Introduction
Dental pulp stem cells (DPSCs) are primarily
isolated from dental pulp tissues (1,2).
These cells exhibit multilineage differentiation potential,
particularly odontogenic differentiation potential. Previous
studies have indicated that DPSCs are capable of differentiating
into an odontoblastic lineage in vitro and forming ectopic
pulp-dentine-like tissue in vivo (2–4).
However, limitations remain for the direct application of DPSCs in
dental tissue regeneration, and optimized issue engineering
strategies are required to improve the odontogenic differentiation
capacity of DPSCs (5).
The administration of cytokines is one of the most
commonly utilized strategies to strengthen the osteogenic
differentiation capacity of cells. Vascular endothelial growth
factor (VEGF) is an important cytokine that can promote the
osteogenic differentiation of cells. A previous study demonstrated
that recombinant human VEGF improved the cell proliferation and
alkaline phosphatase (ALP) expression of dental pulp cells
(6). VEGF culture medium also
promoted the osteogenic differentiation capacity of MC3T3 stem
cells by increasing the expression of ALP and osteocalcin (OCN)
in vitro (7). Compared with
control cells, VEGF-treated human periodontal ligament stem cells
exhibited a significantly higher ALP activity, which further led to
the formation of increased mineralized tissue (8). Furthermore, VEGF culture medium
increased ALP expression and calcium accumulation of DPSCs in
vitro (9). However,
there are limitations in the local delivery of cytokines, including
the short half-life, large dose requirements, high costs, the
necessity for repeated applications and poor expression
distribution (10). Gene
transfection technology may be a solution to some of these
technical caveats. Transient transfection of the VEGF gene into
human periosteal cells could promote the expression of ALP, OCN and
Collagen I (11). VEGF
gene-transfected rat bone marrow stromal cells (BMSCs) were
reported to have higher ectopic osteogenesis in vivo
(12). Another study also
confirmed the improved osteogenic differentiation capacity of rat
BMSCs by lentivirus-mediated VEGF gene transfection (13). Due to the similarity between
osteogenic and odontogenic differentiation among DPSCs (14), lentiviral vector-mediated stable
transfection of the VEGF gene may be an effective strategy to
improve the odontogenic differentiation capacity of DPSCs.
This study aimed to explore the effects of
lentivirus-mediated VEGF gene transfection on the odontogenic
differentiation of human DPSCs in vitro. The proliferation
and odontogenic differentiation capacities of VEGF gene-transfected
DPSCs (DPSCs/VEGF) were analyzed. The results of this study could
enhance the understanding of the biological characteristics of
DPSCs/VEGF, and may provide theoretical evidence for the
application of lentivirus-mediated VEGF gene transfection in dental
tissue engineering.
Materials and methods
Isolation of DPSCs
Patients were recruited for this study from the
Guanghua Hospital of Stomatology (Guangzhou, China). All
participants provided their informed consent. The study was
approved by the Ethical Review Committee of the Hospital of
Stomatology, Sun Yat-Sen University (Guangzhou, China). DPSCs were
isolated from freshly extracted third molars without signs of decay
(Fig. 1A). The extracted teeth
were thoroughly cleaned, then cut at the cementoenamel junction
using a sterile dental fissure bur. The tissues in the pulp chamber
were exposed and gently separated from the crown. The pulp tissues
were subsequently minced and digested with 3 mg/ml collagenase type
I (Worthington Biochem, Freehold, NJ, USA) and 4 mg/ml dispase
(Boehringer Mannheim, Indianapolis, IN, USA) for 1 h at 37°C. The
cells were then passed through a 70-μm strainer (BD Biosciences,
Bedford, MA, USA) to generate a single-cell suspension. The
isolated DPSCs were seeded at densities of between 1×104
and 1×105/well in six-well plates (Corning, New York,
NY, USA) containing Dulbecco’s modified Eagle’s medium (DMEM;
Invitrogen Hong Kong Ltd., Hong Kong, China) supplemented with 10%
fetal bovine serum (FBS; Sijiqing, Hangzhou, China), 100 μM
L-ascorbic acid-2-phosphate (Wako, Tokyo, Japan), 100 U/ml
penicillin-G and 100 mg/ml streptomycin, and were cultured with 5%
CO2 at 37°C.
