Introduction
Traumatic brain injury (TBI) is one of the leading
causes of injury-associated death and disability, particularly in
young individuals (1). Brain
damage following traumatic injury may be a result of direct
mechanisms, for example immediate mechanical disruption of the
brain tissue and primary injury, or indirect mechanisms, such as
secondary or delayed injury. Secondary mechanisms involve the
initiation of an acute inflammatory response, including breakdown
of the blood-brain barrier (BBB), edema and swelling, infiltration
of peripheral blood cells and activation of resident
immunocompetent cells, as well as the intrathecal release of
numerous immune mediators, such as interleukins (ILs) and
chemotactic factors (2).
Pro-inflammatory genes, including IL-1β, tumor necrosis factor
(TNF)-α and IL-6, have been reported to be differentially regulated
following TBI (3–6). Furthermore, high levels of IL-6 and
soluble IL-6 receptor (sIL-6R) have been reported in the
cerebrospinal fluid (CSF) of patients with cerebral trauma
(7).
In response to injury, IL-6 acts as an important
mediator for the initiation and progression of post-traumatic
inflammation, causing additional cell death and neurological
dysfunction. IL-6 may also facilitate reparative processes
(8). Overproduction of IL-6 in the
central nervous system (CNS) has been reported to increase the
production of inflammatory cytokines, and thus, IL-6 may have a
proinflammatory and detrimental role when dysregulated in the CNS.
IL-6 is an essential cytokine required for normal brain function,
not only during injury, but also under normal conditions (9). IL-6 has been shown to exert numerous
effects, both beneficial and destructive, on CNS cells. However,
downregulation of IL-6 is required to maintain its beneficial
functions and prevent its potentially detrimental effects (10).
Anti-IL-6 strategies represent an attractive
therapeutic option in several diseases, including B-cell neoplasia,
osteoporosis and autoimmunity (11,12).
Thus, IL-6 may be a target for therapeutic intervention. Although
the neutralization of IL-6 activity using monoclonal antibodies
(mAbs) or receptor antagonists is beneficial for the treatment of
immune and neoplastic diseases, the necessity for continuous
delivery of these anticytokine agents poses considerable practical
limitations. Furthermore, systemic administration of antibodies may
compromise the host’s capacity to fight infection. In addition,
therapeutic attempts in humans have shown that the administration
of injectable doses of anti-IL-6 mAbs does not provide efficient
neutralization of IL-6 in vivo and the use of humanized
antibodies in the clinic is hindered by a combination of factors,
including their short half-life, rapid blood clearance,
immunogenicity and high cost. Therefore, alternative approaches are
required.
RNA interference (RNAi) is a sequence-specific,
post-transcriptional gene silencing mechanism (13). RNAi-induced gene silencing involves
processing long double-stranded (ds)RNA into 19–21 nucleotide RNA
known as short interfering (si)RNA, which promotes the enzymatic
cleavage of complementary mRNA. RNAi is a powerful and widely used
tool for the analysis of gene function in invertebrates and
vertebrates (14). However, the
effectiveness of RNAi-induced protein expression attenuation
depends on the efficiency of the cellular uptake of the dsRNA, the
half-life of the dsRNA inside the cells and the half-life of the
protein to be silenced. The gene silencing induced by siRNA is
often poor, which limits its application. The pSUPER vector system
has been reported to be capable of directing the synthesis of siRNA
in mammalian cells and to induce efficient, sustained and specific
knockdown of target gene expression (15). Of note, this vector-based siRNA
system has been shown to stably repress gene expression.
In the present study, vector-based siRNA was used to
knockdown IL-6 gene expression in C6 rat glioma cells. Furthermore,
this vector was used to downregulate IL-6 expression in rat brains
exhibiting stab wound injury. The effect of pSUPER-IL-6-induced
siRNA expression on the downregulation of endogenous,
lipopolysaccharide (LPS)-induced IL-6 expression was investigated
in C6 cells. In addition, the effect of small hairpin (sh)RNA
administration on the reduction of IL-6 expression, inhibition of
inflammation and elimination of brain edema was investigated in TBI
rats in vivo.
Materials and methods
Animals
A total of 42 12-week-old, male Sprague-Dawley rats
weighing 250–300 g were purchased from the Center for Experimental
Animals, Chinese Academy of Medical Sciences (Beijing, China). The
animals were maintained at the animal facility of the Beijing
Neurosurgical Institute (Beijing, China) according to the
institute’s guidelines. The study was approved by Laboratory Animal
Welfare Ethics Committee of Beijing Neurosurgical Institute
(Beijing, China).
