Introduction
Crohn’s disease (CD) is a chronic relapsing
inflammatory disorder of the gastrointestinal tract. Although the
mechanisms underlying the pathogenesis of CD are not fully
understood, humoral and cellular immunity are known to be involved
(1).
A new member of the interleukin (IL)-12 cytokine
family, produced mainly by dendritic cells and activated
macrophages, was termed IL-23 in 2000 (2). It consists of two subunits: p40,
which is shared by IL-12, and p19, which is specific to IL-23.
Numerous pathological defects found in animal models of
autoimmunity were initially associated with IL-12, but are now
understood to be caused by IL-23 (3,4). For
example, inactivation of the IL-23p19 gene imparts resistance to
experimental allergic encephalomyelitis and collagen-induced
arthritis in rats, whereas rats deficient in IL-12p35 are
sensitized to these diseases (5).
Furthermore, IL-23 was found to be overexpressed in the intestine
of patients with CD, indicating a correlation between disease
activity and the levels of IL-23 in CD (6).
IL-23 was demonstrated to induce the differentiation
of naïve T CD4 lymphocytes into T helper 17 (Th17) cells, causing
overexpression of the transcription factor retinoic acid-related
orphan receptor-γt (ROR-γt) and production of IL-17, and was
negatively regulated by TNF-γ (7–9). The
proinflammatory cytokine IL-17 resulted in the production of other
cytokines and created a positive feedback loop for Th17
inflammation. The IL-23/IL-17 pathway is considered to be important
in the pathogenesis of CD (10,11).
Over the last decade, the use of cytokine
antagonists, including monoclonal antibodies and soluble receptors,
has become a clinical standard for the treatment of autoimmune
disease, as illustrated by the use of anti-TNF-α antagonists in CD
(12,13). In light of the suspected effect of
IL-23 on the development of CD, the present study evaluated the
efficacy of an immunization specifically targeting the IL-23p19
subunit in a CD rat model induced by 2,4,6-trinitrobenzene sulfonic
acid (TNBS).
Materials and methods
Ethics statement
All experiments were performed in conformity with
the National Institute of Health (NIH) guidelines (NIH Pub. no.
85-23, revised 1996) and the Animal Research Reporting In
Vivo Experiments guidelines (14) and approved by the Animal Care and
Use Committee of Sun Yat-Sen University (Gangzhou, China; no.
20131652101). All surgical and experimental procedures were
performed according to the guidelines for the care and use of
animals approved by the Sun Yat-Sen University and in accordance
with the code of Ethics of the EU Directive 2010/63/EU for animal
experiments. All efforts were made to minimize animal suffering and
to reduce the number of animals used.
Animals
A total of 60 specific pathogen-free male Sprague
Dawley rats (6-weeks-old, weighing ~180–220 g), were obtained from
the Animal Experiment Center of the Sun Yat-Sen University. Animals
were housed in 12 cages with five rats each and were maintained in
a 12:12 h artificial light-dark cycle. All experiments were
performed in the city of Guangzhou, China.
Method
After 1 week habituation, 60 Sprague Dawley rats
were randomly divided into three groups of 20 rats each: control
group, CD model group and anti-IL-23p19 mAb treatment group. The CD
rat models were induced by rectal administration of TNBS (Sigma,
St. Louis, MO, USA; no. 068K5001)/ethanol (TNBS 100 mg/kg + 0.25 ml
50% ethanol), performed four times at 10 day intervals (days 0, 10,
20 and 30) (15). Anti-IL-23p19
mAb (RayBiotech, Inc., Norcross, GA, USA; no: PAB1106) was
administered intramuscularly (1 mg/kg) every week at days 0, 7, 14,
21, 28 and 35. Rats in the control group and in the model group
received rectal administration of 0.9% sodium chloride solution
(0.1 mg/kg).
Necropsy
At the end of the experiment (day 40), rats were
anesthetized and blood samples were collected into tubes by cardiac
puncture for enzyme-linked immunosorbent assay (ELISA). Colon
tissue specimens were assessed macroscopically prior to being
excised and fixed in 10% formaldehyde saline solution for
histological analysis. The remaining tissue was snap-frozen in
liquid nitrogen and stored at −80°C for reverse transcription
polymerase chain reaction (RT-PCR) detection.
