per well. MTT (10
μl, 0.5 mg/ml per well) was added to the culture and incubated for
an additional 4 h at 37°C and 5% CO2. DMSO (150 μl) was
added to each well to solubilize the formazan salt following
removal of the media. Finally, the resultant purple azo-dye was
detected at 570 nm with a SpectraMax M5 plate reader (Molecular
Devices, Sunnyvale, CA, USA). Untreated PC12 cells were used as a
control with 100% viability. The cell viability in treated PC12
cells was calculated as a percentage relative to the untreated PC12
cells.
Determination of cNOS activity
Differentiated PC12 cells were plated onto six-well
plates at a density of 1×106 cells/ml (2 ml/well). The
total protein was extracted from the cells of each group 24 h after
different interventions. iNOS activity and total NOS activity in
the cells were measured using a NOS Activity Detection kit (Nanjing
Jiancheng Bioengineering Institute, Nanjing, China), which assessed
NOS activity by measuring the conversion of L-[14C]-arginine to
L-[14C]-citrulline (21,22). The total NOS activity was
determined by incubating samples (50 μl) for 15 min at 37°C in a
reaction mixture containing buffer solution, 20 μM β-NADPH, 1 mm
CaCl2, 50 μM tetrahydrobiopterin (BH4) and 1 μCi/ml
L-[14C]-arginine. iNOS activity was measured by omitting calcium
and adding 1 mm ethylenediaminetetraacetic acid (EDTA) to the
reaction mixture (50 μl) for 60 min at 37°C. The reaction was
terminated by the addition of 1 ml ice-chilled buffer containing 30
mm HEPES and 3 mm EDTA (pH 5.5), following which the reaction mix
was applied to Dowex 50WX8 columns (Sigma-Aldrich, St. Louis, MO,
USA) to remove L-[14C]-arginine. Columns were eluted two times with
0.5 ml of distilled water and L-[14C]-citrulline was quantified
using a Quantulus Liquid Scintillation Spectrometer (PerkinElmer,
Inc., Waltham, MA, USA). cNOS activity was calculated by
subtracting iNOS activity from total NOS activity. One unit (U) of
total NOS activity was defined as picomoles of L-[14C]-citrulline
produced per minute per microgram protein/milliliter. The activity
of cNOS in cells was expressed as U/mg of cell protein.
Western blotting for nNOS and c-jun
expression
The expression of nNOS and c-jun in PC12 cells was
determined by western immunoblot analysis performed as previously
described (19,20). In brief, differentiated PC12 cells
following different treatments were harvested and lysed at 4°C by
adding 100 μl lysis buffer and 10 μl phenylmethylsulfonyl fluoride
(100 mm/l) per 2×106 cells for 30 min on ice followed by
centrifugation at 13,000 × g for 15 min. Supernatant was collected
for the determination of protein concentrations using the BCA
protein assay using Bio-Rad Protein Assay Reagent (Bio-Rad,
Hercules, CA, USA). The samples were stored at −80°C until analysis
(usually within 2 weeks). For western blotting, cell lysates (30 μg
protein/lane) were separated on 8% sodium dodecyl
sulphate-polyacrylamide gels with a pre-stained protein ladder (5
μl) marker and electrophoretically transferred onto polyvinylidene
difluoride membranes (Pall Corporation, Port Washington, NY, USA).
The membranes were then blocked with 5% non-fat dry milk in
tris-buffered saline (TBS) containing 0.1% Tween-20 at RT for 2 h
and incubated with either c-jun polyclonal anti-rabbit antibody
(1:1,000; Cell Signaling Technology), nNOS polyclonal anti-rabbit
antibody (1:500; Cell Signaling Technology) or anti-β-actin
monoclonal anti-mouse antibody (1:1,000; Wuhan Boster Biological
Technology, Ltd., Wuhan, China) overnight with gentle agitation at
4°C. Following three washes in 0.1% Tween-20 in TBS (TBS-T) and
incubation for 2 h at RT with horseradish peroxidase-conjugated
polyclonal goat anti-rabbit secondary antibody (1:3,000; Cell
Signaling Technology), the membranes were washed five times in
TBS-T and immunocomplexes were visualized using the supersignal
west Pico Trial kit (Pierce Biotechnology, Inc., Rockford, IL, USA)
and the blots were exposed to X-ray film (Fujifilm, Tokyo, Japan).
