Introduction
Prostate cancer (PCa) is one of the most common
malignancies and represents the second most common cause of
cancer-associated mortalities among males in the USA (1). Metastasis is a major cause of
mortality among patients with PCa (2). Clinically insignificant tumors are
widespread in elderly men; however, PCa frequently has an
aggressive phenotype that requires rapid intervention (3). Therefore, PCa is a target for
effective anti-metastatic drugs.
Epithelial-mesenchymal transition (EMT) is a
fundamental process of embryogenesis; however, it is also
associated with the progression of a number of different types of
cancer (4–6). During EMT reprogramming, epithelial
cells acquire mesenchymal phenotypes. In addition, they gain the
expression of mesenchymal markers, including vimentin, fibronectin
and N-cadherin, which results in an enhanced ability for cell
migration and invasion (7),
therefore promoting the metastatic ability of cancer cells.
Following migration, the tumor cells undergo
mesenchymal-to-epithelial transition (MET) and regain the
expression of epithelial markers, including E-cadherin (8). Increasing evidence suggests that EMT
is important during the progression and malignant transformation of
PCa, allowing cancer cells to gain invasive and metastatic
properties (9–11). Therefore, EMT may be a promising
therapeutic target and the inhibition of EMT may prevent or
restrain the invasion and metastasis of PCa cells.
Current anti-cancer therapies only offer modest
benefits (12) and natural
products as chemopreventive agents may provide alternative and safe
cancer treatments (13). Among
several dietary chemopreventive agents, resveratrol has gained
considerable interest (14).
Resveratrol is found in various plants, including grapes, and is
used in traditional Chinese medicine (15). Previous studies have shown that
resveratrol has numerous pharmaceutical properties, including
anti-tumorigenic capabilities (16) against a number of different tumor
cell types, including breast, prostate and esophageal cancer cells
(17–19). Resveratrol is also considered to be
a potent chemopreventive agent. It has been suggested that the
anti-invasive effects of resveratrol may be due to its ability to
inhibit EMT; however, the molecular mechanisms of resveratrol have
yet to be fully elucidated. It has previously been shown that
lipopolysaccharide (LPS) induces EMT in cancer cells (20), therefore, in the present study, LPS
was used to induce EMT. The aim of the present study was to
investigate the ability of resveratrol to inhibit LPS-triggered EMT
in PCa cells. In addition, it was investigated whether this effect
was accompanied by the inhibition of the Hedgehog (Hh) signaling
pathway.
Materials and methods
Reagents and cell culture
Antibodies against glioma-associated oncogene
homolog 1 (Gli1), E-cadherin, vimentin and β-actin were purchased
from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Matrigel
and LPS were purchased from Sigma (St. Louis, MO, USA). Resveratrol
(purity, >98%) was purchased from Shanghai Tauto Biotech Co.,
Ltd. (Shanghai, China). PC-3 and LNCaP prostate cancer cell lines
(obtained from the American Type Culture Collection, Manassas, VA,
USA) were maintained in Dulbecco’s modified Eagle medium (DMEM,
Gibco®, Carlsbad, CA, USA) supplemented with penicillin
(100 U/ml), streptomycin (100 μg/ml), 0.1 mM non-essential amino
acids, 0.2 mM glutamine, 1 mM pyruvate and 10% heat-inactivated
fetal bovine serum and incubated in 5% CO2 humidified
atmosphere at 37°C. Cells were grown to 80% confluence prior to
treatment. The present study was approved by the Institutional
Review Board and Ethics Committee of the Xi’an Jiaotong University,
Xi’an, China.
Cell viability assay
Cells were seeded (5×103/well) in 200 μl
of DMEM into 96-well plates and cultured overnight. The MTT assay
was then used to determine cell viability. Resveratrol (0–50 μM)
was added to the cells and the cells were cultured for 24 h. The
MTT reagent (5 mg/ml) was added and the cells were incubated for a
further 4 h. The reaction was terminated by adding 150 μl
dimethylsulfoxide (Sigma) per well. The absorbance values were
determined using an MRX Revelation 96-well multiscanner (Dynex
Technologies, Chantilly, VA, USA). The cells cultured in DMEM
served as the control group. The cell viability index was
calculated using the following formula: Experimental optical
density (OD) value/control OD value. Each experiment was repeated
three times.
Scanning electron microscopy
The cells treated or untreated with resveratrol were
harvested and washed with phosphate-buffered saline (PBS). Cells
were fixed for 2 h in 4% paraformaldehyde and 1% glutaraldehyde in
0.1 M phosphate buffer (PB; pH 7.4) and then rinsed in PB, prior to
being fixed in 1% osmium tetraoxide for 1 h. The cells were washed
with PB and progressively dehydrated in 10% graded series of
30–100% ethanol. The cells were then dried in an acetonitrile
solution of 70–100%. Finally, cells were sprayed gold and examined
using scanning electron microscopy.