Differentiation stimulation
The multipotent differentiation potential of the
cells was identified by osteogenic and adipogenic differentiation
induction (14). Briefly, the
cells were exposed to osteogenic medium (DMEM supplemented with 10
nmol/l dexamethasone, 10 mmol/l β-glycerophosphate, 50 μg/ml
ascorbate phosphate, 10 nmol/l 1,25 dihydroxyvitamin D3 and 10%
FBS) and adipogenic medium (DMEM supplemented with 1 μmol/l
dexamethasone, 1 μg/ml insulin, 0.5 mmol/l
3-isobutyl-1-methylxantine and 10% FBS) for 28 days. Alizarin Red S
and Oil Red O reagent were used to visualize the calcium
accumulation and oil droplets, respectively.
Flow cytometric analysis
Prior to the conduction of the experiments, the
phenotype of the freshly isolated DPSCs was evaluated by flow
cytometry (FCM) for the expression of STRO-1/Alexa Fluor 647
(BioLegend, San Diego, CA, USA), cluster of differentiation 146
(CD146)/phycoerythrin (PE) (BD Pharmingen, San Diego, CA, USA),
CD34/PE (BD Pharmingen), CD45/fluorescein isothiocyanate (FITC; BD
Pharmingen)) and CD24/FITC (BD Pharmingen).
Construction of lentivirus plasmid
The lentivirus vector pCDH-CMV-MCS-EF1-copGFP (pCDH;
System Biosciences, Mountain View, CA, USA) with green fluorescent
protein (GFP) label was used to visualize the recombinant plasmid
expression. The human VEGF primers were designed with Oligo 7.0
software (Molecular Biology Insights, Plymouth, MN, USA) according
to the National Center for Biotechnology Information GenBank no.
AF486837.1, and were amplified by polymerase chain reaction (PCR).
The PCR primers were designed as follows: Forward, GCCGAATTCATG AACTTTCTGCTGTCTTG
(the underlined sequence indicates an EcoRI site); reverse,
GCCGGATCCTCACCG
CCTCGGCTTGTCAC (the underlined sequence indicates a BamHI
site). For PCR amplification, specific primers were used with
following reaction conditions: Pre-denaturation, 95°C for 3 min; 30
cycles of denaturation, 95°C for 15 sec; primer annealing, 55°C for
30 sec; primer extension, 72°C for 1 min and final extension at
72°C for 7 min, prior to storage at 4°C for 10 min. The amplified
products were digested using EcoRI and BamHI
restriction enzymes. The fragment containing human VEGF gene was
cloned into the vector to produce the pCDH-CMV-MCS-EF1-copGFP-VEGF
recombinant lentiviral plasmid, referred to as pCDH-VEGF.
Gene transfection
293FT cells (System Biosciences) were cultured in
DMEM with 10% FBS, prior to being seeded (1.2×106 cells)
into a 10 cm dish one day before transfection. The recombinant
plasmid pCDH-VEGF, packaging plasmid psPAX.2 (Cyagen, Guangzhou,
China) and envelope plasmid pMD2.G were co-transfected into the
293FT cells using Lipofectamine™ 2000 (Invitrogen Life
Technologies, Carlsbad, CA, USA) (15,16).
The liquid supernatant was collected after 48 h, centrifuged at
1,000 × g at 37°C for 10 min and filtered using a 0.2-μm syringe
(Millipore, Bedford, MA, USA). The third-passage DPSCs were
infected with the supernatant to acquire the recombined DPSCs/VEGF.
By use of the same protocol, the blank pCDH vector was used to
infect DPSCs to construct DPSCs/Vector as a negative control.
Through analyzing the percentage of GFP fluorescence, the
transfection ratio was identified by ImageJ software (NIH,
Bethesda, MD, USA). VEGF expression from the DPSCs/Vector and
DPSCs/VEGF was assessed using a quantitative PCR (qPCR) and western
blot analysis two days after transfection. The DPSCs/Vector and
DPSCs/VEGF were seeded in 25-cm2 culture flasks
containing DMEM supplemented with 10% FBS, and cultured with 5%
CO2 at 37°C. The culture medium was changed at 24-h
intervals.