Disease activity index (DAI) of rats
Symptomatic parameters were observed and recorded
daily during the experimental period. Stools were assessed for
occult blood using the colloidal gold method and a fecal occult
blood test kit (Shanghai Sunred Biological Technology Co., Ltd.,
Shanghai, China). DAI was determined in a blinded manner by scoring
the extent of body weight loss, stool consistency and stool occult
blood positivity or gross bleeding in accordance with the method
described by Murthy et al (16). The results are shown in Table I.
 | Table ICriteria for scoring disease activity
index. |
Table I
Criteria for scoring disease activity
index.
| Score | Weight loss (%) | Stool
consistency | Occult blood or gross
bleeding |
|---|
| 0 | None | Normal | Negative |
| 1 | 1–5 | Loose | Negative |
| 2 | 5–10 | Loose | Hemoccult
positive |
| 3 | 10–15 | Diarrhea | Hemoccult
positive |
| 4 | >15 | Diarrhea | Gross bleeding |
Colon macroscopic damage index (CMDI) of
rats
The distal 8 cm portion of the colon was excised,
opened longitudinally and thoroughly washed in ice-cold
phosphate-buffered saline (PBS; Qiagen, Valencia, CA, USA). The
CMDI was determined according to the scoring system of Wallace and
Keenan (17,18). The criteria for assessing
macroscopic damage and the numerical rating score were as follows:
0, no ulcer or inflammation; 1, local hyperemia without ulcers; 2,
ulceration without hyperemia; 3, ulceration and inflammation at one
site only; 4, two or more sites of ulceration and inflammation
extending >1 cm and 5, ulceration extending >2 cm (19).
Tissue damage index (TDI) of rats
The specimens of colon tissue were stained with
hematoxylin and eosin for evaluation of liver histology. The TDI of
the stained samples was evaluated in a blinded manner according to
the previously described criteria (20,21)
between 0 and 4 (0, no signs of inflammation; 1, low level of
leukocyte infiltration; 2, moderate level of leukocyte
infiltration; 3, high level of leukocyte infiltration, high
vascular density, thickening of the colon wall; 4, on the basis of
score 3, the lesions exceed 50% of the specimens; 5, transmural
infiltration, loss of goblet cells, high vascular density,
thickening of the colon wall; 6, on the basis of score 5, the
lesions exceed 50% of the specimens).
Isolation of total RNA and RT-PCR
analysis
All rats in each group were sampled for RT-PCR
analysis. Total RNA was extracted from the colon tissue using
TRIzol (Qiagen) according to the manufacturer’s instructions. Total
RNA (500 ng) was used for cDNA synthesis and 1 μl of each reverse
transcription product was added to 9 μl Master mix [containing
buffer, SYGB, Hotstart Taq polymerase and deoxyribonucleotide
triphosphates (dNTPs), purchased from Qiagen], 0.2 μl 25 mM dNTPs,
0.5 μl 25 μM corresponding primers and 9.3 μl ddH2O
(Qiagen) for PCR amplification. The number of cycles, annealing
temperature for each primer pair and primer sequences used are
listed in Table II. The relative
levels of target mRNA were normalized to the corresponding levels
of GAPDH mRNA in the same cDNA sample using the standard curve
method recommended in the LightCycler Software, version 3.5 (Roche
Molecular Diagnostics Systems, Meylan, France).
 | Table IIPrimers and product size for each
target gene. |
Table II
Primers and product size for each
target gene.
| Gene | Primer | Length (bp) | Cycles | Annealing temp
(°C) |
|---|
| IL-23p19 | Forward:
5′-GAATCTGGTGGCTGTGGA-3′
Reverse: 5′-CCCTGAAAGGCTTGGTCT-3′ | 230 | 35 | 52 |
| p40 (IL-23/12) | Forward:
5′-GATTATGTGGAGTCTGGA-3′
Reverse: 5′-CTAAGACACCTCAGACCT-3′ | 278 | 35 | 52 |
| ROR-γt | Forward:
5′-CGTATTACGGATAACCGACG-3′
Reverse: 5′-GCATAATGCCTATTGGCTGC-3′ | 266 | 38 | 52 |
| Th17 | Forward:
5′-CATCAGCTACATGCATTCC-3′
Reverse: 5′-GTAGTCGATGTACGTAAGG-3′ | 253 | 35 | 52 |
| IL-17 | Forward:
5′-GCAATGGCCTATACGCCATC-3′
Reverse: 5′-CGTTACCGGATATGCGGTAG-3′ | 233 | 33 | 52 |
| GAPDH | Forward:
5′-GAATCTGGTGGCTGTGGA-3′
Reverse: 5′-CCCTGAAAGGCTTGGTCT-3′ | 207 | 35 | 52 |
ELISA analysis of levels of cytokines in
the serum
The levels of cytokines IL-23p19, p40 (IL-23/12),
ROR-γt and IL-17 in the serum were measured using ELISA kits
(R&D Systems, Minneapolis, MN, USA) according to the
manufacturer’s instructions. A standard curve was generated with
each assay with the limits of detection as follows: IL-23p19, 12
pg/ml; p40 (IL-23/12), 15 pg/ml; ROR-γt = 6.1 pg/ml and IL-17 = 3.0
pg/ml. Each sample was performed in triplicate.
