Introduction
Stromal-derived factor-1α (SDF-1α), also known as
CXCL12, is one of ~50 soluble peptides that have been characterized
as chemokines. SDF-1α is expressed in a wide range of malignant
tissues as well as in various normal tissues. Its physiological
expression has been documented in a number of organs, including
heart, brain, kidney, adrenal glands, liver, lung, skeletal
muscles, lymphoid organs and bone marrow (1,2). In
these tissues, vascular endothelial cells, stromal fibroblasts and
osteoblasts are major SDF-lα producers (2). SDF-lα secretion increases during
tissue damage, limb ischemia, toxic liver damage, excessive
bleeding, total body irradiation and damage associated with
chemotherapy.
SDF-lα was originally described as a pre-B cell
growth factor (3). However, a
number of different functions for this chemokine have subsequently
been determined. Under normal conditions, SDF-1 participates in
regulating hematopoiesis and modulating the immune system via
signaling through C-X-C chemokine receptor 4 (CXCR4), which mediate
chemotaxis and have an important role in cell-homing responses
(4,5). It is also a modulator of cell growth
and survival (6). CXCR4 was
originally considered to be the only receptor for this chemokine.
However, it has been identified that SDF-1α also reacts with the
CXCR7 receptor (7). A considerable
amount of evidence on CXCR4 and CXCR7 receptors has accumulated in
recent years (8).
SDF-1α affects the chemoattraction of
CXCR4+ cells, which is necessary for tissue/organ
regeneration (9,10). For oncological malignancies, the
SDF-1α/CXCR4 axis promotes tumor progression by several different
mechanisms. The most important of these appear to be: (i) directly
supporting the growth of neoplastic cells; (ii) promoting
metastatic spread to organs that highly express SDF-1α; (iii)
supporting tumor angiogenesis by attracting endothelial cells to
the tumor microenvironment and reducing extracellular angiostatin
levels by downregulating phosphoglycerate kinase (PGK) (2,4,9,11–13).
It has also been demonstrated that SDF-1α promotes cytotoxic T-cell
apoptosis (14).
Stromal elements are attractive therapeutic targets
due to their roles in tumor biology (15) and a number of studies that target
the SDF-1α/CXCR4 axis are under way at present. It has already been
demonstrated that CXCR4 antagonists may have anti-tumor activities
in patients with various malignancies (16). This treatment appears to affect
both the capability to disseminate tumor cells and the sensitivity
of a tumor to immunotherapy (17).
In the present study, bcr-abl-transformed mouse 12B1
cells were utilized and the effects of SDF-1α administration on the
efficacy of a DNA vaccine carrying a complete bcr-abl fusion gene
was investigated. The original hypothesis proposed that repeated
intracutaneous administration of a plasmid carrying the SDF-1α gene
may induce immune reactions against this chemokine and result in
enhancing the immunogenicity of the bcr-abl-directed vaccine. The
results indicated that this did not occur. However, administering
this chemokine did result in a change in the oncogenic potential of
the bcr-abl-transformed mouse 12B1 cells.
Materials and methods
Cells
The 12B1 cells derived from BALB/c mouse bone marrow
cells transfected with a human bcr-abl fusion gene and expressing
the BCR-ABL fusion protein (18)
were consistent with those used in previous studies (19–21).
These cells are of early B-cell lineage (Krmencikova-Fliegl;
manuscript in preparation) and induce acute leukemia-like disease
following intravenous inoculation, but form solid tumors when
administered subcutaneously. To monitor the activity of a newly
constructed plasmid (see below), 293T cells were used. These were
cultured as previously described (22). K562 and HeLa cells were used as
positive controls for western blotting. The HeLa cell line was
grown in Dulbecco’s Modified Eagle Medium (DMEM; PAA Laboratories,
Pasching, Austria) supplemented with 10% fetal bovine serum (PAA
Laboratories) and antibiotics. K562 cells were cultured as
described previously (23).
Mice
Female BALB/c mice aged 5–6 weeks were obtained from
Charles River Laboratories (Sulzfeld, Germany). All of the
experimental animal procedures were performed in accordance with
the validated regulations of the Czech Republic. The study was
approved by the Ethics Committee of the First Medical Faculty,
Charles University (Prague, Czech Republic).
Plasmids
The construction of a mammalian expression plasmid,
pBSC, used as a negative control and a pBSC/bcr-abl plasmid
carrying the whole bcr-abl gene, was conducted as described
previously (19,24). A plasmid designated pBSC/SDF1α was
prepared using a pUC57/SDF-1α plasmid (GeneScript, Piscataway, NJ,
USA) carrying the whole SDF1α gene flanked with a Flag sequence. A
fragment encoding for SDF-1α and Flag was obtained by cleavage with
EcoRI and Bgl III (New England Biolabs, Inc., Ipswich, MA, USA) and
subsequently inserted into a pBSC plasmid downstream of the
immediately early human cytomegalovirus promotor using the same
restriction sites. The inserted gene was re-sequenced with
satisfactory results.