Cell proliferation assessment
DPSCs/Vector and DPSCs/VEGF were seeded onto 96-well
plates at the density of 2×103 cells/well and cultured
in DMEM with 10% FBS. The cell proliferation was evaluated using a
Cell Counting kit 8 (CCK8) according to the manufacturer’s
instructions (Dojindo Molecular Technologies, Tokyo, Japan). The
CCK8 reduction/attenuation values of the wells were measured by
spectrophotometer at an optical density (OD) of 520 nm
(BioPhotometer Plus; Eppendorf, Hamburg, Germany). The tests were
performed on the first, second, fourth and eighth days after
seeding. The assay of each well was repeated in triplicate.
qPCR analysis
On the first, second, fourth, eighth and 16th days
after transfection, the total RNA of the DPSCs/Vector and
DPSCs/VEGF was isolated using TRIzol® (Invitrogen Life
Technologies), and the quantity of extracted RNA was evaluated by a
spectrophotometer (BioPhotometer Plus; Eppendorf). For each sample,
2 μg RNA was used to synthesize the cDNA of each gene using the
RevertAid™ First Strand cDNA Synthesis kit (Thermo Scientific™
Molecular Biology, Shenzhen, China). The qPCR reaction mix used was
iQSYBR Green Supermix (BioRad, Hercules, CA, USA) and the reaction
was controlled by the spectrofluorimetric thermal iCycler iQ5
(Bio-Rad). VEGF gene and four odontogenic differentiation genes,
ALP, OCN, dentin sialophosphoprotein (DSPP) and dentin matrix
protein 1 (DMP1), were assessed. For PCR amplification of the cDNA
of each gene, an initial amplification using gene-specific primers
(Table I) was performed with a
denaturation step (95°C for 3 min), followed by 39 cycles of
denaturation (95°C for 10 sec), primer annealing (55°C for 10 sec)
and primer extension (72°C for 30 sec). The amplification
efficiency of these genes was determined relative to the
housekeeping gene, GAPDH. Each sample was performed in
triplicate.
 | Table IHuman-specific primer sequences used
for quantitative polymerase chain reaction. |
Table I
Human-specific primer sequences used
for quantitative polymerase chain reaction.
| Gene | Primer sequence
(5–3′) | Size (bp) |
|---|
| VEGF | Forward:
CTACCTCCACCATGCCAAGT | |
| Reverse:
AGCTGCGCTGATAGACATCC | 104 |
| ALP | Forward:
CTATCCTGGCTCCGTGCTC | |
| Reverse:
GCTGGCAGTGGTCAGATGTT | 100 |
| OCN | Forward:
CTCACACTCCTCGCCCTATT | |
| Reverse:
TTGGACACAAAGGCTGCAC | 107 |
| DSPP | Forward:
GCCACTTTCAGTCTTCAAAGAGA | |
| Reverse:
GCCCAAATGCAAAAATATGTAA | 130 |
| DMP1 | Forward:
AAAATTCTTTGTGAACTACGGAGG | |
| Reverse:
GAGCACAGGATAATCCCCAA | 94 |
| GAPDH | Forward:
AAGGTGAAGGTCGGAGTCAA | |
| Reverse:
AATGAAGGGGTCATTGATGG | 108 |
Western blot analysis
DPSCs/Vector and DPSCs/VEGF were seeded on six-well
plates with 5×104/well density. On the first, second,
fourth, eighth and 16th days after transfection, the cells were
harvested for western blot analysis. The culture media of the
DPSCs/Vector and DPSCs/VEGF were removed from the six-well plates
and the cells were washed with 2 ml phosphate-buffered saline at
4°C for 1 min. The total proteins of these cells were extracted
with 100 μl radio-immunoprecipitation assay buffer (BioTeke,
Beijing, China) and 1 μl phenylmethanesulfonyl fluoride phosphatase
inhibitor, and then collected by centrifugation at 12,000 rpm for 5
min. A bicinchoninic acid protein assay kit (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) was used to detect the total
protein concentrations of the two groups of cells. Equal quantities
of protein from the samples were then subjected to 12% SDS-PAGE for
electrophoresis. Thereafter, the separated proteins were
transferred onto polyvinylidene difluoride membranes (Millipore,
Billerica, MA, USA). The membranes were blocked with 5% (w/v)
non-fat milk for 2 h at room temperature, and incubated
respectively with 1:500 mouse anti-human VEGF antibody (Abcam, MA,
USA), rabbit anti-human DMP1 antibody and goat anti-human dentin
sialoprotein (DSP) antibody (Santa Cruz Biotechnology Inc., Santa
Cruz, CA, USA) overnight at 4°C. The membranes were subsequently
incubated with secondary antibody at 37°C for 2 h. GAPDH was used
as the internal control. The resultant films were visualized by an
Enhanced Chemiluminescence Western Blotting Detection system
(Millipore).