Statistical analysis
Data are expressed as the mean ± standard deviation.
Data were analyzed by one-way analysis of variance (ANOVA),
followed by the Bonferroni multiple comparisons test. The
statistical significance of the expression analysis was also
assessed by ANOVA and the differences identified were assessed
using the unpaired Student’s t-test. P<0.05 was considered to
indicate a statistically significant difference. All statistical
analyses were performed using SPSS version 13.0 (SPSS, Inc.,
Chicago, IL, USA).
Results
DAI scores of rats in each group
DAI, representing symptomatic parameters, including
diarrhea and loss of body weight, was investigated. Rats in the
normal control group gained body weight and defecated normally. As
shown in Fig. 1, following
induction of CD by administration of TNBS, pasty to liquid, gross
blood stools and weight loss were observed with higher DAI scores
in CD model rats (P<0.001, vs. control group). However, the DAI
scores markedly decreased following treatment with anti-IL-23p19
mAb (P<0.005, vs. model group).
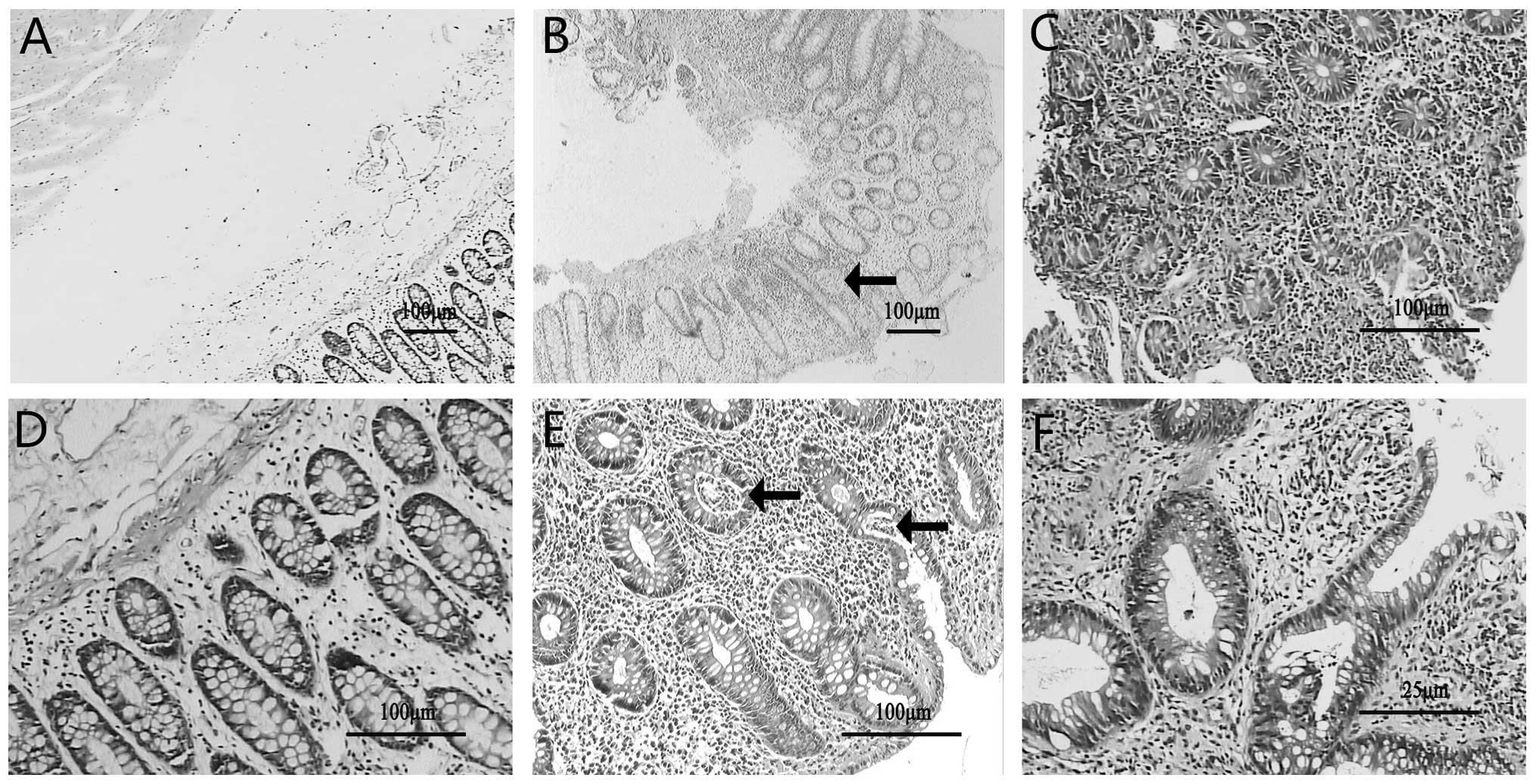
Pathological alterations in rat colon
tissue in each group
The colon tissue from the CD model rats induced by
TNBS was characterized by epithelial hyperplasia and infiltration
of the lamina propria with inflammatory cells, including a large
number of neutrophils (Fig. 2B).