Cell culture
C6 rat glioma cells of Rattus norvegicus
origin, as well as C6-green fluorescent protein (GFP) cells
(Beijing Neurosurgical Institute) were cultured in Dulbecco’s
modified Eagle’s medium supplemented with 10% heat-inactivated
fetal bovine serum, 100 U/ml penicillin and 100 μl/ml
streptomycin (all from Gibco-BRL, New York, NY, USA) at 37°C in 5%
CO2.
Design of siRNA oligos targeting the IL-6
gene
siRNA sequences targeting the rat IL-6 gene (GenBank
accession number: NM_012589; https://www.ncbi.nlm.nih.gov/genbank/) were designed
using http://dharmacon.gelifesciences.com/design-center/?redirect=true.
The siRNA sequences corresponding to the coding region of the IL-6
gene are shown in Table I and
numbering begins from the first nucleotide of the transcription
start site.
 | Table ITargeted and antisense sequences of
rat IL-6 siRNA. |
Table I
Targeted and antisense sequences of
rat IL-6 siRNA.
| Type | siRNA targeted
sequence | Antisense
sequence | Start site
(nt) |
|---|
| T1 (pSUPER-IL-6
1) |
GGACCAAGACCATCCAACT |
AGTTGGATGGTCTTGGTCC | 621 |
| T2 (pSUPER-IL-6
2) |
CAGCGATGATGCACTGTCA |
TGACAGTGCATCATCGCTG | 298 |
| T3 (pSUPER-IL-6
3) |
CTGGCAATATGAATGTTGA |
TCAACATTCATATTGCCAG | 802 |
| T4 (pSUPER-IL-6
4) |
GTCGGAGGCTTAATTACAT |
ATGTAATTAAGCCTCCGAC | 215 |
| T5 (pSUPER-IL-6
5) |
CTGGATATAACCAGGAAAT |
ATTTCCTGGTTATATCCAG | 369 |
| Neg (pSUPER
Neg) |
GATTCAGGTGTAGAACGAG |
CTCGTTCTACACCTGAATC | - |
Plasmid construction
The pSUPER, pSUPER-GFP and pSUPER-siRNA-GFP vector
plasmids were obtained from Dr Hua Wang (Laboratory of Cell
Biology, Bureau of Life Sciences and Biotechnology, Chinese Academy
of Sciences, Beijing, China). An siRNA-expressing construct was
designed using a polymerase-III H1-RNA gene promoter to drive
expression. Two sets of DNA oligonucleotides were used to construct
the coding region of the siRNA. BglII and HindIII
cleavage sites were designed in the hairpin to facilitate
restriction analysis during cloning. The forward and reverse oligos
included a unique 19 nucleotide target in both sense and antisense
orientation, separated by a 9 nucleotide non-complementary spacer
sequence (TCTCTTGA) (Fig. 1). All
five pair DNA oligonucleotides generated for siRNA expression were
chemically synthesized by Shanghai Sangon Biological Engineering
Technology & Services Co., Ltd. (Shanghai, China). The oligos
were dissolved in sterile, nuclease-free H2O to a
concentration of 3 mg/ml. For the annealing reaction, 1 μl
of each oligo (forward and reverse) was mixed with 48 μl
annealing buffer. The solution was incubated at 90°C for 4 min and
then at 70°C for 10 min. The annealed oligos were slowly cooled to
10°C and the annealed sequences were inserted into the
pSUPER backbone following digestion with BglII and
HindIII and transformation into DH5α competent cells
(Tiangen Biotech Co., Ltd., Beijing, China) according to the
manufacturer’s instructions (OligoEngine, Seattle, WA, USA).
Subsequent to amplification, all vector constructs were verified
using digestion with EcoRI and XhoI followed by DNA
sequencing (Sage Science, USA). The constructed plasmid vectors
were referred to as pSUPER IL-6 1–5.
Transfection of C6 cells and
induction
C6 cells were seeded on 24-well plates at a density
of 5×105 cells per well in a final volume of 1 ml. The
cells were grown to ~70% confluency. Immediately prior to
transfection, the growth medium was substituted with fresh DMEM
without serum and antibiotics (1 ml/well). The transfection
mixtures (pSUPER IL-6 1–5 or pSUPER blank vector) were then
pipetted dropwise onto the C6 cell cultures. The cells were exposed
to the transfection agents for 6 h and the medium was then replaced
with normal growth medium. The transfected cells were induced with
LPS (19 ng/ml) to stimulate IL-6 secretion. Each sample was run in
triplicate. C6-GFP cells transfected with the pSUPER-siRNA-GFP
vector and C6 cells transfected with the pSUPER-GFP vector at the
same dose were used as controls.