Statistical analysis
The data are presented as the mean ± standard
deviation. The two-way analysis of variance (ANOVA) test was used
to analyze the differences between the DPSCs/Vector and DPSCs/VEGF
(SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Morphological, multipotent
differentiation and phenotypic characteristics of DPSCs
DPSCs were isolated from third molars and a cellular
suspension was obtained (Fig. 1A and
B). The majority of the adherent DPSCs exhibited a spindle-like
appearance with extending cytoplasmic processes (Fig. 1C). Following in vitro
culture for 10 days, the first passage of DPSCs reached complete
confluence (Fig. 1D). Calcium
accumulation (Fig. 1E) and oil
droplets (Fig. 1F) were observed
in DPSCs subsequent to differentiation induction for 28 days. The
FCM results showed that the expression of STRO-1, CD146, CD34, CD45
and CD24 was 0.59, 73.00, 0.13, 0.01 and 0.01%, respectively
(Fig. 2).
Transfection efficiency
The DPSCs/Vector and DPSCs/VEGF were successfully
constructed (Fig. 3). The
DPSCs/Vector and DPSCs/VEGF exhibited a spindle-like shape with
extending cytoplasmic process, as observed in untransfected DPSCs
(Fig. 3A and D). The green
fluorescence was detected in the majority of the transfected cells
(Fig. 3B and E), and the
transfection ratio was ~90% (Fig. 3C
and F), analyzed using ImageI software. The VEGF gene
expression was higher in the DPSCs/VEGF (8.22±0.59) than that in
the DPSCs/Vector (1.00±0.06) (P<0.01) two days after
transfection (Fig. 3G). The
western blot analysis also showed that VEGF expression in the
DPSCs/VEGF was significantly upregulated, compared with that in the
DPSCs/Vector (Fig. 3H).
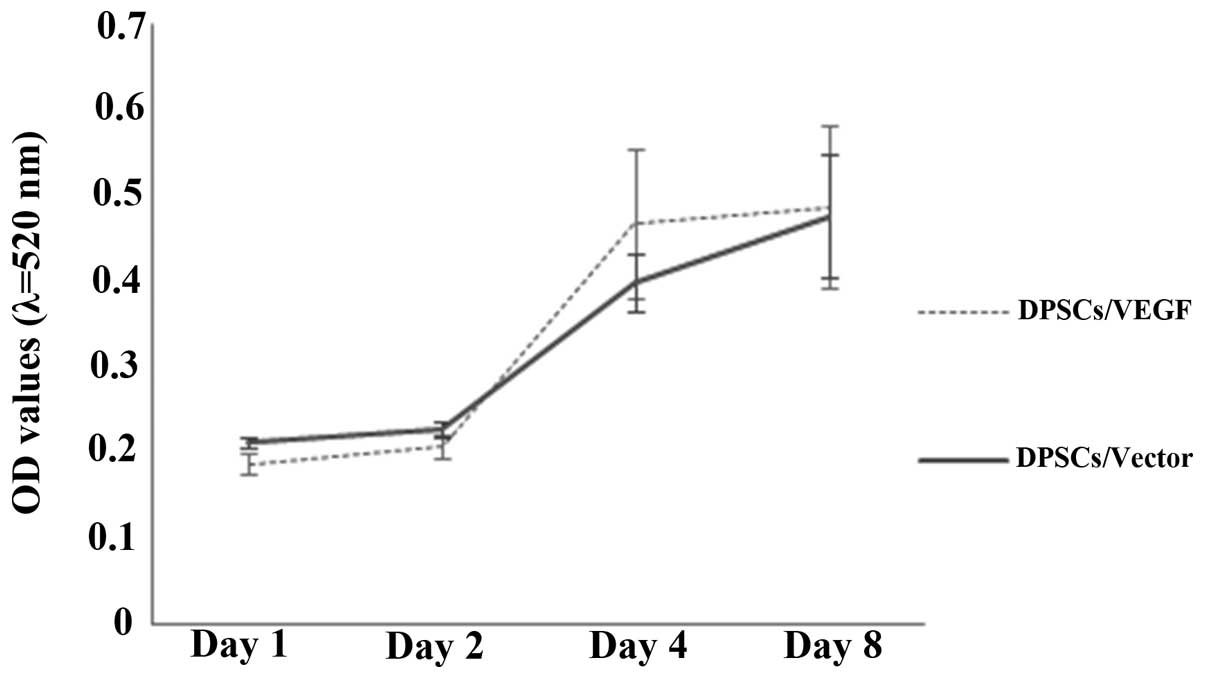
DPSC proliferation characteristics
A similar proliferation profile was observed for the
DPSCs/Vector and DPSCs/VEGF (Fig.
4). The average OD values of the two groups of cells
significantly increased from 0.2 to 0.5 during the observation
period of eight days (P<0.05). However, larger standard
deviations in the OD readings from the DPSCs/VEGF were observed
when compared with those from the DPSCs/Vector over time.
Odontogenic differentiation gene
expression
The relative ALP gene expression of the DPSCs/Vector
and DPSCs/VEGF increased during the first eight days (Fig. 5). The relative ALP gene expression
in the DPSCs/VEGF was significantly higher than that in the
DPSCs/Vector on the first, fourth, eighth and 16th days after gene