Epithelioid granuloma (Arrow, Fig.
2B) and crypt abscesses (Arrows, Fig. 2E) were also frequently observed.
Following treatment with anti-IL-23p19 mAb, the inflammation
present within the colon tissue typically involved the mucosa only,
with little to no inflammation present in the submucosa,
demonstrating a marked improvement in inflammation (Fig. 2C and F).
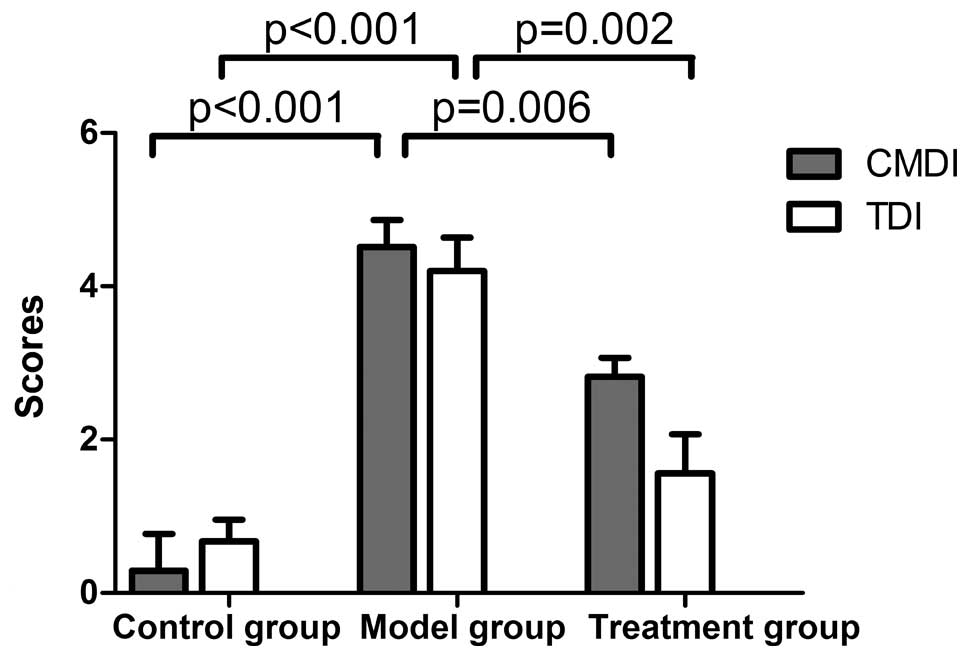
CMDI and TDI were used to assess the degree of
colonic inflammation. The CMDI and TDI were significantly higher in
the model group compared with the control group (P<0.01). The
anti-IL-23p19 mAb treatment group demonstrated a marked reduction
in CMDI and TDI scores compared with the model group (P<0.05),
indicating that the macroscopical and microscopical lesions reduced
following drug intervention (Fig.
3).