ELISA for IL-6 and IL-8
The supernatants of the transfected cells were
collected after 24 and 48 h and analyzed for IL-6 and IL-8 using
ELISA according to the manufacturer’s instructions (Bender
Medsystems, Vienna, Austria). Statistical analysis was performed
using the two-tailed t-test. The most efficiently constructed
vector was selected for the animal studies in vivo.
In vivo animal experiments
Brain injury was induced using Feeney’s free falling
method (16). Subsequent to being
weighed, rats were injected intraperitoneally with 30 g/l sodium
pentobarbital at a dose of 30 g/kg as an anesthetic. Animals were
then fixed in a stereotactic frame. A right parietal craniotomy
(diameter, 4 mm) was drilled under aseptic conditions 2 mm
posterior to the anterior fontanelle and 2 mm from the skull middle
line. The intact cranial dura was exposed to a freefalling weight,
which produced a standardized parietal contusion by allowing a
steel rod with a flat end diameter of 4 mm, weighing 20 g, fall
onto a piston resting on the dura from a height of 30 cm. The
piston was allowed to compress the tissue to a maximum of 2 mm.
Following brain injury, 42 rats were randomly divided into three
equal groups: Control, treatment and blank vector groups.
Transfection in vivo
For periodic injection of the injured area, a tubule
was implanted at the injury site and secured in position using
dental cement. At −24, 0, +24 and +48 h after the induction of TBI,
rats were anesthetized and injected with 5 μl pSUPER-IL-6
(0.5 μg/μl), pSUPER blank vector (0.5
μg/μl) or 0.9% sodium chloride over a period of 5
min. All animals survived the intracerebral injection. At 24 and 72
h after the last injection, seven rats from each group were
decapitated under deep anesthesia (80 mg/kg intraperitoneal
pentobarbital). The brain was quickly removed and placed on a
cooled surface. The cerebrum was coronally divided into three
sections through the needle entry site and the midpoint of the
posterior remnant. The first section (2-mm thick) was cut from 5 mm
ipsilateral and contralateral of the TBI site, and then fixed using
2% paraformaldehyde and 2.5% glutaraldehyde. Frozen brain sections
were prepared for electron microscopy and the two remaining
sections were used for brain edema analysis and ELISA.
Analysis of brain water and sodium
content
Each section was wrapped in pre-weighed aluminum
foil and weighed to obtain the wet weight (WW), then dried for 72 h
in an oven at 110°C and weighed again to obtain the dry
weight (DW). The brain water content was calculated as the
percentage change using the following formula: (WW-DW)/WW ×
100.
The dehydrated brain samples were digested in 1 ml
1N nitric acid for one week. The sodium ion content was then
assessed using the Inductively Coupled Plasma Emission Spectrometer
ICPE-9000 (Thermo Jarrell-Ash Corporation, Franklin, MA, USA).
Sodium ion levels were expressed in milliequivalents per kilogram
of dehydrated brain tissue.
Statistical analysis
All data are presented as the mean ± standard error
of the mean. Data from the different animal groups were analyzed
using the Student’s t-test. SPSS 10.0 (SPSS Inc., Chicago, IL, USA)
was used for statistical analysis. P<0.05 was considered to
indicate a statistically significant difference.
Results
Identification of recombinant
plasmids
The restriction digestion enzymes EcoRI and
XhoI were used to digest the empty pSUPER plasmid and the
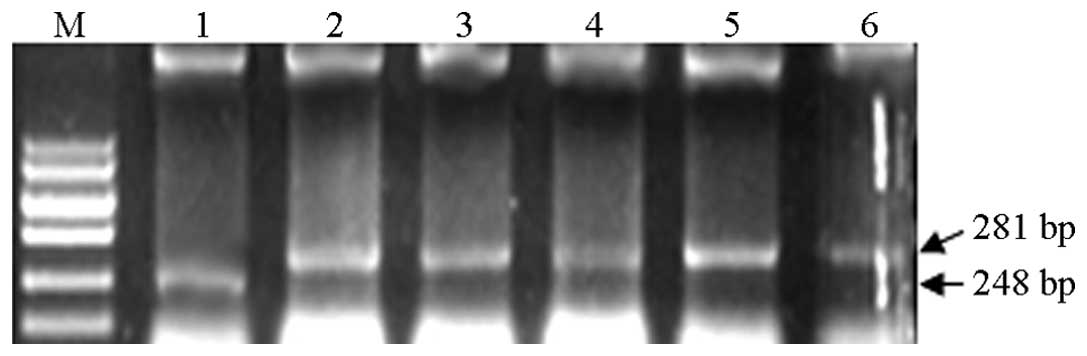
recombinant pSUPER IL-6 1–5 plasmids. Following digestion, the
empty plasmid generated a 248 bp fragment, while the recombinant
plasmid generated a 281 bp fragment, which was consistent with the
expected findings (Fig. 2).