Immunofluorescence assay
Exponentially growing cells were seeded on 25-mm
square glass cover slips and placed in 35-mm diameter culture
dishes. Following treatment, the cells were fixed with 4%
formaldehyde for 5 min, permeabilized with 0.2% solution of Triton
X-100 in PBS and blocked using 2% bovine serum albumin-PBS for 30
min. Slides were then incubated overnight with cyanine 3-labeled
anti-E-cadherin (1:100) and fluorescein isothiocyanate-labeled
anti-vimentin (1:100), respectively. The cell nuclei were
counterstained using DAPI. Fluorescent imaging was performed using
a confocal laser scanning microscope (Carl Zeiss MicroImaging,
Inc., Oberkochen, Germany).
Cell invasion assay
The cell invasion assay was performed using Boyden
chambers with 8 μm porosity polyvinylpyrrolidone-free polycarbonate
filters coated with 50 μg/ml Matrigel solution. The cells were
seeded in 12-well plates at a concentration of
2.5×105/well and were cultured for 48 h with LPS (5
μg/ml). For the co-treatment experiment, 20 μM resveratrol was
added to the cell cultures 1 h prior to the addition of LPS. Normal
culture medium was added to the bottom chamber to induce the cancer
cell lines. Pretreated cells were seeded in the top chamber. The
Matrigel invasion chamber was incubated for 24 h in a humidified
tissue culture incubator, and after 24 h, the non-invasive cells
were removed from the upper surface of the separating membrane
using a cotton swab. The invading cells were then fixed in 100%
methanol and stained with 0.1% crystal violet solution. They were
then counted using a microscope (magnification, ×200; (Carl Zeiss
MicroImaging, Jena, Germany).
Quantitative polymerase chain reaction
(qPCR)
Total cellular RNA was isolated using the Illustra
triplePrep extraction kit in accordance with the manufacturer’s
instructions. The quantity of RNA was determined
spectrophotometrically. The RNA was then reverse transcribed using
the High Capacity RNA-to-cDNA Master Mix (Applied Biosystems™,
Foster City, CA, USA). First-strand cDNA was synthesized from 2 μg
of total RNA. The PCR primer sequences used were as follows:
E-caderin (502 bp) forward, 5′-CGCATTGCCACATACA-3′ and reverse,
5′-CGTTAGCCTCGTTCTCA-3′; Vimentin (690 bp) forward,
5′-CGCTTCGCCAACTAC AT-3′, and reverse, 5′-AGGGCATCCACTTCACAG-3′;
β-actin (179 bp) forward, 5′-ATCGTGCGTGACATTAAGGAGAAG-3′ and
reverse, 5′-AGGAAGGAAGGCTGGAAGAGTG-3′. PCR was performed under the
following conditions: denaturation at 95°C for 30 sec, annealing at
60°C for 30 sec and extension at 72°C for 45 sec. PCR samples were
loaded onto a 1.2% agarose gel containing ethidium bromide. All PCR
experiments were performed in triplicate. The housekeeping gene
β-actin was used as an internal control.
Western blotting
For isolation of total proteins, control and treated
cells were washed in ice cold PBS, lysed in
Radioimmunoprecipitation assay buffer (containing 50 mM Tris-base,
150 mM NaCl, 0.1% SDS, 1% Triton X-100, 0.5% sodium deoxycholate, 1
mM sodium orthovanadate, 10 mM sodium fluoride and 1% protease
inhibitor cocktail) and quantified using the Bradford protein
assay. The cellular lysates were separated using 10% SDS-PAGE, and
transferred onto a nitrocellulose membrane. The membranes were
blocked with 5% non-fat milk in TBST and incubated with primary
antibodies at 4°C overnight. The membranes were then incubated with
1:2,000 horseradish peroxidase-conjugated secondary antibodies for
2 h. Immunoreactive bands were visualized using an enhanced
chemiluminescence kit. Western blot signals were quantitated by
densitometric analysis using Total Lab Nonlinear Dynamic Image
analysis software (MathWorks, Natick, MA, USA).
Statistical analysis
Each experiment was performed ≥three times. Data are
presented as the mean ± standard deviation and the differences were
analyzed using Student’s t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effect of resveratrol on the growth of
PCa cells in vitro
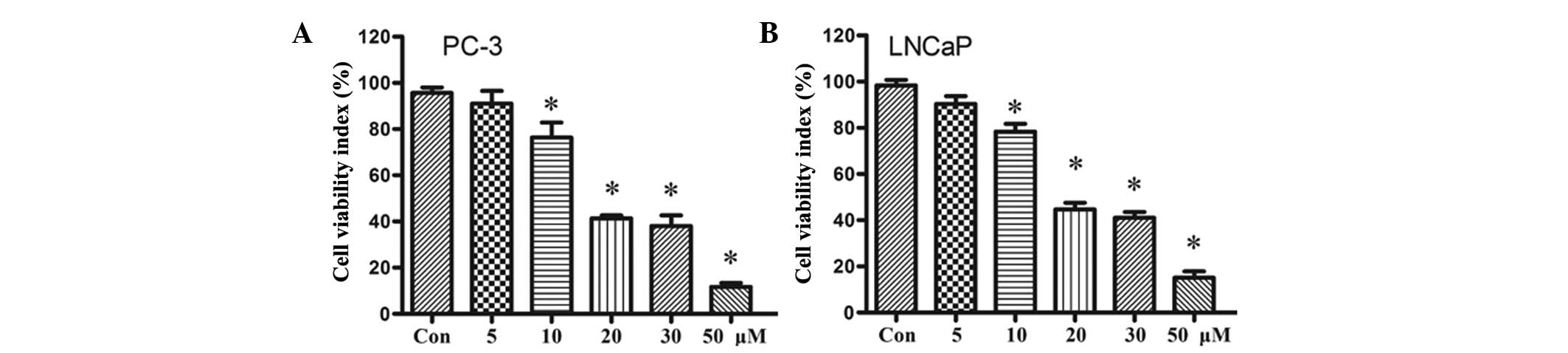
The effect of resveratrol on the proliferation of
PC-3 and LNCaP cells was investigated. The two cell lines were
cultured in vitro with different concentrations of
resveratrol (0–50 μM) for 48 h, and cell viability was measured
using the MTT assay. The results demonstrated that the
proliferative abilities of PC-3 and LNCaP cells decreased in the
presence of resveratrol in a dose-dependent manner. In addition,
the results demonstrated that treatment with resveratrol at
concentrations ≤10 μM exhibited no cytotoxic effects on PC-3 and
LNCaP cells (Fig. 1). Therefore,
lower concentrations of resveratrol, without cytotoxic effects on
PC-3 and LNCaP cells, were used for the subsequent experiments.