The intensity was quantified in the two bands. All bands on the
immunoblots were normalized to their corresponding β-actin bands.
Relative levels of protein in the different lanes were compared by
analyzing scanned images using the NIH Image J software (National
Institutes of Health, Bethesda, MA, USA). All experiments were
performed three times with independent cultures.
Statistical analysis
All variables are presented as the mean ± standard
deviation of at least three independent experiments. Data analysis
was performed using SPSS version 16.0 (SPSS, Inc., Chicago, IL,
USA). One-way analysis of variance was used to analyze the
differences among groups followed by Tukey-Kramer multiple
comparison tests. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of c-jun and nNOS in
differentiated PC12 cells in vitro
Differentiated PC12 cells were cultured as described
in the experimental procedures. At first, the expression of nNOS
and c-jun in differentiated PC12 cells was investigated by
immunofluorescent double labeling detection. Morphologically, a
large soma and two unbranched processes with the length of the
processes being more than twice the length of the cell body, are
indications of differentiation of PC12 cells. Under the DMI4000 B
inverted microscope, it was observed that the bodies of incubated
PC12 cells were round and transparent, and they were suspended in
the cell culture solution evenly. The majority of the cells began
to adhere to the wall of the cell culture dish at 4 h and stretched
processes appeared 12 h after incubation, which were similar to the
axons of neurons and their length was 2-fold longer than the
diameter of cell bodies (Fig. 1).
The results demonstrated that nNOS was expressed in differentiated
PC12 cells, while nNOS immunoreactivity (ir) was present in the
cytoplasm (Fig. 1A); c-jun was
also clearly expressed in differentiated PC12 cells, while c-jun ir
was present in the nuclei (Fig.
1D). All the nuclei of the differentiated PC12 cells were
labeled with Hoechst 33258 (Fig. 1B
and E). Immunofluorescence double labeling further indicated
that c-jun and nNOS were co-expressed in almost all the
differentiated PC12 cells (Fig. 1C and
F). Based on the above findings, the next series of experiments
aimed to determine the correlation between c-jun and nNOS in
differentiated PC12 cells.
nNOS inhibitor 7-NI decreases the
viability and survival of cultured differentiated PC12 cells in
vitro
In order to examine the effectiveness of different
concentrations of 7-NI and verify whether DMSO as the solvent of
7-NI had cell cytotoxicity, the PC12 cells were divided into the
normal PC12 cell control group (normal group), DMSO control group
(DMSO group) and 7-NI intervention group (7-NI group). The 7-NI
group was subdivided into five different concentration groups of
10, 50, 100, 200 and 400 μmol/l. With the 7-NI intervention for 24
h, the cell viability was detected by MTT analysis. Statistical
analysis demonstrated that the cell viability was significantly
lower in the 200 and 400 μmol/l 7-NI treatment group compared with
the normal group (P<0.05), while no significant difference was
observed between the DMSO group and the normal group (P>0.05),
which indicated that the DMSO had no cytotoxicity on differentiated
PC12 cells (Fig. 2). Therefore,
200 μmol/l 7-NI was selected as the optimal concentration for the
following experiments.