Western blotting
To verify the functionality of our new construct,
293T cells were transfected either with the pBSC/SDF-1α plasmid or
with an empty pBSC used as a control. Following 2 days, the cells
were harvested and lysed with Kaufman buffer. The cell lysates were
examined by western blotting as described previously (23). A mouse monoclonal antibody against
Flag (Amersham Biosciences, Little Chalfont, UK) was used as the
primary antibody and a peroxidase labeled anti-mouse antibody (GE
Healthcare, Little Chalfont, UK) was used as the secondary
antibody.
To test for the presence of SDF-1α in the lysates of
12B1 cells, a goat anti-SDF/PBSF antibody (Sigma-Aldrich, St.
Louis, MO, USA), a rabbit anti-SDF-1 antibody (Abcam, Cambridge,
UK), horseradish-peroxidase-labeled donkey anti-goat IgG antibody
(Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and
peroxidase-labeled anti-rabbit IgG antibody (Amersham Biosciences),
respectively, were used. The 293T cells transfected with
pBSC/SDF-1α plasmids and with empty pBSC plasmids were used as
positive and negative controls, respectively. To detect
hypoxia-inducible factor-1α (HIF-1α) a mouse monoclonal anti-HIF-1α
antibody (Sigma, St. Louis, MO, USA) and peroxidase labeled
anti-mouse antibody (GE Healthcare, Little Chalfont, UK) were used.
An anti-CXCR4 rabbit polyclonal antibody (Abcam) and
horseradish-peroxidase-labeled anti-rabbit IgG antibody (GE
Healthcare) were used to detect the CXCR4 protein expression levels
in lysates of 12B1 cells and in lysates from cultures derived from
12B1 cell-induced solid tumors.
To prepare cytosolic and nuclear extracts of 12B1
cells, a total of 107 cells were washed two times in
PBS. A mixture was prepared consisting of 5 ml of buffer A (10 mM
HEPES, pH 7.9, 10 mM KCl and 0.1 mM EDTA), 5 μl of a protease
inhibitor cocktail (Sigma-Aldrich) and 200 μl of 10% IGEPAL
(Sigma-Aldrich). This mixture was added directly to the cell
pellets. Following a 10 min incubation at room temperature, the
mixture was repeatedly pipetted up and down with a P1000 (Nichyrio
America, Inc., Maryland Heights, MO, USA) to disrupt the cell
clumps and then transferred to pre-chilled microcentrifuge tubes.
Following centrifugation (13,000 × g at 4°C for 3 min), the
supernatant (cytosolic fraction) was retrieved and stored at −70°C.
The remaining pellet was resuspended in 150 μl of buffer B (20 mM
HEPES, pH 7.9, 0.4 M NaCl, 1 mM EDTA and 10% glycerol) supplemented
with 1 μl of a protease inhibitor cocktail. The mixture was shaken
vigorously at 4°C for 2 h. The nuclear extract was centrifuged
(13,000 × g at 4°C for 5 min) and stored at −70°C. A Bradford assay
was used to determine the protein concentrations.
To detect tubulin, a mouse monoclonal anti-β tubulin
antibody (Sigma) and peroxidase labeled anti-mouse antibody (GE
Healthcare) were used.
Immunization procedure
The DNA cartridges for a gene gun were prepared
according to the manufacturer’s instructions (Hélios Gene Gun
System; Bio-Rad, Hercules, CA, USA). Each cartridge contained 1 μg
of DNA on gold particles, 1 μm in diameter. DNA was administered
intradermally into a shaven abdominal area. When using pBSC and
pBSC/SDF-1α plasmids, three doses were administered at two week
intervals. The plasmid pBSC/bcr-abl was administered only twice,
simultaneously with the second and third doses of the other
plasmids. This suboptimal immunization scheme (two doses of the
pBSC/bcr-abl vaccine induces only incomplete protection) was
selected to allow for the manifestation of possible SDF-1α
effects.
Two weeks following the last dose, the mice were
challenged with 5×103 12B1 cells (i.e., ~10
TID50). Each experiment included four groups of mice (6
mice/group): (i) mice that received the empty pBSC plasmid (control
group); (ii) mice that received only the pBSC/SDF-1α plasmid; (iii)
mice that received only the pBSC/bcr-abl vaccine; and (iv) mice
that were inoculated with both the pBSC/bcr-abl and pBSC/SDF-1α
plasmids. These experiments were repeated four times. The mice were
followed for 50 days following these challenges. When the tumors
reached a size of 400 mm2, the mice were humanely
sacrificed.