Resveratrol inhibits LPS-induced EMT
morphological changes in PCa cells
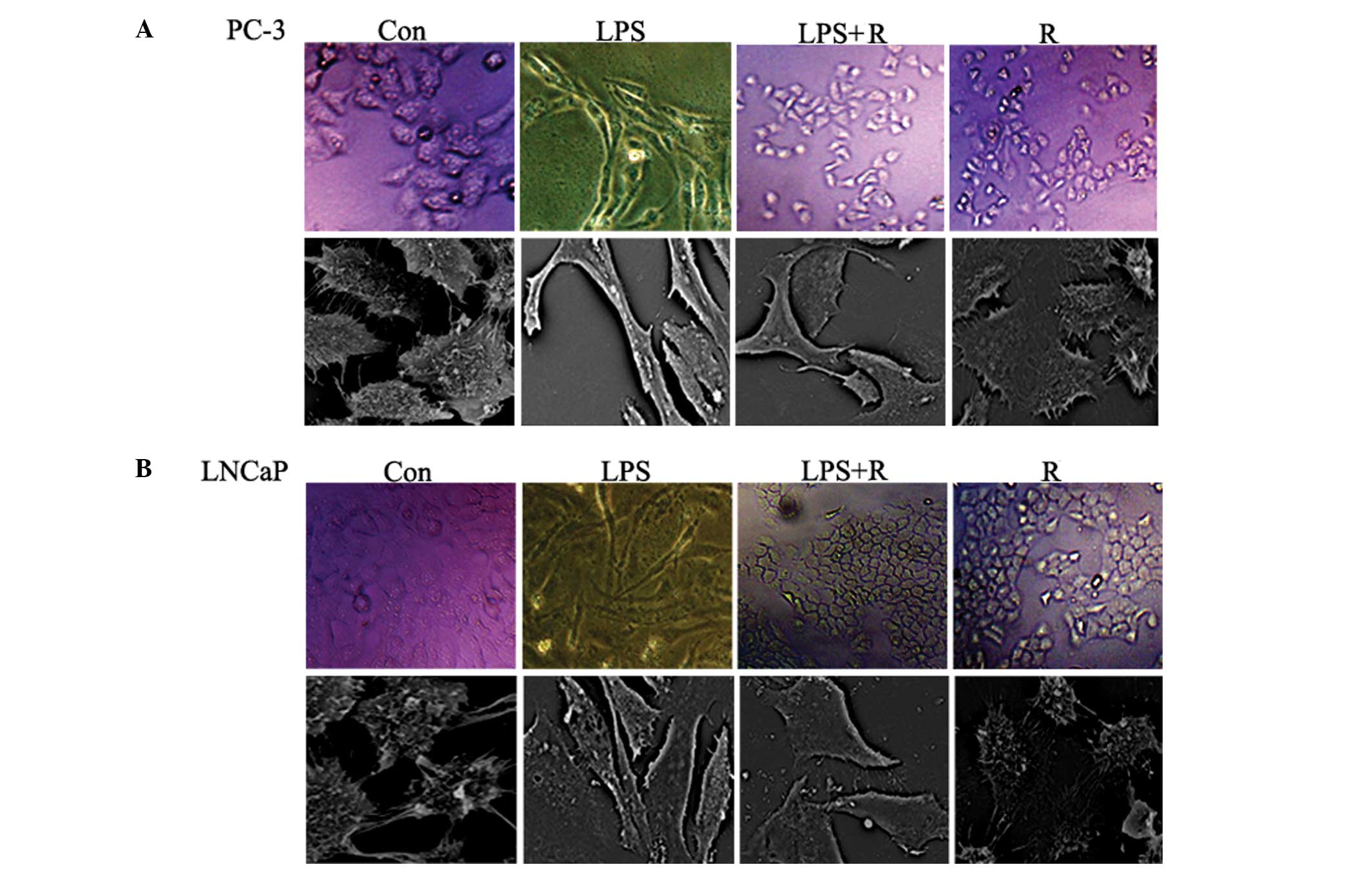
In the present study, it was investigated whether
resveratrol may inhibit EMT. LPS-treated PC-3 and LNCaP cell lines
were used since LPS (5 μg/ml) has been previously demonstrated to
induce EMT (20). Optical and
scanning electron microscopy was used to investigate changes in the
morphology of PC-3 and LNCaP human PCa cells exposed to LPS, in the
presence or absence of resveratrol. Cells were treated with LPS for
48 h. As shown in Fig. 2A and B,
the two cell lines underwent typical EMT morphological changes in
response to LPS: there was a loss of cell-to-cell contact leaving
scattered clusters of cells, the cells acquired a spindle-shaped
and fibroblast-like phenotype and scanning electron microscopy
revealed that the number of extracellular microvilli increased in
certain cells. It was then investigated whether resveratrol was
capable of inhibiting these LPS-induced phenomena. The mesenchymal
phenotype was less marked in cells co-treated with LPS and
resveratrol compared with cells treated with LPS alone (Fig. 2A and B). These results indicate
that resveratrol inhibits LPS-induced EMT.