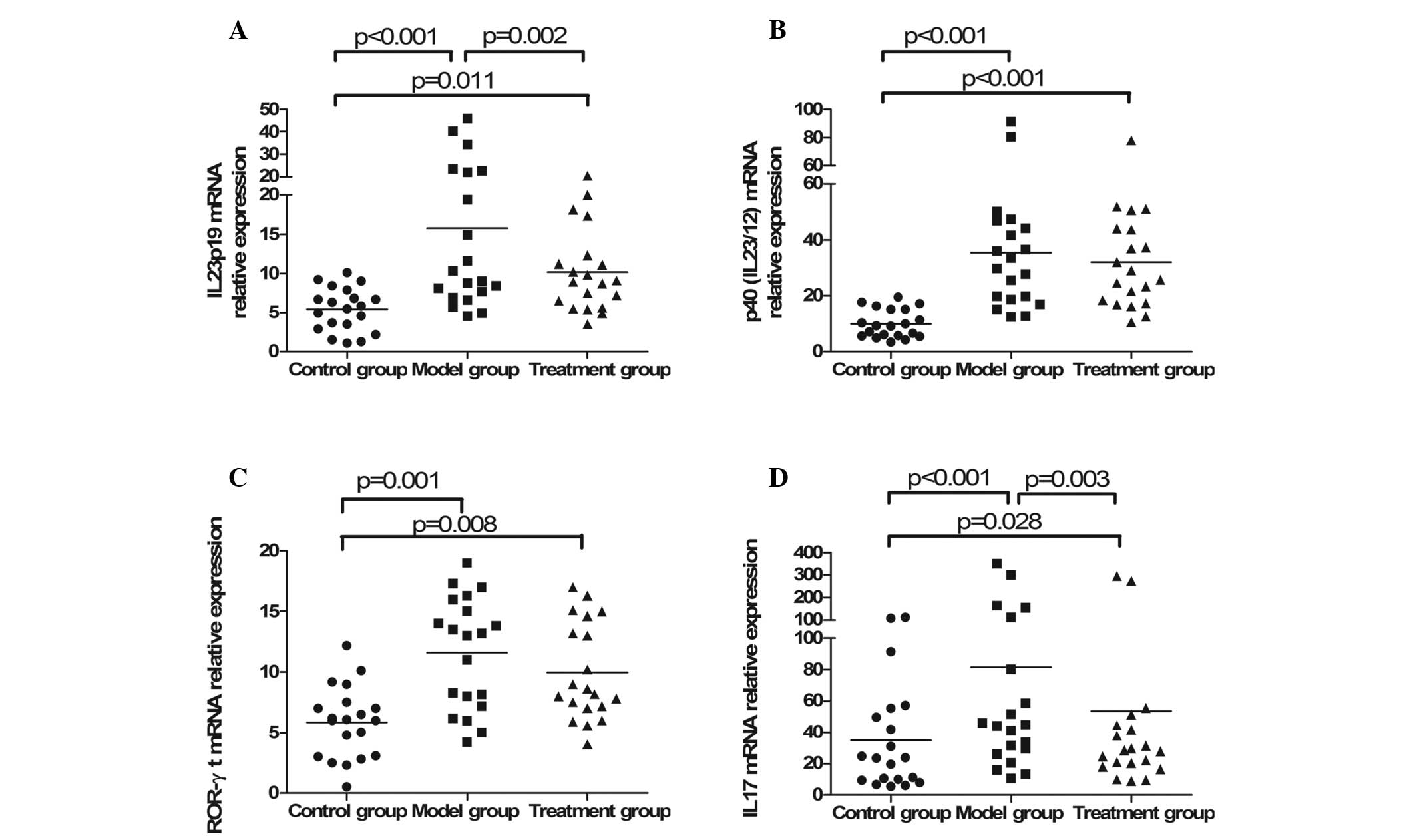
Expression of cytokines in colon tissue
detected by RT-PCR
A significant increase in all cytokines [IL-23p19,
p40 (IL-23/12), ROR-γt and IL-17] was observed in the CD model
group induced by TNBS (P<0.001). The expression of IL-23p19
(P=0.002) and IL-17 (P=0.003) was inhibited following
administration of anti-IL-23p19 mAb as a targeted therapy. There
was also a decrease in the expression of p40 (IL23/12) and ROR-γt
in the treatment group compared with the model group, however, this
was not statistically significant (Fig. 4).
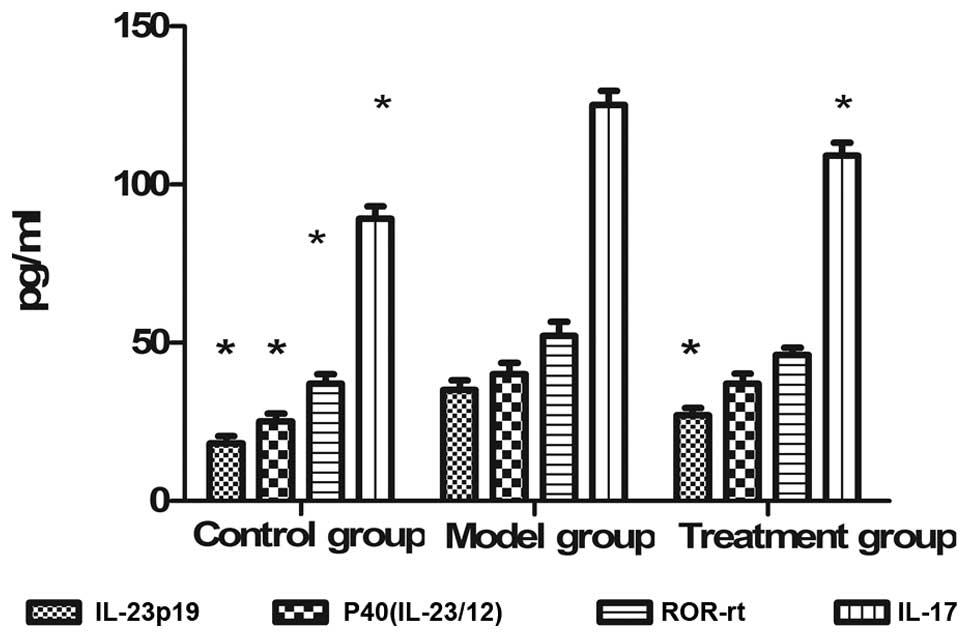
Serum cytokines in each group detected by
ELISA
The concentration of secreted serum cytokines of
rats in each group was measured using ELISA. A significant increase
in IL-23p19, P40 (IL-23/12), ROR-γt and IL-17 content was observed
in the model group compared with the control group. A significant
decrease in the concentration of IL-23p19 and IL-17 was observed in
the treatment group (Fig. 5).