transfection (P<0.05). The increasing trend in the relative OCN
expression in the DPSCs/Vector was no longer observed on the 16th
day after transfection; however, the increasing trend remained in
the DPSCs/VEGF. The relative expression of OCN was statistically
higher in the DPSCs/VEGF as compared with that in the DPSCs/Vector
on the fourth, eighth and 16th days after gene transfection
(P<0.05). Both the DPSCs/Vector and the DPSCs/VEGF showed an
enhancement in DSPP expression over time. The DSPP expression
levels were statistically higher in the DPSCs/VEGF than those in
DPSCs/Vector at the four time-points (P<0.01). Furthermore, the
relative expression levels of DMP1 gene in the DPSCs/VEGF were
significantly higher than those in the DPSCs/Vector on the first,
fourth and 16th days after gene transfection (P<0.05). For the
expression of ALP, OCN, DSPP and DMP1 genes, the two-way ANOVA
showed a significant main effect for the cell types and culture
times (P<0.05), and the interaction between the cell types and
culture times was also significant (P<0.05).
 | Figure 5Odontogenic differentiation gene
expression. The gene expression of DPSCs/Vector and DPSCs/VEGF was
assessed by quantitative polymerase chain reaction on the first,
fourth, eighth and 16th days of lentivirus-mediated gene
transfection. The relative expression levels of ALP, OCN, DSPP and
DMP1 genes were generally upregulated in DPSCs/VEGF as compared
with those in DPSCs/Vector. *P<0.05 and
**P<0.01. Error bars represent the mean ± standard
deviation. VEGF, vascular endothelial growth factor; ALP, alkaline
phosphatase; OCN, osteocalcin, DSPP, dentin sialophosphoprotein;
DMP1, dentin matrix acidic phosphoprotein 1; DPSCs, dental pulp
stem cells. |
Odontogenic differentiation protein
expression
Western blot analysis confirmed that the odontogenic
differentiation-specific marker DSP was significantly upregulated
(P<0.05) in the DPSCs/VEGF as compared with the DPSCs/Vector on
the first, fourth, eighth and 16th days after transfection, and the
odontogenic differentiation-specific marker DMP1 was also
significantly upregulated (P<0.05) in the DPSCs/VEGF as compared
with the DPSCs/Vector on the first, fourth and 16th days after
transfection (Fig. 6). For the
expression of DSP and DMP1, two-way ANOVA showed a significant main
effect for the cell types and culture times (P<0.05), and the
interaction between the cell types and culture times was also
significant (P<0.05).