ELISA
To examine the possible development of SDF-1α
specific antibodies, the plasma samples of the treated and control
mice were investigated using an in-house ELISA. The sequences of
the peptides used are presented in Table I. These were purchased from Peptide
2.0, Inc. (Chantilly, VA, USA). The peptides used covered the whole
amino acid sequence of the SDF-1 protein and mutually overlapped by
~10 amino acids. This was performed to avoid the possible loss of
any important epitopes and was conducted as described previously
(25).
 | Table IPeptide sequences used for in-house
ELISAs. These peptides covered the whole amino-acid sequence of the
SDF-1 protein and overlapped by 10 amino acids. |
Table I
Peptide sequences used for in-house
ELISAs. These peptides covered the whole amino-acid sequence of the
SDF-1 protein and overlapped by 10 amino acids.
| Peptide
designation | Peptide sequence | Peptide length |
|---|
| SDF-1α 1/3 |
MDAKVVAVLALVLAALCISDGKPVSLSYRCPCRFFE | 36aa |
| SDF-1α 2/3 |
SYRCPCRFFESHIARANVKHLKILNTPNCALQIVARL | 37aa |
| SDF-1α 3/3 |
NCALQIVARLKNNNRQVCIDPKLKWIQEYLEKALNK | 36aa |
To examine for the presence of SDF-1α in the media
of the 12B1 cell cultures, a mouse CXCL12/SDF-1α ELISA kit (Cell
Sciences, Inc., Canton, MA, USA) was used following the
manufacturer’s instructions.
ELISPOT assay
To examine for the possible development of
cell-mediated immune responses against SDF-1α, an ELISPOT assay was
used as described previously (26). Lymphocytes (8×105) from
the mice inoculated with either pBSC or pBSC/SDF-1α plasmids were
incubated with several concentrations (0.1 μg, 1 μg and 10 μg) of
KVVAVLAL peptides. This sequence located at the N-terminus of the
SDF-1α protein had a high T-epitope score using both ‘Syfpeithi’
and ‘Rankpep’ prediction software. Lymphocytes that produced
interferon-γ (INF-γ) were detected with an ImmunoSpot S5 UV Lite
Analyzer (Cellular Technology Limited, Shaker Heights, OH,
USA).
Statistical analysis
Survival analysis for the experimental groups used
log-rank tests. Statistical analysis was performed using Prism
software version 5.0 (Graph-Pad Software, La Jolla, CA, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Functionality of the pBSC/SDF-1α
plasmid
Firstly, the functionality of the newly constructed
pBSC/SDF-1α plasmid was examined. The western blotting results
using lysates of transfected and mock-transfected 293T cells are
revealed in Fig. 1. This
demonstrated that SDF-1α was present in the lysates of
pBSC/SDF-1α-transfected cells but not in the lysates of control
pBSC-transfected cells.
Absence of SDF-1 and presence of CXCR4
and HIF-1α in 12B1 cells
The culture media and the cell lysates of 12B1 cells
were examined for the presence of SDF-1α, by ELISA and western
blotting, respectively. SDF-1α was not detected in either of these
preparations using two different antibody systems (results not
shown). By contrast, the lysates of 12B1 cells and the lysates
obtained from the subcutaneous tumors, and from cultures derived
from them, contained substantial amounts of CXCR4 (Fig. 2A). CXCR4 was detected both in the
cytosolic and nuclear fractions of the 12B1 cells (Fig. 2B). HIF-1α was also detected in the
lysates of the 12B1 cells (Fig.
2C).
 | Figure 2(A) CXCR4 in lysates. Lane 1, HeLa
cells; lane 2, 12B1 cells. CXCR4 in tumors induced by 12B1 cells
and cell lines derived from them. Lanes 3, 5, 7, the samples
obtained from tumors immediately following necropsy; lanes 4, 6, 8,
the samples obtained from cell cultures derived from tumors. (B)
CXCR4 protein in 12B1 cells. Lysates of 12B1 cells: Lane 1, whole
cells; lane 2, cytosolic fraction; lane 3 nuclear fraction. Tubulin
was used as a control to assess the purity of nuclear fractions.