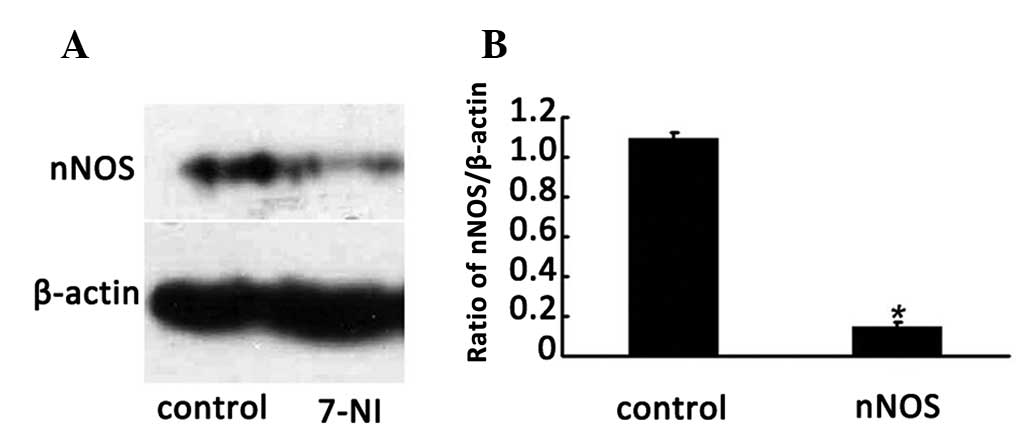
nNOS inhibitor 7-NI downregulates the
nNOS gene in differentiated PC12 cells
To investigate whether the nNOS inhibitor 7-NI
reduces nNOS expression in differentiated PC12 cells, the protein
levels of nNOS in the cells following treatment with 200 μmol/l
7-NI for 24 h were evaluated by western blotting. The results
indicated that the expression level of nNOS in the cells of the 200
μmol/l 7-NI group for 24 h was significantly lower than that of the
normal group (P<0.05; Fig. 3A and
B), which indicated that the expression of the nNOS protein was
able to be effectively inhibited by 200 μmol/l 7-NI.
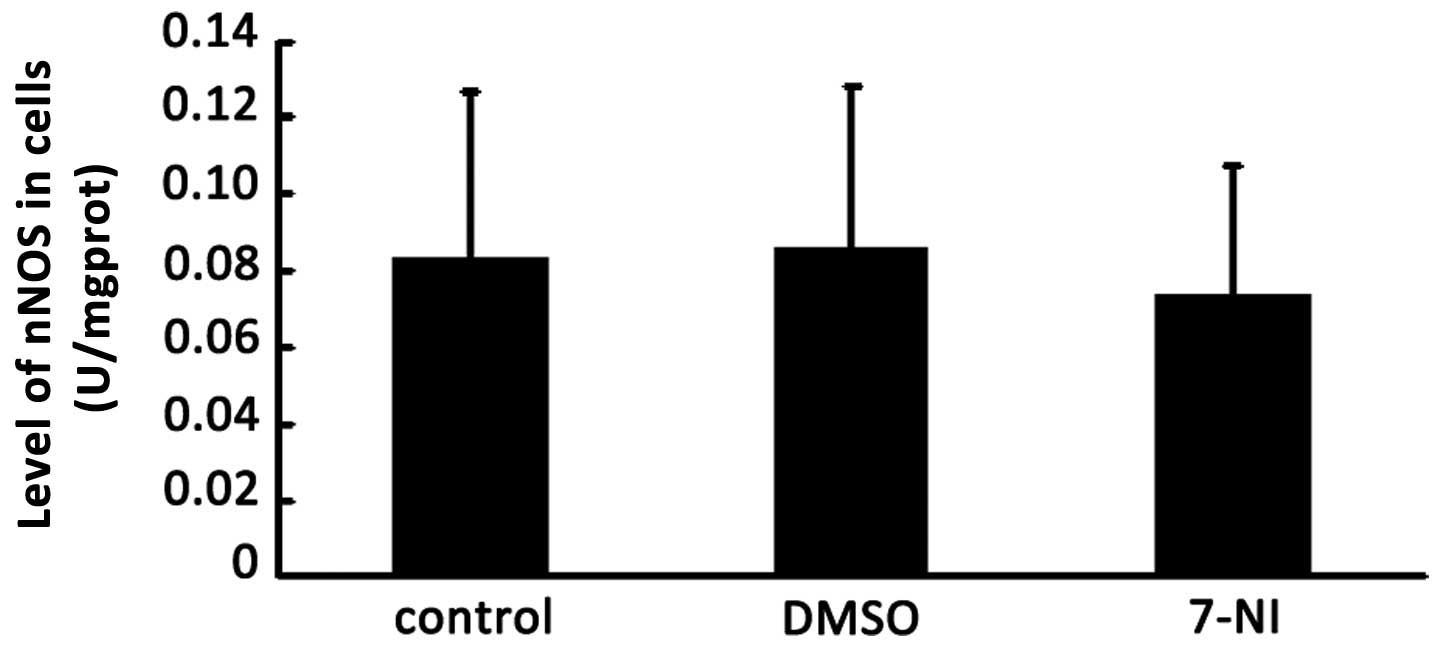
Effect of the nNOS inhibitor 7-NI on the
activity of cNOS in differentiated PC12 cells
The nNOS inhibitor 7-NI also may result in a change
in cNOS activity in various cell and tissue samples. The activity
of cNOS was detected 24 h after treatment with 200 μmol/l 7-NI in
differentiated PC12 cells. The total protein was extracted from the
cells of the normal group, DMSO group and 200 μmol/l 7-NI group,
and then the activity of cNOS in differentiated PC12 cells of these
three groups were detected using the NOS Activity Detection kit. No
significant differences were observed in the activity of cNOS among
the 200 μmol/l 7-NI group, DMSO group and the control group
following intervention for 24 h (Fig.