Resveratrol inhibits the expression of
EMT markers in PCa cells
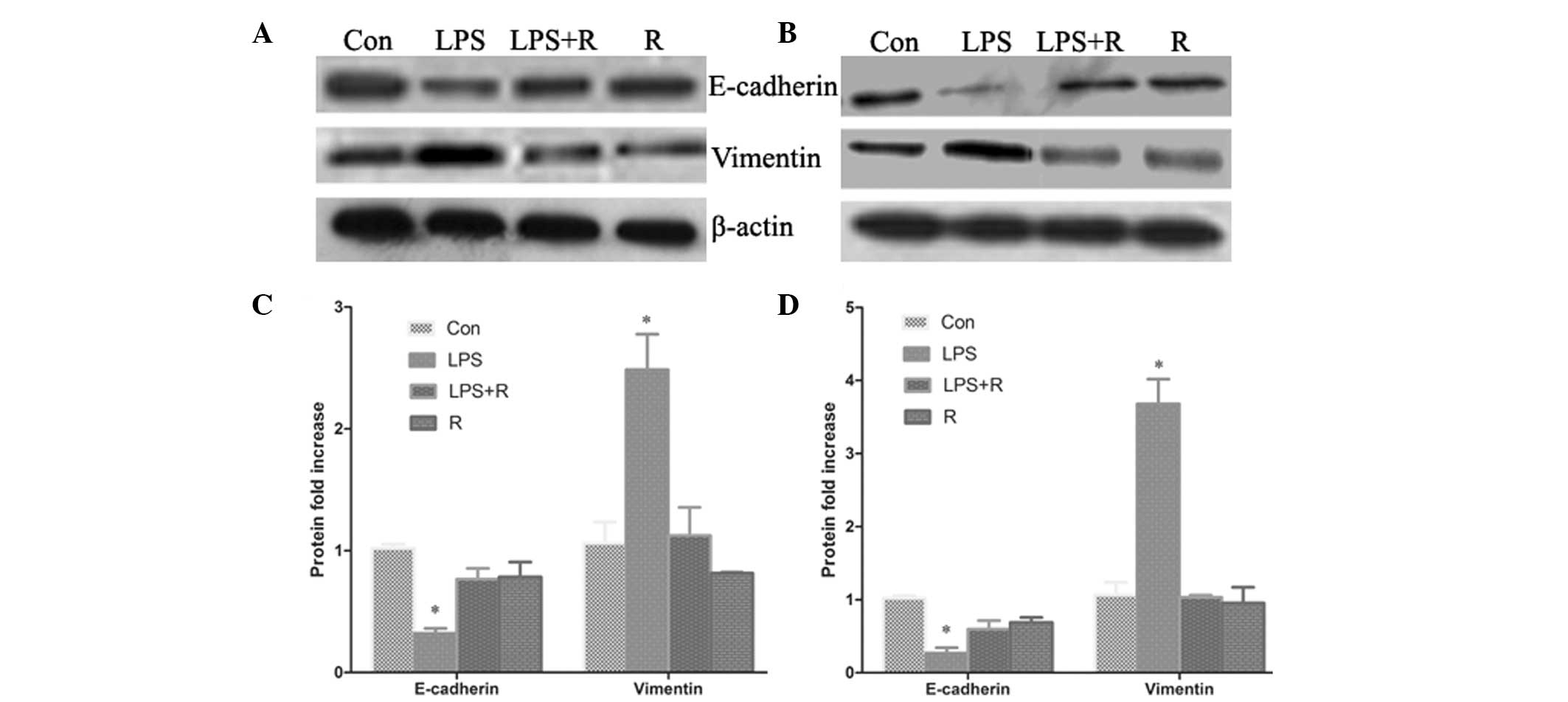
In addition to the morphological changes, the
expression of EMT phenotypic markers was detected using qPCR and
western blot analysis. The results from the qPCR (Fig. 3A–F) demonstrate that the mRNA
levels of vimentin and E-cadherin in LPS-treated cells were
significantly increased and suppressed, respectively. Western blot
analysis (Fig. 4A–D) revealed that
the protein expression of E-cadherin was also significantly
downregulated in the LPS-treated cells compared with control cells,
whilst vimentin protein expression was significantly increased
(P<0.05). In cells treated with resveratrol, LPS-induced EMT was
found to be reversed, resulting in the induction of E-cadherin
expression and the inhibition of vimentin expression (Fig. 3 and 4). These results further suggest that
resveratrol has an inhibitory effect on cellular EMT.