(C) HIF-1α in lysates; lane 1, human K562 cells; lane 2, 12B1
cells. CXCR4, C-X-C chemokine receptor type 4; HIF-1α,
hypoxia-inducible factor 1α. |
Survival of SDF-1 treated and untreated
mice following challenge with 12B1 cells
A total of four repeat experiments were performed
with each, including 24 mice divided into four groups as described
in Materials and methods. Although the immunization effects
differed somewhat from experiment to experiment, for each
experiment, more mice survived (2, 1, 1 and 3, respectively) that
were treated with both the pBSC/bcr-abl and pBSC-SDF-1α plasmids
than those that only received the pBSC/bcr-abl plasmid. A summary
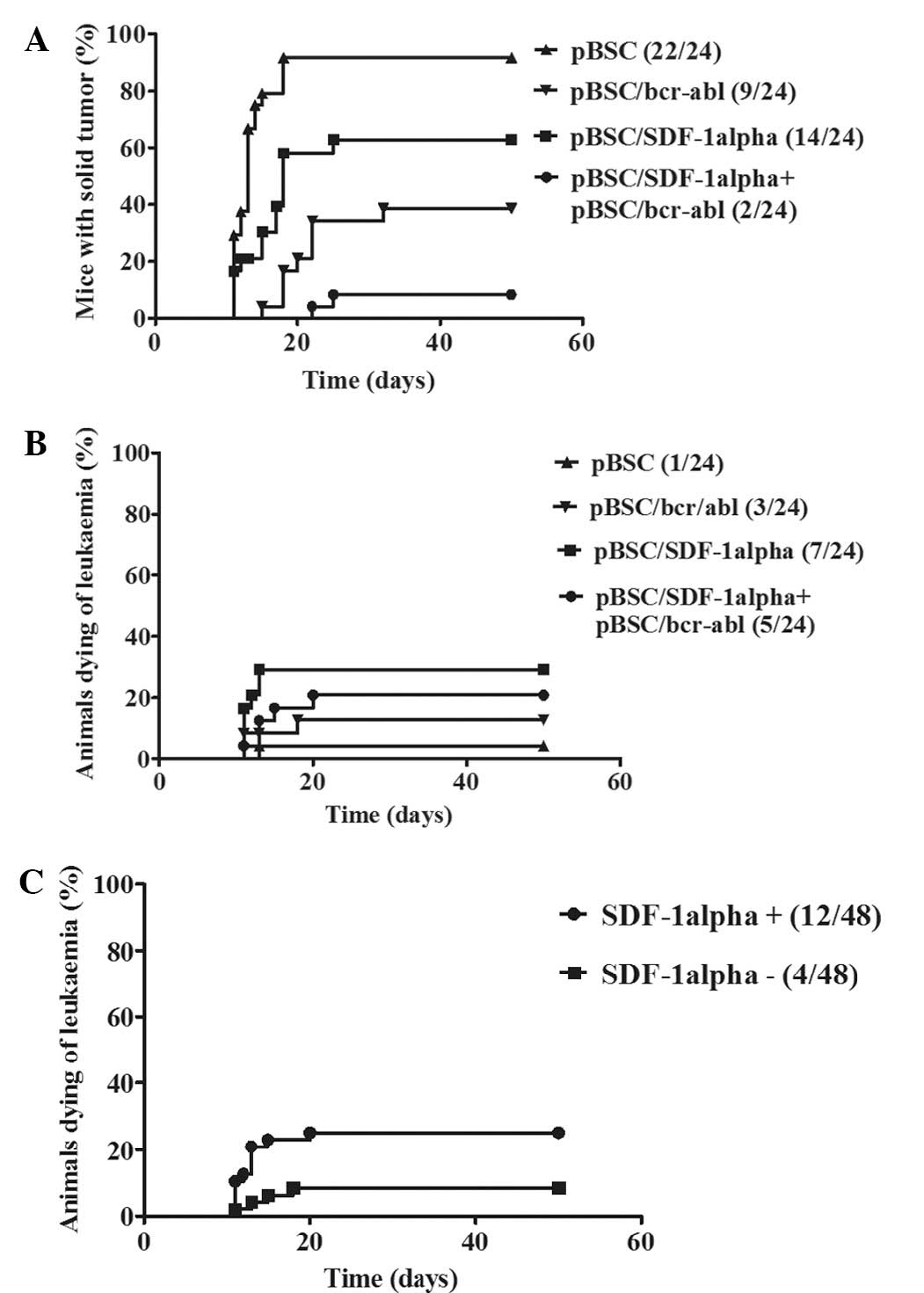
of these four experiments is illustrated in Fig. 3.
Close to all of the control mice (23/24) that were
treated with the empty plasmid were dead on day 24 following
challenge; mice were humanly sacrificed either due to a large tumor
size (22/23) or because they were dying of leukemia (1/23). For
mice that only received the pBSC-SDF-1α plasmid, several survived
(3/24). Furthermore, a number of these mice achieved a prolonged
survival.
As expected, more mice survived following receiving
the specific vaccine. Of those immunized with the pBSC/bcr-abl
vaccine alone, 12/24 (50%) survived. The number of survivors in the
group that received both the pBSC/bcr-abl and pBSC/SDF1α plasmids
was higher. A total of 17/24 (70.8%) mice survived, which suggested
that SDF-1α plasmid administration had a protective effect. The
difference in survival between pBSC/bcr-abl immunized and
non-immunized mice was highly significant (P<0.001). However,
the differences in survival between the SDF-1α treated and
untreated mice noted above were not statistically significant.