Sequence determination of the inserted
fragment
DNA sequencing was performed on the fragment
inserted into the pSUPER-IL-6 1–5 plasmids. The findings showed
that the siRNA-IL-6 sequence cloned into the pSUPER vector was the
same as the designed and synthesized sequence.
IL-6 expression in C6 cells
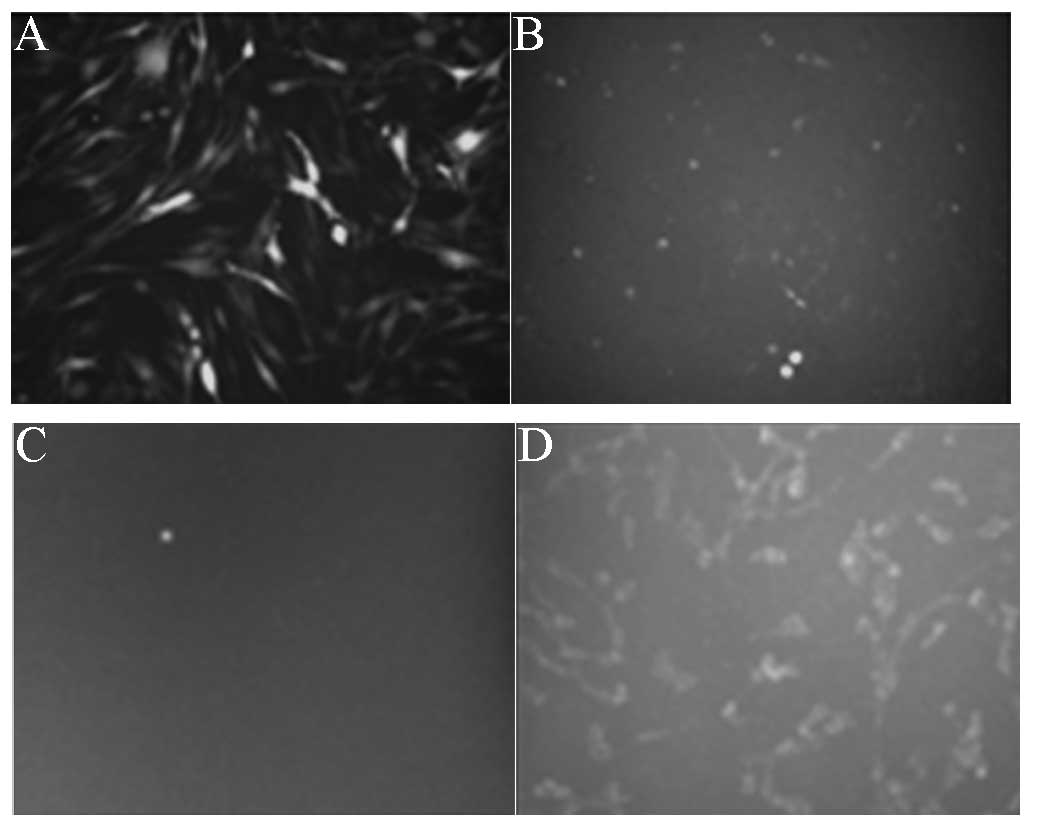
In the positive control groups (Fig. 3A and B), following C6-GFP cell
transfection with pSUPER-siRNA-GFP, the fluorescence intensity was
significantly reduced (Fig. 3B),
while in the negative control groups (Fig. 3C and D), following C6 cell
transfection with pSUPER-GFP, the cytoplasm was observed to exhibit
fluorescence (Fig. 3D).