Discussion
The TNBS model of experimental CD is a well
established model of chronic colonic inflammation and ulceration in
rats, which is characterized by transmural inflammation associated
with severe diarrhea and histopathological features that resemble
the immune characteristics of CD in humans (22,23).
It has been used extensively in the evaluation of new therapeutic
strategies for human cases of CD since Morris’ preliminary
description in 1989 (15). The
present study successfully established CD rat models induced by
TNBS. This was confirmed by the observation of pathological
changes, including extensive transmural infiltration of
inflammatory cells, thickening of the colon wall and formation of
typical epithelioid granulomas and crypt abscesses. Furthermore,
the model rats demonstrated a marked increase in CMDI, TDI and DAI
scores, indicating the presence of severe colonic damage at the
macroscopic and microscopic levels and clinical symptoms. Disorders
in the expression of cytokines in the IL-23/IL-17 pathway were also
observed in the model rats, revealing the importance of cytokines
in the pathogenesis of CD.
Currently, an effective therapy for patients with CD
is lacking. Aminosalicylates, corticosteroids and immunomodulating
drugs have multiple adverse effects and a high relapse percentage
(24,25). Biological agents, including
anti-TNF-α mAb may induce the alleviation of inflammatory bowel
disease (26), but at a high cost
and with an increased risk to patients in developing
treatment-associated cancer (27,28).
The ultimate alternative is often surgical resection of the colon
and ileostomy (29). Therefore,
there is a continuing interest in novel therapeutic approaches for
controlling CD, thus the present study focused on targeted therapy
using anti-IL-23p19 mAb. The present study demonstrated that the
administration of anti-IL-23p19 mAb effectively attenuated colonic
inflammation induced by TNBS in rats and significantly reduced DAI,
CMDI and TDI scores. Pathological evaluation of colon tissue
demonstrated improvements in inflammation with a reduction in
inflammatory cell infiltration. The above revealed a favorable
efficacy of anti-IL-23p19 mAb in curing TNBS-induced CD in
rats.
The results from the present study demonstrated a
marked increase in the expression of IL-23p19, p40 (IL-23/12),
ROR-γt and the downstream cytokine IL-17 in CD rats, indicating
that the IL-23/IL-17 pathway was important in the pathogenesis of
CD. The present study focused on the efficacy of targeted therapy
by inhibition of the IL-23/IL-17 pathway using anti-IL-23p19 mAb.
As expected, anti-IL-23p19 mAb effectively inhibited the expression
of IL-23p19 and the proinflammatory cytokine IL-17, however, no
significant effect on the expression of p40, the common subunit
shared by IL-23 and IL-12, was observed. The results of the present
study verified the hypothesis that IL-23, not IL-12, is associated
with autoimmune disease. The expression of serum cytokines detected
by ELISA demonstrated similar results. Notably, regulation of the
expression of ROR-γt, considered to be a key transcription factor
in the differentiation of Th17 cells and regulation of IL-17
expression in vivo and in vitro, was not affected by
anti-IL-23p19 mAb. ROR-γt knockout mice demonstrated a reduction in
Th17 cells and in the incidence of autoimmune diseases (30,31).
In the present study, intervention by anti-IL-23p19 mAb was
associated with a decrease in ROR-γt, although this was not
considered to be statistically significant. This indicated that
anti-IL-23p19 mAb may inhibit the differentiation of Th17 through
the mediator ROR-γt. The detailed and specific pathway of
anti-IL-23p19 mAb in treating CD model rats therefore requires
further investigation.
IL-12 and IL-23 are members of a heterodimeric
cytokine family, which share a common IL-12p40 subunit (2,32,33).
IL-12 is the driving force behind Th1 inflammation, while IL-23
maintains the effector function of Th17 cells (34,35).