However, when the mice that developed solid tumors
were only taken into consideration, the results were markedly
different. As demonstrated in Fig.
4A, the occurrence of solid tumors was considerably lower in
mice that were treated with both the pBSC/bcr-abl vaccine and the
pBCS/SDF-1α plasmid than in those that were immunized only with the
pBSC/bcr-abl vaccine. This difference was statistically significant
(P=0.0115). There were also differences in solid tumor formation
between the pBSC/bcr-abl non-immunized mice that were either
treated or untreated with the SDF-1 plasmid. The occurrence of
solid tumors was higher and the tumors appeared earlier in mice
that did not receive the pBSC/SDF-1α plasmid than in mice to which
this plasmid was administered. This difference was also
statistically significant (P=0.0023). The occurrence of fatal
leukemia is revealed in Fig. 4B.
Although the difference within bcr-abl immunized mice was not
statistically significant (P=0.0517), the development of fatal
leukemia in pBSC/bcr-abl non-immunized, pBSC/SDF-1α treated and
untreated mice was significantly different (P=0.0191). As is also
revealed in Fig. 4C, when
comparing all of the mice (i.e., including those immunized with the
pBSC/bcr-abl vaccine) that were either treated or not treated with
the pBSC/SDF-1α plasmid, the development of leukemia was
statistically significantly different (P=0.0265).
Possible immune responses to SDF-1
To determine whether the observed differences noted
above were associated with the development of immune responses
against SDF-1α induced by the intradermally administered
pBSC/SDF-1α plasmid, we assayed for the presence of both specific
antibodies using ELISA with SDF-1α-derived peptides and cell
mediated immunity using an ELISPOT assay with an SDF-1α-derived
peptide carrying a putative T epitope, as predicted by two
different programs. Both of these tests were negative (results not
shown).
Discussion
The aim of the present study was to induce an immune
response against SDF-1α and to monitor its effects on the efficacy
of the pBSC/bcr-abl vaccine. However, with the methods used, it was
not possible to detect either a humoral or a cell-mediated immune
response against SDF-1. Despite this, a number of notable effects
of administering the pBSC/SDF-1α plasmid were evident. These were
associated with the oncogenic potential of bcr-abl-transformed 12B1
cells.
Administering the SDF-1α-expressing plasmid
simultaneously with specific vaccination against these cells
resulted in an increase in the survival among the vaccinated mice,
although this difference was not significant. However, when only
the development of solid tumors was considered, the results were
different; the increased protection in doubly inoculated mice were
significantly different.
A critical look at the present data suggests that
this effect may be, at least in part, an ‘artifact’. The increased
leukemogenic potential that resulted in early leukemia onset in the
SDF-1α-treated mice and the rapid death of the respective mice may
have obscured the induction of solid tumors, which usually appears
later than does fatal leukemia. Therefore, it is reasonable to
assume that at least a number of the mice that died early of
leukemia would have developed solid tumors later on. However, these
results strongly suggest that SDF-1α plasmid administration
resulted in a significant enhancement of the leukemogenic potential
of 12B1 cells.
The results obtained in the present study were
associated with the nature of the cells used. The 12B1 cells are of
pre-B origin, they express the BCR-ABL protein, produce substantial
amounts of CXCR4 and also produce HIF-1α. This transcription factor
is known to upregulate CXCR4 expression (27) and has been demonstrated to be
required for the survival of leukemic stem cells in a mouse CML
model (28). The role of the
chemokine SDF-1α in patients with leukemia has been most frequently
discussed in association with the role of the SDF-1α/CXCR4 axis
during the pathogenic process (29). It has been assumed that SDF-1α
interactions with CXCR4 are crucial for leukemic stem cell adhesion
to stromal cells, which would enable their self-renewal,
proliferation and differentiation arrest. However, the current
model is possibly more closely associated with the experimental
diseases induced in NOD-SCID mice following administration of human
acute leukemia cells (30) or
T-leukemia cells (31). In these
two studies, it was demonstrated that extramedullar niches had a
significant role in the development of the experimentally induced
disease. According to these authors, tumor cell replication was
demonstrated in the neighborhood of bile ducts, with their
subsequent accumulation in the portal area. When these liver-homed
cells were exposed to SDF-1α in vitro, the number of
colonies that formed significantly increased. It is likely that in
the present study, additional SDF-1α formed following pBSC/SDF-1α
plasmid administration, contributed to the activities of these
niches and possibly to the formation of other extramedullar niches,
thereby facilitating the leukemogenic process. It has been
suggested that in acute myeloid leukemia, SDF-1 has a role in the
development of extramedullar disease (32).