Discussion
In the present study, evidence from the formation of
calcium accumulation and oil droplets following osteogenic and
adipogenic induction showed that the isolated DSPCs were
multipotent. The expression of STRO-1 and CD146 in the DPSCs was
0.59 and 73.00% respectively, indicating that the isolated cells
conformed to the phenotypic characteristics of DSPCs. STRO-1 has
been previously observed in pulp tissues and is regarded as a
marker for stromal stem cells, recognizing the trypsin-insensitive
epitope on perivascular cells (4).
In the majority of previous studies, STRO-1 expression has been
observed in DPSCs, with an expression level ranging between 0.46
and 26.14% (4,14,17).
CD146 is a stem cell marker identified in adult bone marrow and
dental pulp tissue, associated with blood vessels (18). Studies have shown positive
expression of CD146 among DPSCs, with a range in expression of
between 20.4 and 98.0% (2,14,19,20).
CD34 is a marker expressed in the majority of stem and progenitor
cells, involved in the maintenance of the plastic state of
undifferentiated cells (21). The
expression of CD34 in this study was 0.13%, suggesting that
undifferentiated cells were present in the DPSC culture. CD45 and
CD24 are markers of hematopoietic/endothelial stem cells (14). Consistent with discussions in a
previous review (4), no CD45 and
CD24 expression was observed in this study.
In the present study, cell-culture conditions
without L-ascorbic acid-2-phosphate and other external supplements
following lentivirus-mediated transfection were selected. Cells
that are cultured with L-ascorbic acid-2-phosphate have been shown
to exhibit a significant improvement in cell proliferation, ALP
expression and osteogenic differentiation capacity (22–25).
Culturing the DPSCs with L-ascorbic acid-2-phosphate may cause
hybrid bias in the analysis and confound the interpretation of the
role of the VEGF expressed in the described genetically modified
DPSCs.
ALP has been widely used as a marker for
differentiated cells producing mineralized matrix (7,14).
OCN, a non-collagenous protein, is usually expressed during the
late stages of osteogenic/odontogenic differentiation (7,14).
In this study, the expression of ALP and OCN was significantly
enhanced in the DPSCs/VEGF as compared with that in the
DPSCs/Vector. This result was consistent with that from other
reports on cells with VEGF overexpression in vitro (11,13).
The expression of odontogenic differentiation-specific markers,
such as DSPP and DMP1 genes, was also enhanced among the DPSCs/VEGF
in this study. DSPP is a dentine non-collagenous protein, mainly
expressed by odontoblasts in the mineralized nodules and organized
structures (14). DMP1 plays an
important role in the maturation of ameloblasts, osteoblasts and
odontoblasts, as well as in the mineralization of these cells
(26). The increased expression of
odontogenic differentiation genes identified in this study is
consistent with an improvement in the odontogenic differentiation
capacity of the DPSCs due to the overexpression of VEGF.
DSP, the amino-terminal part of DSPP, is a specific
protein involved in the odontogenic differentiation of DPSCs, and
it is always expressed by newly-formed odontoblasts associated with
the secretion of pre-dentin matrix (27,28).
The expression of DSP occurs prior to the initiation of the dentin
mineralization, not yet present in pre-odontoblasts (27). An in vivo study has
additionally shown that DSP is involved in the initiation of dentin
mineralization, but not in the maturation of dentin (29). DSP appeared not only in the
odontoblasts of the primary dentin but also in the odontoblast-like
cells of reparative dentin. The function of DSP could be associated
with the synthesis of the dentin matrix and the conversion of
pre-dentin to mineralized dentin (28). In the present study, western blot
analysis indicated that DSP expression in the DPSCs/VEGF was
significantly higher than that in the DPSCs/Vector at the four
selected time-points. Furthermore, DMP1 expression in the
DPSCs/VEGF was also significantly higher than that in the
DPSCs/Vector at three time-points. These results were consistent
with the qPCR data, and therefore further confirmed that VEGF gene
transfection improved the odontogenic differentiation of DPSCs.