In 2009, a biological drug termed ustekinumab, which targets the
p40 subunit of IL-12 and IL-23, was developed and approved by the
US FDA and the European Medicines Agency for the treatment of
moderate to severe psoriasis. However, the high incidence of
adverse events (51.6–57.6%) restricted its application in CD
patients (36,37). The present study focused on another
targeted therapy using anti-IL-23p19 mAb. In the rats administered
with anti-IL-23p19 mAb, a reduction in colon pathology and improved
clinical symptoms were observed compared with the model rats. In
addition, no mortality or serious adverse reactions were observed
in the rats treated with anti-IL-23p19 mAb. However, as the
prevention of CD was incomplete, the results suggested that there
may have been a dosing and/or timing issue associated with the
therapeutic effectiveness of anti-IL-23p19 mAb treatment.
In conclusion, the present study demonstrated that
anti-IL-23p19 mAb attenuated TNBS-induced CD in rats. The possible
mechanisms may be associated with inhibition of the IL-23/IL-17
pathway by inhibiting the expression of IL-23p19 and downregulating
the downstream proinflammatory cytokine IL-17. Anti-IL-23p19 mAb as
a new targeted therapy may provide a novel therapeutic approach for
patients with CD, however, this requires further investigation.
Acknowledgements
The present study was supported by a grant from the
Medical Research Foundation of Guangdong, China (no. B2013159) to
Jiayin Yao.
References
|
1
|
Geremia A, Biancheri P, Allan P, Corazza
GR and Di Sabatino A: Innate and adaptive immunity in inflammatory
bowel disease. Autoimmun Rev. 13:3–10. 2013. View Article : Google Scholar
|
|
2
|
Oppmann B, Lesley R, Blom B, et al: Novel
p19 protein engages IL-12p40 to form a cytokine, IL-23, with
biological activities similar as well as distinct from IL-12.
Immunity. 13:715–725. 2000. View Article : Google Scholar
|
|
3
|
Cua DJ, Sherlock J, Chen Y, et al:
Interleukin-23 rather than interleukin-12 is the critical cytokine
for autoimmune inflammation of the brain. Nature. 421:744–748.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vignali DA and Kuchroo VK: IL-12 family
cytokines: immunological playmakers. Nat Immunol. 13:722–728. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Duvallet E, Semerano L, Assier E,
Falgarone G and Boissier MC: Interleukin-23: a key cytokine in
inflammatory diseases. Ann Med. 43:503–511. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Duerr RH, Taylor KD, Brant SR, et al: A
genome-wide association study identifies IL23R as an inflammatory
bowel disease gene. Science. 314:1461–1463. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McGeachy MJ, Chen Y, Tato CM, et al: The
interleukin 23 receptor is essential for the terminal
differentiation of interleukin 17-producing effector T helper cells
in vivo. Nat Immunol. 10:314–324. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rovedatti L, Kudo T, Biancheri P, et al:
Differential regulation of interleukin 17 and interferon gamma
production in inflammatory bowel disease. Gut. 58:1629–1636. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sarra M, Pallone F, Macdonald TT and
Monteleone G: IL-23/IL-17 axis in IBD. Inflamm Bowel Dis.
16:1808–1813. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Siakavellas SI and Bamias G: Role of the
IL-23/IL-17 axis in Crohn’s disease. Discov Med. 14:253–262.
2012.
|
|
11
|
Toussirot E: The IL23/Th17 pathway as a
therapeutic target in chronic inflammatory diseases. Inflamm
Allergy Drug Targets. 1:159–168. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Imaeda H, Takahashi K, Fujimoto T, et al:
Clinical utility of newly developed immunoassays for serum
concentrations of adalimumab and anti-adalimumab antibodies in
patients with Crohn’s disease. J Gastroenterol. 49:100–109.
2014.PubMed/NCBI
|
|
13
|
Peyrin-Biroulet L and Danese S: Stopping
infliximab in Crohn’s disease: still an ongoing STORI. Inflamm
Bowel Dis. 18:2201–2202. 2012.
|
|
14
|
Kilkenny C, Browne WJ, Cuthill IC, Emerson
M and Altman DG: Improving bioscience research reporting: the
ARRIVE guidelines for reporting animal research. PLoS Biol.
8:e10004122010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Morris GP, Beck PL, Herridge MS, et al:
Hapten-induced model of chronic inflammation and ulceration in the
rat colon. Gastroenterology. 96:795–803. 1989.PubMed/NCBI
|
|
16
|
Murthy SN, Cooper HS, Shim H, Shah RS,
Ibrahim SA and Sedergran DJ: Treatment of dextran sulfate
sodium-induced murine colitis by intracolonic cyclosporin. Dig Dis
Sci. 38:1722–1734. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Murano M, Maemura K, Hirata I, et al:
Therapeutic effect of intracolonically administered nuclear factor
kappa B (p65) antisense oligonucleotide on mouse dextran sulphate
sodium (DSS)-induced colitis. Clin Exp Immunol. 120:51–58. 2000.