In attempting to interpret the present data, the
presence of the BCR-ABL protein in 12B1 cells must be taken into
consideration. The results of the present study as evidence for its
effect on SDF-1α activity is controversial, and are inconsistent
with the results of Salgia et al (33), which suggested that
bcr-abl-transformed cell lines become refractory to SDF-1α. It has
been repeatedly demonstrated that the BCR-ABL protein inhibits the
chemotactic response to SDF-1α (34–37).
Several events have been implicated for these effects, including
the downregulation of class II phosphoinositide 3-kinase (P13KC2γ)
(38), the increased expression of
the β2 integrin LFA-1 (37),
activation of Cdc42 GTPase (39)
and the activation of Lyn kinase (40). Tyrosine-kinase inhibitors, such as
imatinib mesylate, reversed this phenomenon, which indicated a
causal connection between the BCR-ABL protein and reduced
CXCR4-associated activity. However, there have been other studies
that, to a certain degree, question the role of the BCR-ABL protein
in SDF 1α-induced migration and homing of transformed cells, and
that appear to be relevant to the present observations. According
to one study, the effects of SDF-1α on pseudopodia formation and
migration from these niches were not suppressed by BCR-ABL activity
(41). In fact, the authors
observed increased pseudopodia formation and the transmigration of
bcr-abl-transformed cells following their exposure to SDF-1α. It
has also been demonstrated that SDF-1α, which inhibits the
proliferation of primitive haematopoietic cells, failed to abrogate
the proliferation of primitive human bcr-abl-positive cells
(42). Another study reported that
in bcr-abl-transformed cells, a reduced chemotactic response to
SDF-1α was paradoxically accompanied by increased spontaneous
migration (35).
Based on these previously reported results, a
tentative, simple mechanistic explanation of the present
observations may be proposed. The SDF-1α that was formed
accelerated the migration of 12B1 cells to extramedullar niches
where the proliferation of leukemic cells occurred that, in turn,
was accelerated by the ability of this chemokine to support the
growth of pre-B 12B1 cells. The rapid migration of these cells from
the site of inoculation, if this truly occurred, reduced the number
of cells that were capable of inducing subcutaneous tumors, thereby
leading to a delay in their appearance or even to reducing the
number of remaining cells below a tumor-inducing dose. These events
may also be associated with the decreased adhesion of tumor cells
to stromal cells, which would reduce the capability of 12B1 cells
to induce solid tumors.
To be certain, this proposed explanation is subject
to substantial corrections. In addition to the controversies
already mentioned, other factors may be involved. For example, in
addition to its chemotactic activity, SDF-1α has multiple other
functions, a number of which may act in concert to the phenomena
observed. Furthermore, as suggested by Salanga et al
(43), chemokines are not isolated
entities, but act in complex networks with other signaling systems.
In addition, it is impossible to neglect the expression of the
BCR-ABL protein identified in the cells used in the present study.
As previously noted, this protein is known to affect the
functionality of the SDF-1α/CXCR4 axis. It has also been
demonstrated that a number of factors, including prostaglandin E,
hyaluronic acid, fibrinogen, cleavage of the C3 component of
complement, and others, prime or enhance the responsiveness of
cells to SDF-1α (44). To the best
of our knowledge, their effect has not yet been adequately
investigated.
All of the aspects outlined above make forming
reliable conclusions from the present data particularly difficult.
To establish extrapolations from the discrepant and frequently
contrasting results of the previous studies on SDF-1α, which were
performed using a wide spectrum of cell systems and were based on
varying experimental designs, is not simple, and thus further
studies are required to elucidate its role.
Acknowledgements
The authors are grateful to Miss Katerina Kernova
and Miss Tereza Novakova for their excellent technical assistance.
This study was supported by MZCR IGA NS 10634-3/2009, MZCR IGA NT
12363-4/2011, by grant ERDF OPPK CZ.2.16/3.1.00/24001, and by the
project (Ministry of Health, Czech Republic) for Conceptual
development of research organization (00023736 UHKT).