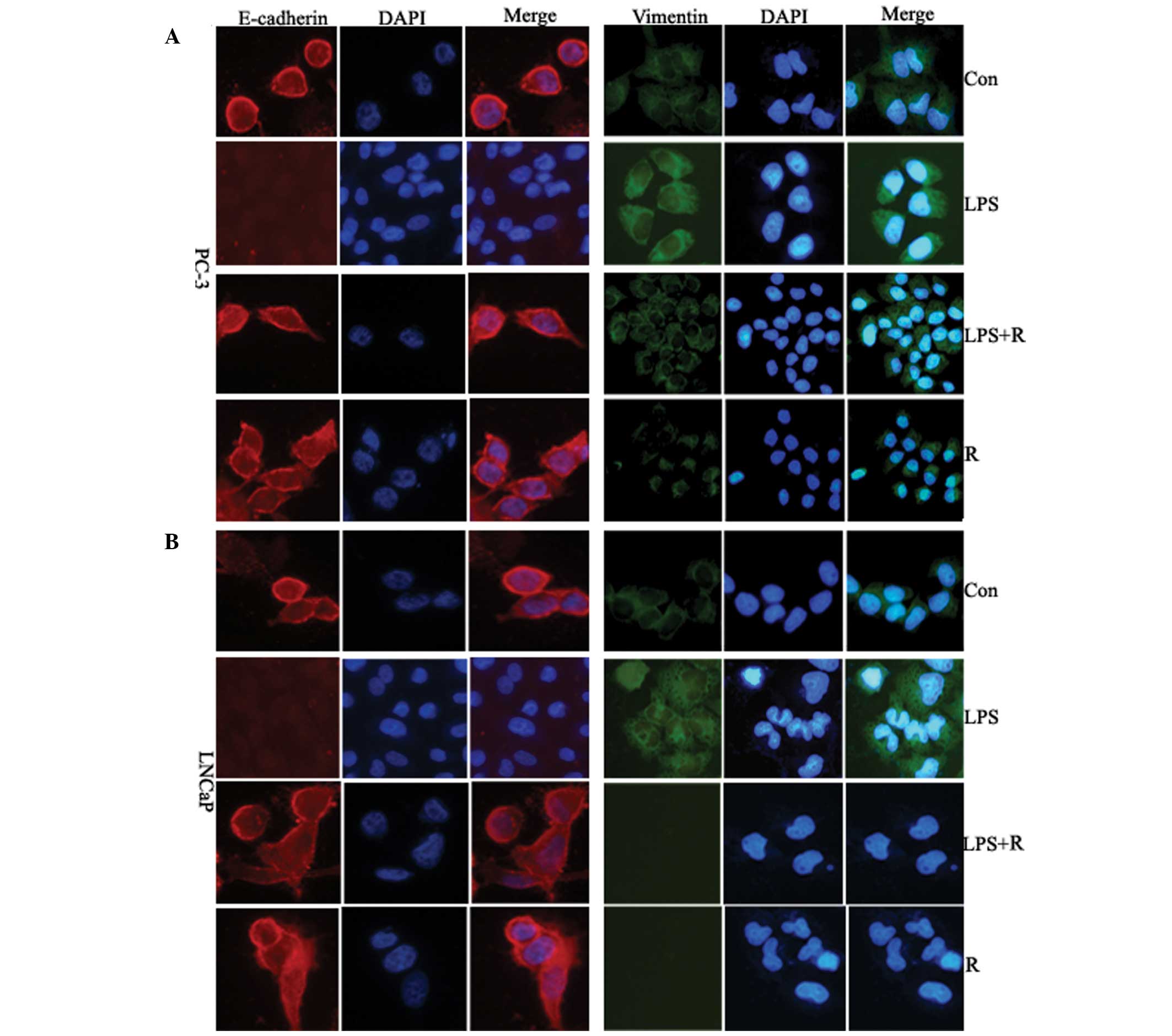
To further determine possible alterations in
E-cadherin and vimentin, PC-3 and LNCaP cells treated with
resveratrol were stained with fluorescence immunostaining and
analyzed using confocal microscopy. The E-cadherin fluorescence
signal in the resveratrol treated-cells was higher after 48 h
compared with untreated cells, whilst the vimentin fluorescence
signal was substantially lower (Fig.
5). These results further suggest that resveratrol has
inhibitory effect on cellular EMT.
Hh signaling is required to decrease
E-cadherin and increase vimentin expression levels
Previous studies have suggested that the Hh
signaling pathway may induce EMT of cancer cells. To investigate
whether the inhibitory effect of resveratrol is associated with the
inhibition of Hh signaling activation, the expression of Gli1
transcription factor was measured in PCa cells using western blot
analysis. The results demonstrated that LPS promotes the expression
of Gli1 protein, accompanied by a decrease in E-cadherin expression
and an increase in vimentin expression. It was found that
resveratrol inhibits this effect (Fig.