IL-6 secretion was found to be significantly
increased 24 and 48 h after LPS stimulation in C6 cells, peaking at
24 h. pSUPER-IL-6 1–5 were observed to affect IL-6 secretion in
LPS-stimulated C6 cells, with IL-6 protein levels in the culture
supernatant in the pSUPER and pSUPER-IL-6 1–5 transfected cells
observed to be 369±16.7, 121±12, 155±13.3, 198±11.3, 176±9 and
166±11 pg/ml, respectively. Moreover, the interference effect of
pSUPER-IL-6 1–5 was found to be stronger 24 h after LPS stimulation
compared with 48 h after LPS stimulation. ELISA revealed that
pSUPER-IL-6 1 exhibited the strongest RNAi effect, inducing a 66.6%
reduction in IL-6 secretion compared with the control, thus this
plasmid was selected for the subsequent animal experiments
(Fig. 4).
IL-8 expression in C6 cells
IL-8 basic levels were very low (5±0.5 pg/ml),
however, following transfection after LPS stimulation, IL-8 levels
were found to significantly increase (377±13 pg/ml). Compared with
the control group, no significant difference was observed in IL-8
expression in LPS-stimulated C6 cells transfected with pSUPER-IL-6
1–5 (P>0.05; Fig. 5).
IL-6 expression in the brain tissue
The IL-6 levels in the peripheral brain tissues
around the focal traumatic lesions after 24 h were 79±4, 76±5 and
58±6.3 pg/ml in the negative control, empty plasmid and pSUPER-IL-6
1 groups, respectively. Furthermore, after 72 h, the IL-6 levels
were 72±5, 71±4 and 66±5.3 pg/ml in the negative control, empty
plasmid and pSUPER-IL-6 1 groups, respectively. Compared with the
control group and the empty plasmid group, IL-6 was observed to be
significantly lower in the pSUPER-IL-6 1 group (P<0.05; Fig. 6).
Brain edema
Statistically significant differences were observed
in the water and sodium content in the peripheral brain tissues
around the focal traumatic lesions among the control, empty plasmid
and experimental groups (P<0.05; Table II).
 | Table IIWater and sodium content of the
peripheral brain tissues. |
Table II
Water and sodium content of the
peripheral brain tissues.
| Group | 24 h | 72 h |
|---|
| Control |
| Water content
(%) | 78.68±0.76 | 81.67±0.67 |
| Sodium content
(mmol/kg) | 671.67±40.04 | 594.39±29.17 |
| Empty plasmid |
| Water content
(%) | 77.49±0.59 | 85.55±0.75 |
| Sodium content
(mmol/kg) | 668.13±56.11 | 599.36±33.71 |
| Experimental |
| Water content
(%) | 69.03±0.55a | 75.45±0.37a |
| Sodium content
(mmol/kg) |
590.24±38.54b |
510.89±39.60b |
Discussion
The present study identified that IL-6 was
overexpressed in C6 rat glioma cells treated with the microbial
product LPS. This finding is in agreement with a previous study,
which analyzed the association between IL-6 and post-injury
treatment of C6 glioma cells (17). pSUPER-IL-6 vector-based siRNA was
found to downregulate endogenous IL-6 expression in C6 rat glioma
cells induced by the microbial product LPS. Furthermore,
vector-based IL-6 siRNA was also found to downregulate the
expression of IL-6 in rats with TBI. Thus, the administration of
siRNA may inhibit brain edema in rats with TBI.
IL-6 is a cytokine with broad immunomodulatory
effects. IL-6 consists of 212 amino acids, has a molecular
weight of 26 kDa and is primarily produced by T and B lymphocytes,
monocytes and endothelial cells. In the brain, IL-6 is
predominantly produced by astrocytes and glial cells. Under normal
circumstances, the expression of IL-6 in the brain is low,
mediating a variety of physiological functions, including central
immune and nervous reparation (18). However, under pathological stress,
the secretion of cerebral astrocytes and microglia increases, thus
IL-6 expression increases (2,3,19,20),
which mediates a series of pathophysiological reactions. Such
IL-6-induced pathophysiological reactions include: (i) Changes in
cerebral microcirculation, resulting in increases in vascular
permeability and damage to the BBB, thus promoting the formation
and progression of cerebral edema; (ii) increases in leukocyte
adhesion and endothelial cells, promoting extravascular
infiltration and activation of inflammatory cells (4,19);
(iii) increases in the proliferation and reparation of cerebral
glial cells in damaged brain regions (5,22);
(iv) induction of complications associated with TBI, as well as
multiple organ failure, for example, increases in IL-6 have a
negative muscle strength effect and cytotoxic effect, which causes
damage to the structure and function of the left ventricle,
promoting deterioration of patient cardiac function and
hemodynamics (7,8,23,24);
and (v) problems associated with fever and local metabolism,
affecting patient prognosis and rehabilitation. As an inflammatory
cytokine, IL-6 expression has been found to increase significantly
in the CNS following TBI and the levels of IL-6 in the CSF has been
negatively correlated with Glasgow Coma Scale and Glasgow Outcome
Scale scores following injury (9,25).