References
|
1
|
Raman D, Baugher PJ, Thu YM and Richmond
A: Role of chemokines in tumor growth. Cancer Lett. 256:137–165.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kryczek I, Wei S, Keller E, Liu R and Zou
W: Stroma-derived factor (SDF-1/CXCL12) and human tumor
pathogenesis. Am J Physiol Cell Physiol. 292:C987–C995. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nagasawa T, Kikutani H and Kishimoto T:
Molecular cloning and structure of a pre-B-cell growth-stimulating
factor. Proc Natl Acad Sci USA. 91:2305–2309. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Burger JA and Kipps TJ: CXCR4: a key
receptor in the crosstalk between tumor cells and their
microenvironment. Blood. 107:1761–1767. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Karin N: The multiple faces of CXCL12
(SDF-1alpha) in the regulation of immunity during health and
disease. J Leukoc Biol. 88:463–473. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Duda DG, Kozin SV, Kirkpatrick ND, et al:
CXCL12 (SDF1alpha)-CXCR4/CXCR7 pathway inhibition: an Emerging
sensitizer for anticancer therapies? Clin Cancer Res. 17:2074–2080.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Burns JM, Summers BC, Wang Y, et al: A
novel chemokine receptor for SDF-1 and I-TAC involved in cell
survival, cell adhesion, and tumor development. J Exp Med.
203:2201–2213. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hattermann K and Mentlein R: An infernal
trio: the chemokine CXCL12 and its receptors CXCR4 and CXCR7 in
tumor biology. Ann Anat. 195:103–110. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kucia M, Reca R, Miekus K, et al:
Trafficking of normal stem cells and metastasis of cancer stem
cells involve similar mechanisms: pivotal role of the SDF-1-CXCR4
axis. Stem Cells. 23:879–894. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brzoska E, Kowalewska M,
Markowska-Zagrajek A, et al: Sdf-1 (CXCL12) improves skeletal
muscle regeneration via the mobilisation of CXCR4 and CD34
expressing cells. Biol Cell. 104:722–737. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Orimo A, Gupta PB, Sgroi DC, et al:
Stromal fibroblasts present in invasive human breast carcinomas
promote tumor growth and angiogenesis through elevated SDF-1/CXCL12
secretion. Cell. 121:335–348. 2005. View Article : Google Scholar
|
|
12
|
Wang J, Wang J, Dai J, et al: A glycolytic
mechanism regulating an angiogenic switch in prostate cancer.
Cancer Research. 67:149–159. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cojoc M, Peitzsch C, Trautmann F, et al:
Emerging targets in cancer management: role of the CXCL12/CXCR4
axis. Onco Targets Ther. 6:1347–1361. 2013.PubMed/NCBI
|
|
14
|
Schimanski CC, Galle PR and Moehler M:
Chemokine receptor CXCR4-prognostic factor for gastrointestinal
tumors. World J Gastroenterol. 14:4721–4724. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Felix AS, Weissfeld J, Edwards R and
Linkov F: Future directions in the field of endometrial cancer
research: the need to investigate the tumor microenvironment. Eur J
Gynaecol Oncol. 31:139–144. 2010.
|
|
16
|
Burger JA and Peled A: CXCR4 antagonists:
targeting the microenvironment in leukemia and other cancers.
Leukemia. 23:43–52. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee Ch, Kakinuma T, Wang J, et al:
Sensitization of B16 tumor cells with a CXCR4 antagonist increases
the efficacy of immunotherapy for established lung metastases. Mol
Cancer Ther. 5:2592–2599. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
McLaughlin J, Chianese E and Witte ON: In
vitro transformation of immature hematopoietic cells by the P210
BCR/ABL oncogene product of the Philadelphia chromosome. Proc Natl
Acad Sci USA. 84:6558–6562. 1987. View Article : Google Scholar
|
|
19
|
Lucansky V, Sobotkova E, Tachezy R,
Duskova M and Vonka V: DNA vaccination against bcr-abl-positive
cells in mice. Int J Oncol. 35:941–951. 2009.PubMed/NCBI
|
|
20
|
Jelínek F1, Sobotková E and Vonka V:
Characteristics of two mouse bcr-abl-transformed cell lines. II
Pathological lesions induced in mice. Folia Biol (Praha).
51:93–102. 2005.PubMed/NCBI
|
|
21
|
Sobotkova E1, Ludvíková V, Petrácková M,
et al: Characteristic of two mouse bcr-abl-transformed cell lines:
I. General properties of the cells. Folia Biol (Praha). 51:12–18.
2005.PubMed/NCBI
|
|
22
|
Jinoch P, Zak R, Janousková O, et al:
Immunization with live HPV-16-transformed mouse cells expressing
the herpes simplex thymidine kinase and either GM-CSF or IL-2. Int
J Oncol. 23:775–783. 2003.PubMed/NCBI
|
|
23
|
Ludvíková V1, Hamsíková E, Sobotková E, et
al: Use of polyclonal rabbit antibodies for detection of the
bcr-abl fusion zone in cells transfected with experimental bcr-abl
DNA vaccines. Int J Oncol. 27:265–274. 2005.PubMed/NCBI
|
|
24
|
Smahel M, Síma P, Ludvíková V and Vonka V:
Modified HPV16 E7 genes as DNA vaccine against E7-containing
oncogenic Cells. Virology. 281:231–238. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hrbacek J, Urban M, Hamsikova E, et al:
Serum antibodies against genitourinary infectious agents in
prostate cancer and benign prostate hyperplasia patients: a
case-control study. BMC Cancer. 11:532011. View Article : Google Scholar
|
|
26
|
Poláková I1, Pokorná D, Dusková M and
Smahel M: DNA vaccine against human papillomavirus type 16:
modifications of the E6 oncogene. Vaccine. 28:1506–1513.