View Article : Google Scholar
|
|
18
|
Wallace JL and Keenan CM: An orally active
inhibitor of leukotriene synthesis accelerates healing in a rat
model of colitis. Am J Physiol. 258:G527–G534. 1990.PubMed/NCBI
|
|
19
|
Guo L, Ye C, Hao X, et al:
Carboxyamidotriazole ameliorates experimental colitis by inhibition
of cytokine production, nuclear factor-κB activation, and colonic
fibrosis. J Pharmacol Exp Ther. 342:356–365. 2012.PubMed/NCBI
|
|
20
|
Zingarelli B, Hake PW, Burroughs TJ,
Piraino G, O’Connor M and Denenberg A: Activator protein-1
signalling pathway and apoptosis are modulated by poly(ADP-ribose)
polymerase-1 in experimental colitis. Immunology. 113:509–517.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Neurath MF, Fuss I, Kelsall BL, Stüber E
and Strober W: Antibodies to interleukin 12 abrogate established
experimental colitis in mice. J Exp Med. 182:1281–1290. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Strober W, Lúdvíksson BR and Fuss IJ: The
pathogenesis of mucosal inflammation in murine models of
inflammatory bowel disease and Crohn disease. Ann Intern Med.
128:848–856. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Torres MI, Garcia-Martin M, Fernandez MI,
Nieto N, Gil A and Rios A: Experimental colitis induced by
trinitrobenzenesulfonic acid: an ultrastructural and histochemical
study. Dig Dis Sci. 44:2523–2529. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lakatos PL and Lakatos L: Ulcerative
proctitis: a review of pharmacotherapy and management. Expert Opin
Pharmacother. 9:741–749. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Burger D and Travis S: Conventional
medical management of inflammatory bowel disease. Gastroenterology.
140:1827–1837. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Magro F and Portela F: Management of
inflammatory bowel disease with infliximab and other anti-tumor
necrosis factor alpha therapies. BioDrugs. 24:3–14. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mariette X, Matucci-Cerinic M, Pavelka K,
et al: Malignancies associated with tumour necrosis factor
inhibitors in registries and prospective observational studies: a
systematic review and meta-analysis. Ann Rheum Dis. 70:1895–1904.
2011. View Article : Google Scholar
|
|
28
|
Deepak P, Sifuentes H, Sherid M, Stobaugh
D, Sadozai Y and Ehrenpreis ED: T-cell non-Hodgkin’s lymphomas
reported to the FDA AERS with tumor necrosis factor-alpha (TNF-α)
inhibitors: results of the REFURBISH study. Am J Gastroenterol.
108:99–105. 2013.
|
|
29
|
Huang R, Valerian BT and Lee EC:
Laparoscopic approach in patients with recurrent Crohn’s disease.
Am Surg. 78:595–599. 2012.
|
|
30
|
Ivanov II, McKenzie BS, Zhou L, et al: The
orphan nuclear receptor RORgammat directs the differentiation
program of proinflammatory IL-17+ T helper cells. Cell.
126:1121–1133. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ono Y, Kanai T, Sujino T, et al: T-helper
17 and interleukin-17-producing lymphoid tissue inducer-like cells
make different contributions to colitis in mice. Gastroenterology.
143:1288–1297. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Guan Q, Ma Y, Hillman CL, et al: Targeting
IL-12/IL-23 by employing a p40 peptide-based vaccine ameliorates
TNBS-induced acute and chronic murine colitis. Mol Med. 17:646–656.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
De Nitto D, Sarra M, Cupi ML, Pallone F
and Monteleone G: Targeting IL-23 and Th17-cytokines in
inflammatory bowel diseases. Curr Pharm Des. 16:3656–3660.
2010.PubMed/NCBI
|
|
34
|
Monteleone I, Pallone F and Monteleone G:
Interleukin-23 and Th17 cells in the control of gut inflammation.
Mediators Inflamm. 2009:2976452009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cho JH and Brant SR: Recent insights into
the genetics of inflammatory bowel disease. Gastroenterology.
140:1704–1712. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kurzeja M, Rudnicka L and Olszewska M: New
interleukin-23 pathway inhibitors in dermatology: ustekinumab,
briakinumab and secukinumab. Am J Clin Dermatol. 12:113–125. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tzellos T, Kyrgidis A, Trigoni A and
Zouboulis CC: Association of ustekinumab and briakinumab with major
adverse cardiovascular events: An appraisal of meta-analyses and
industry sponsored pooled analyses to date. Dermatoendocrinol.
4:320–323. 2012. View Article : Google Scholar
|