6). These results indicate that Hh signaling has an important
role in the EMT process.
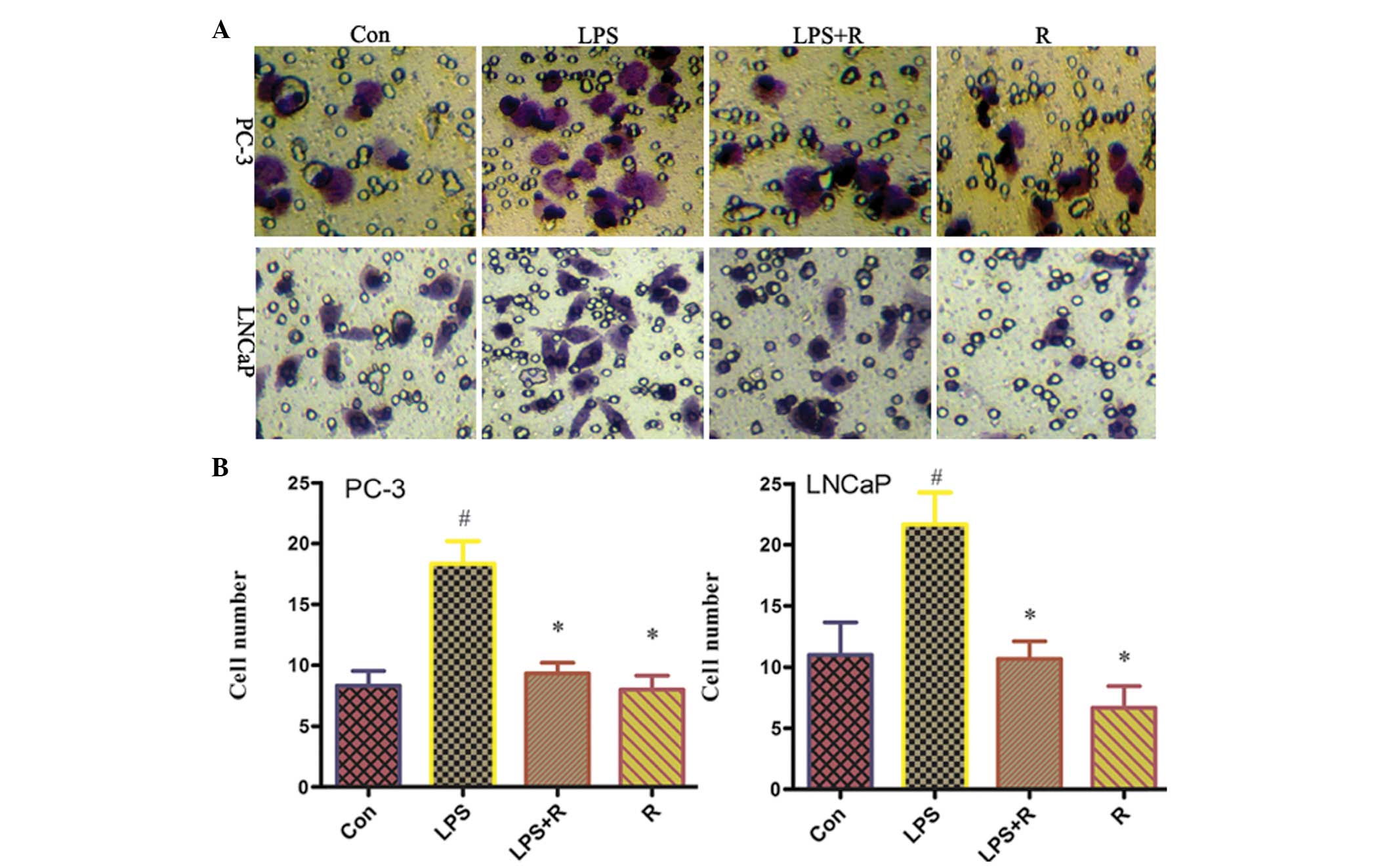
Resveratrol inhibits invasion of PCa
cells
EMT is associated with enhanced cellular
progression. To investigate whether resveratrol may inhibit tumor
invasion, an in vitro invasion assay, using a Matrigel
model, was performed. As shown in Fig.
7, following treatment with LPS, the number of invasive cells
increased significantly compared with untreated cells. However, the
number of invasive cells was significantly reduced in cells
co-treated with LPS and resveratrol. These results suggest that
resveratrol blocks the ability of LPS to increase the invasiveness
of human PCa cells.
Discussion
EMT has an important role in embryonic development
(21); however, it has also
recently been implicated in tumor invasiveness (22). The acquisition of EMT phenotype
allows cells to metastasize in distant sites, therefore enhancing
tumor progression (4).
Previous studies have suggested that LPS may have a
role in mediating EMT. The results from the present study
demonstrated that PC-3 and LNCaP cells treated with LPS exhibit a
spindle-shaped, fibroblastic morphology and express EMT markers.