Clinical and experimental studies have shown that during acute
brain injury, IL-6 levels in the CSF increase rapidly, peaking
within 24 h and gradually declining three days following injury,
indicating that inflammatory cytokines are involved in local
inflammatory responses at an early stage following the induction of
TBI. Furthermore, the levels of inflammatory cytokines exhibit a
significant positive correlation with disease severity and are an
important indicator of condition reflection and prognosis (11,12,26,27).
Fundamentally, inflammation is an anti-damage response in the body;
however, if the inflammatory response is too strong, severe damage
to tissues and organs may be caused. Therefore, inhibiting
excessive inflammatory responses has become the focus of much
research (14,28).
At present, studies investigating IL-6 inhibition
are primarily focused on inhibiting IL-6/IL-6R or neutralizing
IL-6/IL-6R antibodies and are still in the experimental stages
(15–19,29–31).
Based on the 3-dimensional structure of the IL-6 protein, novel
antagonist proteins have been designed, including the pertussis
toxin protein (32). Furthermore,
non-protein antagonists of IL-6R have been generated, including
20R,21R-epoxyresibufogenin-3-formate (21,33).
In studies on breast cancer, the IL-6 Sant 7 mutant (22,34)
has been used to bind IL-6R and inhibit its interactions with the
glycoprotein 130 signal transduction protein, thereby inhibiting
the activity of macrophages and lymphocyte-derived aromatase,
reducing the synthesis of cellular estradiol. Due to the short
half-life and rapid metabolism of the IL-6 antibody, as well as
other factors, including immunogenicity and high cost, there are
several problems associated with its clinical application. As a
novel and powerful research tool, RNAi has shown great potential in
the field of functional genomics, with advantages including its
fast, effective, easy to operate and sequence-specific application.
Therefore, the present study aimed to investigate the effect of
RNAi on the regulation and inhibition of IL-6 following
transcription.
In the present study, a targeted rat IL-6 siRNA
sequence was successfully designed and pSUPER-IL-6 1–5 plasmids
were successfully constructed. Moreover, pSUPER-IL-6 was observed
to transfect the C6 rat cerebral glioma cells effectively. LPS was
used to stimulate the release of inflammatory mediators in the
transfected C6 cells and ELISA revealed that pSUPER-IL-6 1–5 all
effectively inhibited IL-6 expression, with pSUPER-IL-6 1 exerting
the strongest inhibitory effect, achieving a 66.6% reduction in
IL-6 secretion compared with the control. The interference effect
24 h after LPS induction was stronger than that 48 h after LPS
induction, while the pSUPER empty plasmid had no effect. IL-6 and
−8 expression is activated through the nuclear factor
κ-light-chain-enhancer of activated B cell pathway, by IL-1β and
TNF-α (35). In the present study,
to investigate whether pSUPER-IL-6 transfection exerted a
non-specific effect and affected IL-8 secretion, IL-8 protein
levels were also assessed following transfection. Prior to LPS
stimulation, IL-8 levels were observed to be very low (5±0.5
pg/ml); however, following transfection and 24 h after LPS
stimulation, IL-8 levels were found to significantly increase
(377±13 pg/ml). No significant differences in IL-8 expression were
observed among the groups, indicating that the interference was
specific to IL-6.