4), which indicated that 200 μmol/l 7-NI had no effect on the
activity of cNOS in the differentiated PC12 cells.
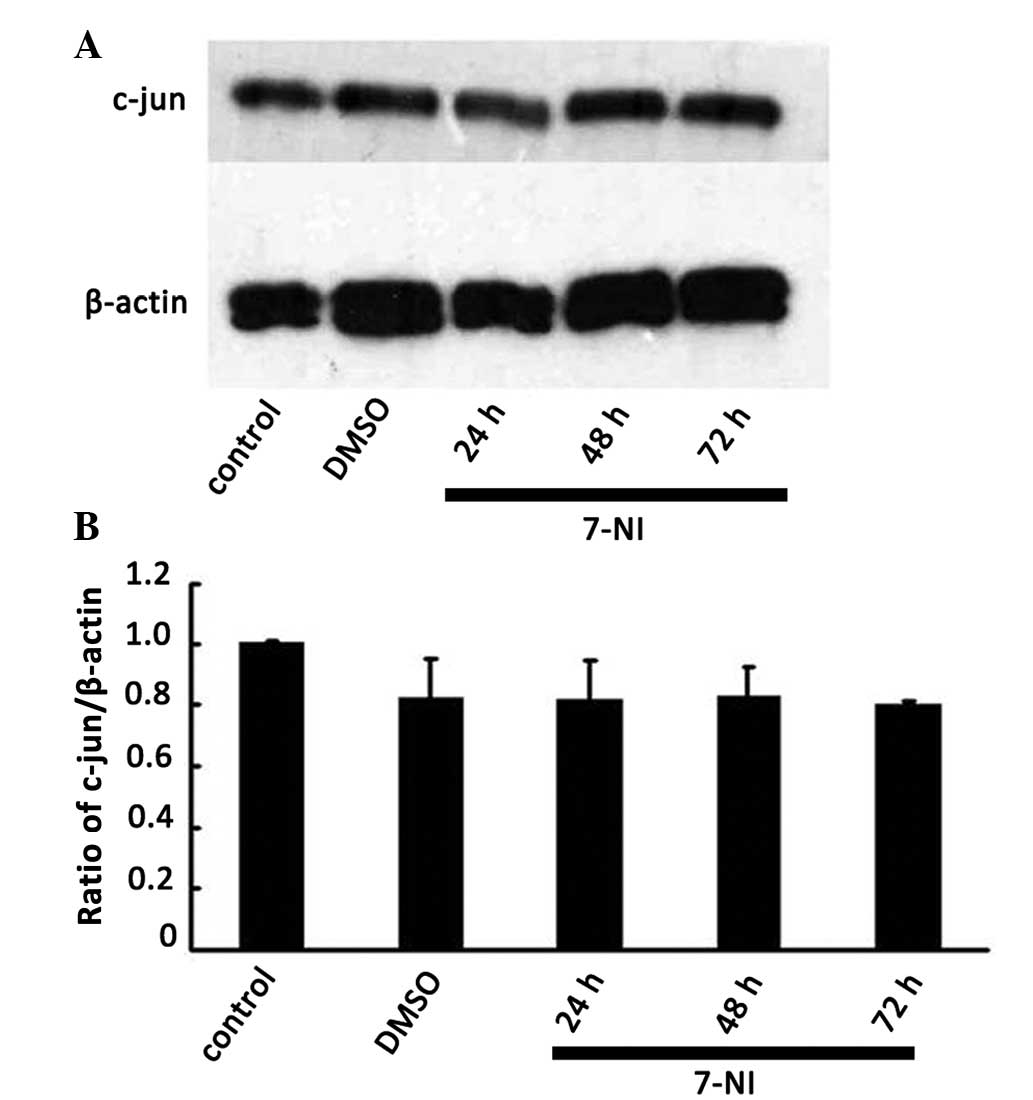
Effect of the nNOS inhibitor 7-NI on the
expression of c-jun in differentiated PC12 cells
Our previous study revealed that suppression of
c-jun expression by c-jun siRNA decreased nNOS expression in
differentiated PC12 cells (18).