However, LPS-induced EMT in PCa cells (PC-3 and LNCaP) treated with
resveratrol was no longer observed. Resveratrol was found to
restore the epithelial phenotype in mesenchymal cells and inhibit
the expression of LPS-induced EMT markers. It was also shown that
resveratrol upregulates the expression of E-cadherin, whilst
downregulating the expression of vimentin. In addition, resveratrol
inhibits the expression of LPS-induced Gli1 protein. Gli1 is a
transcription factor in the Hh signaling pathway, therefore
suggesting that it mediates the expression of E-cadherin and
vimentin (23). It is likely that
the Hh signaling may be involved in LPS-induced EMT in PC-3 and
LNCaP PCa cells. These results extend the current understanding of
the mechanism by which resveratrol may act to inhibit cancer cell
invasiveness.
Resveratrol is a polyphenolic phytoalexin found in
grapes and other fruits. Currently, numerous preclinical studies
have found that resveratrol is a potential therapeutic agent for
cancer prevention and/or treatment. Resveratrol has been shown to
retard the growth of various cancer cells through multiple cellular
signaling pathways, including the Src-STAT3, NF-κB, Wnt and Hh
signaling pathways (17–19,24).
Vergara et al (25)
demonstrated that resveratrol inhibits the epidermal growth
factor-induced EMT in MCF-7 cells (25). Li et al (26) also reported that resveratrol
inhibits EMT in pancreatic cancer cells via suppression of the
PI-3K/Akt/NF-κB pathway, and Wang et al (27) demonstrated that resveratrol
inhibits TGF-β1-induced EMT and suppresses lung cancer invasion and
metastasis (27). Furthermore,
Chen et al (28) reported
that resveratrol inhibits LPS-induced EMT in a mouse melanoma
model. In the present study, it was found that resveratrol inhibits
the expression of Gli1 and downregulates or upregulates the
expression of the EMT markers E-cadherin and vimentin,
respectively. Additionally, resveratrol inhibits cancer cell
invasion, and these results are in accordance with the results from
Chen et al (28).
Furthermore, in the present study, it was demonstrated for the
first time, to the best of our knowledge, that the anti-metastatic
effects of resveratrol are associated with EMT in PCa cells. These
results provide a novel perspective on the role of resveratrol in
preventing the progression of cancer.
Hh proteins were first identified in Drosophilia
melanogaster, and were found to regulate embryonic cell growth
and carcinogenesis in certain vertebrate tissues (23). As a signaling pathway, Hh firstly
binds to Patched (Ptch), leading to the activation of Gli1
transcription factors and an upregulation of Gli1 target genes
(29). The Gli1 transcription
factor is an important mediator in the Hedgehog pathway that
regulates genes essential for tumor progression (30). Xu et al (31) previously demonstrated that sonic
hedgehog-Gli1 signals promote EMT by mediating a complex signaling
network in pancreatic tumors (31). Gli1 is an important positive
regulator of epithelial differentiation and decreased levels of
Gli1 are likely to contribute to the highly metastatic phenotype
observed in pancreatic ductal adenocarcinoma. In the present study,
the effect of resveratrol on Gli1 protein expression, was
investigated. It was found that the expression of Gli1 was
inhibited, accompanied by an increase in expression of E-cadherin
and a decrease in expression of vimentin. Therefore, this suggests
that Hh signaling may regulate EMT. However, other mechanisms of
regulation that may explain the ability of resveratrol to suppress
the EMT process cannot be ruled out. Multiple proteins are targeted
by resveratrol, therefore, high-throughput methods may be used in
the future. In addition, although it was demonstrated in the
present study that resveratrol inhibits EMT, further studies are
required to fully elucidate the regulatory mechanism in
vivo.
Taken together, the results from the present study
suggest that the ability of resveratrol to inhibit tumor invasion
is associated with the EMT, possibly by inhibiting the activation
of the Hh signaling and regulating the expression of the important
downstream EMT markers, E-cadherin and vimentin. These results
provide a novel mechanistic base for the therapeutic application of
resveratrol in patients with PCa.
Acknowledgements
The authors would like to thank the staff of the
Biology and Genetics Laboratory, Xi’an Jiaotong University
(Shannxi, China) for their technical assistance.
References
|
1
|
Snyder A, Tepper JE and Slovin SF:
Perspectives on immunotherapy in prostate cancer and solid tumors:
where is the future? Semin Oncol. 40:347–360. 2013. View Article : Google Scholar
|
|
2
|
Sandhu GS, Nepple KG, Tanagho YS and
Andriole GL: Prostate cancer chemoprevention. Semin Oncol.
40:276–285. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bhavsar T, McCue P and Birbe R: Molecular
diagnosis of prostate cancer: are we up to age? Semin Oncol.
40:259–275. 2013. View Article : Google Scholar
|
|
4
|
Franco-Chuaire ML, Magda Carolina CS and
Chuaire-Noack L: Epithelial-mesenchymal transition (EMT):
principles and clinical impact in cancer therapy. Invest Clin.