2010.PubMed/NCBI
|
|
27
|
Cronin P, Wang J and Redmond HP: Hypoxia
increases the metastatic ability of breast cancer cells via
upregulation of CXCR4. BMC Cancer. 10:2252010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang H, Li H, Xi HS and Li S: HIF1α is
required for survival maintenance of chronic myeloid leukemia stem
cells. Blood. 119:2595–2607. 2012.
|
|
29
|
Kittang AO, Hatfield K, Sand K, Reikvam H
and Bruserud Ø: The chemokine network in acute myelogenous
leukemia: molecular mechanisms involved in leukemogenesis and
therapeutic implications. Curr Top Microbiol Immunol. 341:149–172.
2010.
|
|
30
|
Kato I, Niwa A, Heike T, et al:
Identification of hepatic niche harboring human acute lymphoblastic
leukemic cells via the SDF-1/CXCR4 axis. PLoS One. 6:e270422011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kawaguchi A, Orba Y, Kimura T, et al:
Inhibition of the SDF-1alpha-CXCR4 axis by the CXCR4 antagonist
AMD3100 suppresses the migration of cultured cells from ATL
patients and murine lymphoblastoid cells from HTLV-I Tax transgenic
mice. Blood. 114:2961–2968. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sison EA and Brown P: The bone marrow
microenvironment and leukemia: biology and therapeutic targeting.
Expert Rev Hematol. 4:271–283. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Salgia R, Quackenbush E, Lin J, et al: The
BCR/ABL oncogene alters the chemotactic response to stromal-derived
factor-1alpha. Blood. 94:4233–4246. 1999.PubMed/NCBI
|
|
34
|
Dürig J, Rosenthal C, Elmaagacli A, et al:
Biological effects of stroma-derived factor-1 alpha on normal and
CML CD34+ haemopoietic cells. Leukemia. 14:1652–1660.
2000.PubMed/NCBI
|
|
35
|
Ptasznik A, Urbanowska E, Chinta S, et al:
Crosstalk between BCR/ABL oncoprotein and CXCR4 signaling through a
Src family kinase in human leukemia cells. J Exp Med. 196:667–678.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Geay JF, Buet D, Zhang Y, et al:
p210BCR-ABL inhibits SDF-1 chemotactic response via alteration of
CXCR4 signaling and down-regulation of CXCR4 expression. Cancer
Res. 65:2676–2683. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen YY, Malik M, Tomkowicz BE, Collman RG
and Ptasznik A: BCR-ABL1 alters SDF-1alpha-mediated adhesive
responses through the beta2 integrin LFA-1 in leukemia cells.
Blood. 111:5182–5186. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yu W, Sun X, Tang H, Tao Y and Dai Z:
Inhibition of class II phosphoinositide 3-kinase gamma expression
by p185(Bcr-Abl) contributes to impaired chemotaxis and aberrant
homing of leukemic cells. Leuk Lymphoma. 51:1098–1107. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chang YC, Tien SC, Tien HF, et al:
p210Bcr-Abl desensitizes Cdc42 GTPase signaling for
SDF-1alpha-directed migration in chronic myeloid leukemia cells.
Oncogene. 28:4105–4115. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tabe Y, Jin L, Iwabuchi K, et al: Role of
stromal microenvironment in nonpharmacological resistance of CML to
imatinib through Lyn/CXCR4 interactions in lipid rafts. Leukemia.
26:883–892. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fruehauf S, Srbic K, Seggewiss R, Topaly J
and Ho AD: Functional characterization of podia formation in normal
and malignant hematopoietic cells. J Leukoc Biol. 71:425–432.
2002.PubMed/NCBI
|
|
42
|
Cashman J, Clark-Lewis I, Eaves A and
Eaves C: Stromal-derived factor 1 inhibits the cycling of very
primitive human hematopoietic cells in vitro and in NOD/SCID mice.
Blood. 99:792–799. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Salanga CL, O’Hayre M and Handel T:
Modulation of chemokine receptor activity through dimerization and
crosstalk. Cell Mol Life Sci. 66:1370–1386. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ratajczak MZ, Serwin K and Schneider G:
Innate immunity derived factors as external modulators of the
CXCL12-CXCR4 axis and their role in stem cell homing and
mobilization. Theranostics. 3:3–10. 2013. View Article : Google Scholar : PubMed/NCBI
|