To further investigate the inhibitory effect of
pSUPER-IL-6 on IL-6 protein levels in vivo, a severe rat
brain injury model was generated. pSUPER-IL-6 was found to reduce
IL-6 levels in the focal cerebral damage lesions. Local
inflammatory response levels are directly associated with the
severity of brain edema, which impacts disease progression
and prognosis; therefore, the water and sodium content in the
traumatic brain tissue was also examined. The water and sodium
content of the traumatic brain tissue in the experimental group was
found to be lower than that in other groups, suggesting that IL-6
was directly involved in the inflammatory response of the focal
brain injury and increased brain tissue edema through the
inflammatory response. Although the inflammatory response is an
anti-damage response in the body, it is capable of inducing severe
tissue and organ damage. Thus, controlling excessive inflammatory
reactions may become a new target for studying and treating
inflammation-associated diseases. In the present study, although
IL-6 RNAi was observed to significantly downregulate IL-6
expression in vitro, IL-6 expression in vivo was only
slightly reduced. Furthermore, only slight significant differences
in brain water and sodium content were found among the groups. It
is possible that the delivery of plasmids to neural cells using
Lipofectamine® 2000 has a poor transfection efficiency,
particularly in vivo. The delivery of siRNA using viral
transduction may be one way to overcome this problem. Furthermore,
the continuous delivery of vectors may provide higher transfection
efficiency. Brain trauma causes the expression of a variety of
genes that are involved in the early post-traumatic inflammatory
response (36). The large number
of in vivo inflammatory cytokines form an extensive network
of interactions; therefore, the identification of key inflammatory
factors requires further investigation.
In conclusion, the present study showed that siRNA
targeting of IL-6 mRNA using a plasmid-based system effectively
sustains the knockdown of IL-6 gene expression in C6 neural cells.
However, the potential for IL-6 RNAi as a therapeutic strategy to
control inflammation requires further investigation.
References
|
1
|
Dewall J: The ABCs of TBI. Evidence-based
guidelines for adult traumatic brain injury care. JEMS. 35:54–61.
2010.PubMed/NCBI
|
|
2
|
Mustafa AG and Alshboul OA:
Pathophysiology of traumatic brain injury. Neurosciences (Riyadh).
18:222–234. 2013.
|
|
3
|
Stein DM, Lindell A, Murdock KR, et al:
Relationship of serum and cerebrospinal fluid biomarkers with
intracranial hypertension and cerebral hypoperfusion after severe
traumatic brain injury. J Trauma. 70:1096–1103. 2011. View Article : Google Scholar
|
|
4
|
Zhang R, Liu Y, Yan K, et al:
Anti-inflammatory and immunomodulatory mechanisms of mesenchymal
stem cell transplantation in experimental traumatic brain injury. J
Neuroinflammation. 10:1062013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Roberts DJ, Jenne CN, Léger C, et al:
Association between the cerebral inflammatory and matrix
metalloproteinase responses after severe traumatic brain injury in
humans. J Neurotrauma. 30:1727–1736. 2013. View Article : Google Scholar
|
|
6
|
Yang SH, Gangidine M, Pritts TA, Goodman
MD and Lentsch AB: Interleukin 6 mediates neuroinflammation and
motor coordination deficits after mild traumatic brain injury and
brief hypoxia in mice. Shock. 40:471–475. 2013. View Article : Google Scholar
|
|
7
|
Nakamura M, Okada S, Toyama Y and Okano H:
Role of IL-6 in spinal cord injury in a mouse model. Clin Rev
Allergy Immunol. 28:197–204. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hildebrand F, Pape HC and Krettek C: The
importance of cytokines in the posttraumatic inflammatory reaction.
Unfallchirurg. 108:793–794. 796–803. 2005.(In German).
|
|
9
|
Poulsen CB, Penkowa M, Borup R, et al:
Brain response to traumatic brain injury in wild-type and
interleukin-6 knockout mice: a microarray analysis. J Neurochem.
92:417–432. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Van Wagoner NJ and Benveniste EN:
Interleukin-6 expression and regulation in astrocytes. J
Neuroimmunol. 100:124–139. 1999.PubMed/NCBI
|
|
11
|
Campo S, Serlupi-Crescenzi O, Arseni B, et
al: Comparative activity of Sant7 and anti-IL-6, IL-6R monoclonal
antibodies in a murine model of B-cell lymphoma. Cytokine.
31:368–374. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Srirangan S and Choy EH: The role of
interleukin 6 in the pathophysiology of rheumatoid arthritis. Ther
Adv Musculoskelet Dis. 2:247–256. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tuschl T, Zamore PD, Lehmann R, et al:
Targeted mRNA degradation by double-stranded RNA in vitro. Genes
Dev. 13:3191–3197. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Coelho T, Adams D, Silva A, et al: Safety
and efficacy of RNAi therapy for transthyretin amyloidosis. N Engl
J Med. 369:819–829. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brummelkamp TR, Bernards R and Agami R: A
system for stable expression of short interfering RNAs in mammalian
cells. Science. 296:550–553. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Feeney DM, Boyeson MG, Linn RT, Murray HM
and Dail WG: Responses to cortical injury: I. Methodology and local
effects of contusions in the rat. Brain Res. 211:67–77. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bingham D, John CM, Panter SS and Jarvis
GA: Post-injury treatment with lipopolysaccharide or
lipooligosaccharide protects rat neuronal and glial cell cultures.