To clarify whether there was an association of up and downstream
regulation or a crosstalk between c-jun and nNOS in neurons, the
protein expression level of c-jun was detected by western blot
analysis 24, 48 and 72 h after treatment with 200 μmol/l 7-NI. No
significant differences were observed compared with the normal
group and the DMSO group (Fig. 5),
which indicated that the expression level of c-jun in
differentiated PC12 cells was not affected by the inhibition of
nNOS. The above results further verified that nNOS, as a downstream
signaling molecule of c-jun, regulated the survival of
differentiated PC12 cells.
Discussion
In order to examine the association between c-jun
and nNOS in neurons, the expression of c-jun and nNOS in
differentiated PC12 cells was detected. In addition, the nNOS
inhibitor 7-NI was used to downregulate the protein level of nNOS
in order to observe the changes in the expression level of c-jun.
The results demonstrated that c-jun and nNOS were co-expressed in
PC12 cells. Furthermore, the expression level of nNOS instead of
the activity of nNOS was inhibited by 200 μmol/l 7-NI. In addition,
the inhibited nNOS was not able to regulate the expression level of
c-jun. Combined with our previous correlative research results,
this indicated that the nNOS gene was a downstream signaling
molecule of the JNK/c-jun signaling pathway in the model of
differentiated PC12 cells. c-jun as an upstream signaling molecule
was able to regulate the expression of nNOS downstream. Therefore,
the association between them involved up and downregulation instead
of crosstalk (1,16–19).
PC12 cells exhibit characteristic phenotypes of
neurons following intervention with nerve growth factor (NGF),
which is necessary for the differentiation of PC12 cells. In a
study by Palmada et al (23), it was demonstrated that c-jun was
required for sympathetic neuron degeneration following NGF
withdrawal. It was reported that apoptosis of differentiated PC12
cells without NGF was inhibited by the complex dominant mutation of
the c-jun gene and the injection of the specific antibody of c-jun
(24). In addition, the
constructed activity mutation of c-jun was a requirement for
apoptosis caused by cerebellar granule cells (25). These findings indicated that the
apoptosis observed in differentiated PC12 cells or other cells was
mediated by the activation of c-jun whose mechanism remains to be
elucidated. It was generally accepted that the above procedures
were mainly due to the downstream target gene activated by c-jun in
different types of cells or the protein that interacts with c-jun.
Previous studies have demonstrated that the induced expression of
c-jun and nNOS appeared in several animal models of neural diseases
(15). For instance, nNOS and
c-jun were co-expressed in the lateral geniculate nucleus in a rat
model of experimental glaucoma (26) and the survival of hippocampal
neurons was promoted by the NO-induced activated JNK/c-jun in an
animal model of ischemia (27).