In conclusion, DPSCs/VEGF were successfully
constructed in the present study by lentivirus-mediated VEGF gene
transfection of human DPSCs. No significant difference was observed
between the DPSCs/VEGF and the DPSCs/Vector with regard to the
proliferation characteristics of the cells; however, the DPSCs/VEGF
showed a significantly enhanced expression of odontogenic
differentiation genes and proteins. The results demonstrate that
VEGF gene transfection may be a strategy to improve the efficacy of
DPSCs for odontogenic differentiation. Further evaluation on the
effectiveness of the VEGF gene in promoting the odontogenic
differentiation of DPSCs in vivo is now required.
Acknowledgements
The authors would like to thank Dr Chenfei Zhang
(Clinical Associate Professor in Endodontics, The University of
Hong Kong) for his help in the initial phases of this study and the
staff and postgraduate students of the Guanghua School of
Stomatology, Sun Yat-sen University, who helped in the collection
of clinical samples and the laboratory analysis. This study was
supported by the Guangdong Medical Science Research Fund (B2012142)
and the National Natural Science Foundation of China
(81170932).
References
|
1
|
Gronthos S, Mankani M, Brahim J, Robey PG
and Shi S: Postnatal human dental pulp stem cells (DPSCs) in vitro
and in vivo. Proc Natl Acad Sci USA. 97:13625–13630. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kawashima N: Characterisation of dental
pulp stem cells: a new horizon for tissue regeneration? Arch Oral
Biol. 57:1439–1458. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rodríguez-Lozano FJ, Bueno C, Insausti CL,
et al: Mesenchymal stem cells derived from dental tissues. Int
Endod J. 44:800–806. 2011.PubMed/NCBI
|
|
4
|
Huang GT, Gronthos S and Shi S:
Mesenchymal stem cells derived from dental tissues vs. those from
other sources: their biology and role in regenerative medicine. J
Dent Res. 88:792–806. 2009.PubMed/NCBI
|
|
5
|
Yang X, van der Kraan PM, van den Dolder
J, et al: STRO-1 selected rat dental pulp stem cells transfected
with adenoviral-mediated human bone morphogenetic protein 2 gene
show enhanced odontogenic differentiation. Tissue Eng.
13:2803–2812. 2007. View Article : Google Scholar
|
|
6
|
Matsushita K, Motani R, Sakuta T, et al:
The role of vascular endothelial growth factor in human dental pulp
cells: induction of chemotaxis, proliferation, and differentiation
and activation of the AP-1-dependent signaling pathway. J Dent Res.
79:1596–1603. 2000. View Article : Google Scholar
|
|
7
|
Tan YY, Yang YQ, Chai L, Wong RW and Rabie
AB: Effects of vascular endothelial growth factor (VEGF) on
MC3T3-E1. Orthod Craniofac Res. 13:223–228. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee JH, Um S, Jang JH and Seo BM: Effects
of VEGF and FGF-2 on proliferation and differentiation of human
periodontal ligament stem cells. Cell Tissue Res. 348:475–484.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
D’Alimonte I, Nargi E, Mastrangelo F, et
al: Vascular endothelial growth factor enhances in vitro
proliferation and osteogenic differentiation of human dental pulp
stem cells. J Biol Regul Homeost Agents. 25:57–69. 2011.
|
|
10
|
Wozney JM and Rosen V: Bone morphogenetic
protein and bone morphogenetic protein gene family in bone
formation and repair. Clin Orthop Relat Res. 26–37. 1998.PubMed/NCBI
|
|
11
|
Samee M, Kasugai S, Kondo H, Ohya K,
Shimokawa H and Kuroda S: Bone morphogenetic protein-2 (BMP-2) and
vascular endothelial growth factor (VEGF) transfection to human
periosteal cells enhances osteoblast differentiation and bone
formation. J Pharmacol Sci. 108:18–31. 2008. View Article : Google Scholar
|
|
12
|
Liu B, Li X, Liang G and Liu X: VEGF
expression in mesenchymal stem cells promotes bone formation of
tissue-engineered bones. Mol Med Rep. 4:1121–1126. 2011.PubMed/NCBI
|
|
13
|
Jiang J, Fan CY and Zeng BF: Osteogenic
differentiation effects on rat bone marrow-derived mesenchymal
stromal cells by lentivirus-mediated co-transfection of human BMP2
gene and VEGF165 gene. Biotechnol Lett. 30:197–203. 2008.