Brain Res Bull. 85:403–409. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Spooren A, Kolmus K, Laureys G, et al:
Interleukin-6, a mental cytokine. Brain Res Rev. 67:157–183. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kotzerke K, Mempel M, Aung T, et al:
Immunostimulatory activity of murine keratinocyte-derived exosomes.
Exp Dermatol. 22:650–655. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ziebell JM and Morganti-Kossmann MC:
Involvement of pro- and anti-inflammatory cytokines and chemokines
in the pathophysiology of traumatic brain injury.
Neurotherapeutics. 7:22–30. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lu J, Goh SJ, Tng PY, Deng YY, Ling EA and
Moochhala S: Systemic inflammatory response following acute
traumatic brain injury. Front Biosci (Landmark Ed). 14:3795–3813.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Frugier T, Morganti-Kossmann MC, O’Reilly
D and McLean CA: In situ detection of inflammatory mediators in
post mortem human brain tissue after traumatic injury. J
Neurotrauma. 27:497–507. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Harhay MO, Tracy RP, Bagiella E, et al:
Relationship of CRP, IL-6, and fibrinogen with right ventricular
structure and function: the MESA-Right Ventricle Study. Int J
Cardiol. 168:3818–3824. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Caselli C, D’Amico A, Caruso R, et al:
Impact of normalization strategy on cardiac expression of
pro-inflammatory cytokines: evaluation of reference genes in
different human myocardial regions after Left Ventricular Assist
Device support. Cytokine. 63:113–122. 2013. View Article : Google Scholar
|
|
25
|
Chiaretti A, Antonelli A, Mastrangelo A,
et al: Interleukin-6 and nerve growth factor upregulation
correlates with improved outcome in children with severe traumatic
brain injury. J Neurotrauma. 25:225–234. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Woodcock T and Morganti-Kossmann MC: The
role of markers of inflammation in traumatic brain injury. Front
Neurol. 4:182013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Papa L, Ramia MM, Kelly JM, Burks SS,
Pawlowicz A and Berger RP: Systematic review of clinical research
on biomarkers for pediatric traumatic brain injury. J Neurotrauma.
30:324–338. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Patterson ZR and Holahan MR: Understanding
the neuroinflammatory response following concussion to develop
treatment strategies. Front Cell Neurosci. 6:582012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Erta M, Quintana A and Hidalgo J:
Interleukin-6, a major cytokine in the central nervous system. Int
J Biol Sci. 8:1254–1266. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu Y, Fuchs J, Li C and Lin J: IL-6, a
risk factor for hepatocellular carcinoma: FLLL32 inhibits
IL-6-induced STAT3 phosphorylation in human hepatocellular cancer
cells. Cell Cycle. 9:3423–3427. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mukaino M, Nakamura M, Yamada O, et al:
Anti-IL-6-receptor antibody promotes repair of spinal cord injury
by inducing microglia-dominant inflammation. Exp Neurol.
224:403–414. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang Z, Feng J, Li Y, Hu M, et al:
Structure-based design and characterization of a Novel IL-6
antagonist peptide. Mol Immunol. 42:1015–1021. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Boos TL, Cheng K, Greiner E, Deschamps JR,
Jacobson AE and Rice KC: Configurational reassignment and improved
preparation of the competitive IL-6 receptor antagonist
20R,21R-epoxyresibufogenin-3-formate. J Nat Prod. 75:661–668. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Purohit A, Singh A, Ghilchik MW,
Serlupi-Crescenzi O and Reed MJ: Inhibition of IL-6+IL-6 soluble
receptor-stimulated aromatase activity by the IL-6 antagonist, Sant
7, in breast tissue-derived fibroblasts. Br J Cancer. 88:630–635.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ekström M, Halle M, Bjessmo S, et al:
Systemic inflammation activates the nuclear factor-kappaB
regulatory pathway in adipose tissue. Am J Physiol Endocrinol
Metab. 299:E234–E240. 2010.PubMed/NCBI
|
|
36
|
Israelsson C, Wang Y, Kylberg A, Pick CG,
Hoffer BJ and Ebendal T: Closed head injury in a mouse model
results in molecular changes indicating inflammatory responses. J
Neurotrauma. 26:1307–1314. 2009. View Article : Google Scholar : PubMed/NCBI
|