54:186–205. 2013.
|
|
5
|
De Craene B and Berx G: Regulatory
networks defining EMT during cancer initiation and progression. Nat
Rev Cancer. 13:97–110. 2013.PubMed/NCBI
|
|
6
|
Wu Q, Hou X, Xia J, et al: Emerging roles
of PDGF-D in EMT progression during tumorigenesis. Cancer Treat
Rev. 39:640–646. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Evdokimova V, Tognon CE and Sorensen PH:
On translational regulation and EMT. Semin Cancer Biol. 22:437–445.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tiwari N, Gheldof A, Tatari M and
Christofori G: EMT as the ultimate survival mechanism of cancer
cells. Semin Cancer Biol. 22:194–207. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hance MW, Dole K, Gopal U, et al: Secreted
Hsp90 is a novel regulator of the epithelial to mesenchymal
transition (EMT) in prostate cancer. J Biol Chem. 287:37732–37744.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lu T, Lin WJ, Izumi K, et al: Targeting
androgen receptor to suppress macrophage-induced EMT and benign
prostatic hyperplasia (BPH) development. Mol Endocrinol.
26:1707–1715. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Clyne M: Prostate cancer: androgen
deprivation causes EMT in the prostate. Nat Rev Urol. 9:42012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fryer RA, Galustian C, Dalgleish AG and
Dalgelish AG: Recent advances and developments in treatment
strategies against pancreatic cancer. Curr Clin Pharmacol.
4:102–112. 2009. View Article : Google Scholar
|
|
13
|
Surh YJ: Cancer chemoprevention with
dietary phytochemicals. Nat Rev Cancer. 3:768–780. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hasan MM, Yun HK, Kwak EJ and Baek KH:
Preparation of resveratrol-enriched grape juice from
ultrasonication treated grape fruits. Ultrason Sonochem.
21:729–734. 2014. View Article : Google Scholar
|
|
15
|
Pollack RM and Crandall JP: Resveratrol:
therapeutic potential for improving cardiometabolic health. Am J
Hypertens. Sep 11–2013.(Epub ahead of print).
|
|
16
|
Ren Z, Wang L and Cui J: Resveratrol
inhibits NF-kB signaling through suppression of p65 and IkappaB
kinase activities. Pharmazie. 68:689–94. 2013.
|
|
17
|
Whitlock NC and Baek SJ: The anticancer
effects of resveratrol: modulation of transcription factors. Nutr
Cancer. 64:493–502. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Aluyen JK, Ton QN, Tran T, Yang AE,
Gottlieb HB and Bellanger RA: Resveratrol: potential as anticancer
agent. J Diet Suppl. 9:45–56. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vang O, Ahmad N, Baile CA, et al: What is
new for an old molecule? Systematic review and recommendations on
the use of resveratrol. PLoS One. 6:e198812011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen MC, Chang WW, Kuan YD, Lin ST, Hsu HC
and Lee CH: Resveratrol inhibits LPS-induced epithelial-mesenchymal
transition in mouse melanoma model. Innate Immun. 18:685–693. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Colas E, Pedrola N, Devis L, et al: The
EMT signaling pathways in endometrial carcinoma. Clin Transl Oncol.
14:715–720. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu DZ, Wang YS and Ji AG: The role of
TGF-beta in the EMT of tumor cells. Sheng Li Ke Xue Jin Zhan.
42:463–466. 2011.(In Chinese).
|
|
23
|
Ruat M, Angot E and Traiffort E: Shh
signal and its functional roles in normal and diseased brain. Med
Sci (Paris). 27:979–985. 2011.(In French).
|
|
24
|
Shankar S, Nall D, Tang SN, et al:
Resveratrol inhibits pancreatic cancer stem cell characteristics in
human and KrasG12D transgenic mice by inhibiting pluripotency
maintaining factors and epithelial-mesenchymal transition. PLoS
One. 6:e165302011. View Article : Google Scholar
|
|
25
|
Vergara D, Valente CM, Tinelli A, et al:
Resveratrol inhibits the epidermal growth factor-induced epithelial
mesenchymal transition in MCF-7 cells. Cancer Lett. 310:1–8. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li W, Ma J and Ma Q: Resveratrol inhibits
the epithelial-mesenchymal transition of pancreatic cancer cells
via suppression of the PI-3K/Akt/NF-κB pathway. Curr Med Chem.
20:4185–4194. 2013.PubMed/NCBI
|
|
27
|
Wang H, Zhang H, Tang L, et al:
Resveratrol inhibits TGF-β1-induced epithelial-to-mesenchymal
transition and suppresses lung cancer invasion and metastasis.
Toxicology. 303:139–146. 2013.
|
|
28
|
Chen MC, Chang WW, Kuan YD, Lin ST, Hsu HC
and Lee CH: Resveratrol inhibits LPS-induced epithelial-mesenchymal
transition in mouse melanoma model. Innate Immun. 18:685–693. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vaillant C and Monard D: SHH pathway and
cerebellar development. Cerebellum. 8:291–301. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Panman L and Zeller R: Patterning the limb
before and after SHH signalling. J Anat. 202:3–12. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xu QR, Zheng X, Zan XF, Yao YM, Yang W and
Liu QG: Gli1 expression and its relationship with the expression of
Shh, Vimentin and E-cadherin in human hepatocellular carcinoma. Xi
Bao Yu Fen Zi Mian Yi Xue Za Zhi. 28:536–539. 2012.(In
Chinese).
|