The impairment of proteasome activity and consequent increases in
nNOS levels led to nitrative stress that involved the coordinated
response of the JNK/c-jun cytosolic signaling pathway (28). All the above revealed that there
was a certain functional association between c-jun and nNOS, which
was consistent with our findings in differentiated PC12 cells in
vitro (18). Previous studies
have demonstrated that c-jun and nNOS were co-expressed in
motoneurons. In addition, the expression of nNOS was effectively
downregulated by the activation of c-jun as an upstream signal
(15). Furthermore, the expression
of c-jun was present prior to that of nNOS due to the fact that the
expression of nNOS was also downregulated by utilizing the c-jun
siRNA to downregulate the expression level of c-jun in
differentiated PC12 cells. However, the expression level of c-jun
was not affected by utilizing 7-NI to downregulate the expression
level of nNOS in the present study, which indicated that the nNOS
gene was a downstream signaling molecule of the JNK/c-jun signal
pathway. Additionally, c-jun as an upstream signaling molecule was
able to regulate the expression of nNOS downstream, thus the
association between them involved up and downregulation instead of
crosstalk. The present study verified that nNOS was able to be
regulated by c-jun as an upstream molecule, which expanded the
theory of interaction between apoptosis and regenerated molecules
in neurons. Although, this viewpoint requires further investigation
by utilizing nNOS siRNA and the transgenic animal model of c-jun
and nNOS.
In the present study, the expression level of nNOS
was inhibited by an effective, cell-permeable, reversible nNOS
inhibitor-7-NI. The results demonstrating that the activity of cNOS
was not affected by 7-NI were mainly due to the following
explanations: Firstly, the protein expression instead of the
activity of nNOS protein was able to be regulated by 7-NI. In
addition, cNOS could be subdivided into eNOS and nNOS, therefore
the activity of cNOS was not equal to nNOS.
The present study revealed that c-jun and nNOS were
co-expressed in differentiated PC12 cells, the expression level of
nNOS protein was effectively inhibited by 7-NI and the expression
level of c-jun was not affected by inhibition of nNOS. This
indicated that the nNOS gene was a downstream signaling molecule of
the JNK/c-jun signaling pathway, and that c-jun, as an upstream
signaling molecule, was able to regulate the expression of nNOS
downstream, thus the association between them involved up and
downregulation instead of crosstalk. The current study clarified
c-jun and nNOS as mediators of neuronal apoptosis, providing a
greater understanding and enabling the application of molecular
target therapy in neurodegenerative diseases.
Acknowledgements
This study was supported by research grants from the
Natural Science Foundation Council of China (no. 81303115) and the
Natural Science Foundation Council of Guangdong province (no.
S2013040016915).
References
|
1
|
Wu W, Li L, Yick LW, et al: GDNF and BDNF
alter the expression of neuronal NOS, c-Jun, and p75 and prevent
motoneuron death following spinal root avulsion in adult rats. J
Neurotrauma. 20:603–612. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ozdemir M, Attar A and Kuzu L:
Regenerative treatment in spinal cord injury. Curr Stem Cell Res
Ther. 7:364–369. 2012. View Article : Google Scholar
|
|
3
|
Förstermann U and Sessa WC: Nitric oxide
synthases: regulation and function. Eur Heart J. 33:829–837.
2012.PubMed/NCBI
|
|
4
|
Brown GC: Nitric oxide and neuronal death.
Nitric Oxide. 23:153–165. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Umar S and van der Laarse A: Nitric oxide
and nitric oxide synthase isoforms in the normal, hypertrophic, and
failing heart. Mol Cell Biochem. 333:191–201. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schonhoff CM and Bulseco DA: The Ras-ERK
pathway is required for the induction of neuronal nitric oxide
synthase in differentiating PC12 cells. J Neurochem. 78:631–639.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Raivich G: Transcribing the path to
neurological recovery - from early signals through transcription
factors to downstream effectors of successful regeneration. Ann
Anat. 193:248–258. 2011. View Article : Google Scholar
|
|
8
|
Kenney AM and Kocsis JD: Peripheral
axotomy induces long-term c-Jun amino-terminal kinase-1 activation
and activator protein-1 binding activity by c-Jun and junD in adult
rat dorsal root ganglia in vivo. J Neurosci. 18:1318–1328.
1998.PubMed/NCBI
|
|
9
|
Mruthyunjaya S, Rumma M, Ravibhushan G, et
al: c-Jun/AP-1 transcription factor regulates laminin-1-induced
neurite outgrowth in human bone marrow mesenchymal stem cells: role
of multiple signaling pathways. FEBS Lett. 585:1915–1922. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ferrer I, Barrón S, Rodríquez-Farré E, et
al: Ionizing radiation-induced apoptosis is associated with c-Jun
expression and c-Jun/AP-1 activation in the developing cerebellum
of the rat. Neurosci Lett. 202:105–108. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fonseca MB, Nunes AF and Rodrigues CM:
c-Jun regulates the stability of anti-apoptotic deltaNp63 in
amyloid-beta-induced apoptosis. J Alzheimers Dis. 28:685–694.