View Article : Google Scholar
|
|
14
|
Bakopoulou A, Leyhausen G, Volk J, et al:
Comparative analysis of in vitro osteo/odontogenic differentiation
potential of human dental pulp stem cells (DPSCs) and stem cells
from the apical papilla (SCAP). Arch Oral Biol. 56:709–721. 2011.
View Article : Google Scholar
|
|
15
|
Lahmy R, Soleimani M, Sanati MH, Behmanesh
M, Kouhkan F and Mobarra N: Pancreatic islet differentiation of
human embryonic stem cells by microRNA overexpression. J Tissue Eng
Regen Med. Jul 30–2013.(Epud ahead of print).
|
|
16
|
Hwang SY, Foley J, Numaga-Tomita T,
Petranka JG, Bird GS and Putney JW Jr: Deletion of Orai1 alters
expression of multiple genes during osteoclast and osteoblast
maturation. Cell Calcium. 52:488–500. 2012. View Article : Google Scholar
|
|
17
|
Pereira LO, Rubini MR, Silva JR, et al:
Comparison of stem cell properties of cells isolated from normal
and inflamed dental pulps. Int Endod J. 45:1080–1090. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shi S and Gronthos S: Perivascular niche
of postnatal mesenchymal stem cells in human bone marrow and dental
pulp. J Bone Miner Res. 18:696–704. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jiang L, Peng WW, Li LF, Yang Y and Zhu
YQ: Isolation and identification of CXCR4-positive cells from human
dental pulp cells. J Endod. 38:791–795. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee UL, Jeon SH, Park JY and Choung PH:
Effect of platelet-rich plasma on dental stem cells derived from
human impacted third molars. Regen Med. 6:67–79. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Trubiani O, Tripodi D, Delle Fratte T,
Caputi S and Di Primio R: Human dental pulp vasculogenesis
evaluated by CD34 antigen expression and morphological arrangement.
J Dent Res. 82:742–747. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hata R and Senoo H: L-ascorbic acid
2-phosphate stimulates collagen accumulation, cell proliferation,
and formation of a three-dimensional tissuelike substance by skin
fibroblasts. J Cell Physiol. 138:8–16. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hitomi K, Torii Y and Tsukagoshi N:
Increase in the activity of alkaline phosphatase by L-ascorbic acid
2-phosphate in a human osteoblast cell line, HuO-3N1. J Nutr Sci
Vitaminol (Tokyo). 38:535–544. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Torii Y, Hitomi K and Tsukagoshi N:
L-ascorbic acid 2-phosphate promotes osteoblastic differentiation
of MC3T3-E1 mediated by accumulation of type I collagen. J Nutr Sci
Vitaminol (Tokyo). 40:229–238. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shima N, Kimoto M, Yamaguchi M and
Yamagami S: Increased proliferation and replicative lifespan of
isolated human corneal endothelial cells with L-ascorbic acid
2-phosphate. Invest Ophthalmol Vis Sci. 52:8711–8717. 2011.
View Article : Google Scholar
|
|
26
|
MacDougall M, Gu TT and Simmons D: Dentin
matrix protein-1, a candidate gene for dentinogenesis imperfecta.
Connect Tissue Res. 35:267–272. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ritchie HH, Berry JE, Somerman MJ, et al:
Dentin sialoprotein (DSP) transcripts: developmentally-sustained
expression in odontoblasts and transient expression in
pre-ameloblasts. Eur J Oral Sci. 105:405–413. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee SY, Kim SY, Park SH, Kim JJ, Jang JH
and Kim EC: Effects of recombinant dentin sialoprotein in dental
pulp cells. J Dent Res. 91:407–412. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Suzuki S, Sreenath T, Haruyama N, et al:
Dentin sialoprotein and dentin phosphoprotein have distinct roles
in dentin mineralization. Matrix Biol. 28:221–229. 2009. View Article : Google Scholar : PubMed/NCBI
|