2012.PubMed/NCBI
|
|
12
|
Maritz MF, van der Watt PJ, Holderness N,
et al: Inhibition of AP-1 suppresses cervical cancer cell
proliferation and is associated with p21 expression. Biol Chem.
392:439–448. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Raivich G: c-Jun expression, activation
and function in neural cell death, inflammation and repair. J
Neurochem. 107:898–906. 2008.PubMed/NCBI
|
|
14
|
Dragunow M, Xu R, Walton M, et al: c-Jun
promotes neurite outgrowth and survival in PC12 cells. Brain Res
Mol Brain Res. 83:20–33. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang LL, Zhao XC, Yan LF, et al: C-jun
phosphorylation contributes to down regulation of neuronal nitric
oxide synthase protein and motoneurons death in injured spinal
cords following root-avulsion of the brachial plexus. Neuroscience.
189:397–407. 2011. View Article : Google Scholar
|
|
16
|
Zhou LH, Han S, Xie YY, et al: Differences
in c-jun and nNOS expression levels in motoneurons following
different kinds of axonal injury in adult rats. Brain Cell Biol.
36:213–227. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou L and Wu W: Antisense oligos to
neuronal nitric oxide synthase aggravate motoneuron death induced
by spinal root avulsion in adult rat. Exp Neurol. 197:84–92. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cheng X, Liu S, Wang YQ, et al:
Suppression of c-jun influences nNOS expression in differentiated
PC12 cells. Mol Med Rep. 6:750–754. 2012.PubMed/NCBI
|
|
19
|
Cheng X, Fu R, Gao M, et al: Intrathecal
application of short interfering RNA knocks down c-jun expression
and augments spinal motoneuron death after root avulsion in adult
rats. Neuroscience. 241:268–279. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li M, Wang L, Peng Y, et al: Knockdown of
the neuronal nitric oxide synthase gene retard the development of
the cerebellar granule neurons in vitro. Dev Dyn. 239:474–481.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cheng X, Liu FL, Zhang J, et al: EGb761
protects motoneurons against avulsion-induced oxidative stress in
rats. J Brachial Plex Peripher Nerve Inj. 5:122010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li R, Wang WQ, Zhang H, et al: Adiponectin
improves endothelial function in hyperlipidemic rats by reducing
oxidative/nitrative stress and differential regulation of eNOS/iNOS
activity. Am J Physiol Endocrinol Metab. 293:1703–1708. 2007.
View Article : Google Scholar
|
|
23
|
Palmada M, Kanwal S, Rutkoski NJ, et al:
c-jun is essential for sympathetic neuronal death induced by NGF
withdrawal but not by p75 activation. J Cell Biol. 158:453–461.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ham J, Babij C, Whitfeld J, et al: A c-Jun
dominant negative mutant protects sympathetic neurons against
programmed cell death. Neuron. 14:927–939. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Watson A, Eilers A, Lallemand D, et al:
Phosphorylation of c-Jun is necessary for apoptosis induced by
survival signal withdrawal in cerebellar granule neurons. J
Neurosci. 18:751–762. 1998.PubMed/NCBI
|
|
26
|
Wang X, Tay SS and Ng YK: C-fos and c-jun
expressions in nitric oxide synthase immunoreactive neurons in the
lateral geniculate nucleus of experimental glaucomatous rats. Exp
Brain Res. 144:365–372. 2002. View Article : Google Scholar
|
|
27
|
Zeng XW, Li MW, Pan J, et al: Activation
of c-Jun N-terminal kinase 1/2 regulated by nitric oxide is
associated with neuronal survival in hippocampal neurons in a rat
model of ischemia. Chin Med J (Engl). 124:3367–3372. 2011.
|
|
28
|
Lam PY and Cadenas E: Compromised
proteasome degradation elevates neuronal nitric oxide synthase
levels and induced apoptotic cell death. Arch Biochem Biophys.
478:181–186. 2008. View Article : Google Scholar
|