Introduction
Scinderin (or adseverin), a member of the gelsolin
superfamily, is a Ca2+-dependent filamentous actin
(F-actin) severing and capping protein with three actin-, two
PIP2- and two Ca2+-binding sites (1–4).
Scinderin is expressed largely in endocrine tissues and secretory
cells. During human development, scinderin expression is different
from that in adults: It is highly expressed in the human fetal
kidney, very weakly in the brain and intestine but nowhere else; in
human adult tissues, scinderin exhibits strong expression in the
kidney and low expression in the heart; however, no expression is
observed in the adrenal gland (5).
It has been demonstrated that scinderin has
important roles in exocytosis, megakaryopoiesis, autoimmune
disorders and tumorigenesis. Scinderin is known to regulate
translocation of secretory vesicles by controlling F-actin dynamics
(disassembly ↔ assembly) during secretion (6–8).
Overexpression of scinderin on human airway epithelial cells may
inhibit mucin secretion (9).
Scinderin has also been found in platelets. Previous studies have
suggested that the expression of scinderin in the megakaryoblastic
cell line MEG-01 induces cell differentiation, polyploidization,
maturation and apoptosis with release of plateletlike particles,
while cell proliferation and tumorigenesis in nude mice are
inhibited (10). It has been
reported that single-nucleotide polymorphisms (SNPs) in
scinderin have a prominent effect in multiple sclerosis
(11). Overexpression of scinderin
triggers human non-small-cell lung carcinoma cell line IGR-Heu
resistance to cytolytic T lymphocytes (12). Furthermore, scinderin is capable of
preventing mitochondria-mediated apoptosis by directly binding to
voltage-dependent anion channels in ciplatin-resistant cells
(13).
Gastric cancer is one of the most common primary
gastrointestinal cancers in the world. Despite improvements in
diagnostic modalities, outcomes for patients with gastric cancer
remain extremely poor, with the majority of cancer patients dying
from the occurrence of metastases rather than their primary tumors.
Due to its complexity, the process of tumor metastasis remains
poorly understood. Epithelial-mesenchymal transition (EMT) is a
transdifferentiation process during which epithelial cells lose
their epithelial polarity and cell-cell contacts, gain mesenchymal
properties, and become motile and invasive (14,15).
In this process, fundamental changes in gene expression are
involved in the disruption of epithelial polarity (e.g.,
E-cadherin and cytokeratins) and establishment of a mesenchymal
phenotype (e.g. N-cadherin and vimentin) (16). EMT is physiologically relevant
during embryogenesis and in adult tissue for wound healing.
However, EMT is also associated with tumor metastasis. Of note, the
majority of solid carcinoma cells (including breast, prostate,
pancreatic, colon and gastric cancer, etc.) have been reported to
undergo either partial or complete EMT so as to become motile and
invasive. Currently, EMT is regarded as an important mechanism of
tumor metastasis, having crucial roles in the early process of
therioma metastases (15).
Therefore, it is essential to find molecules involved in both the
positive and negative regulation of EMT. It has been demonstrated
that signaling pathways, including TGF-β, Wnt/β-catenin, Notch,
Hedgehog, interleukin-6/signal transducer and activator of
transcription 3 and nuclear factor-κB trigger EMT by inducing
Snail1, Snail2, Twist1, Twist2, zinc finger E-box binding homeobox
(ZEB)1 and ZEB2 expression (17–19).
Furthermore, non-coding RNAs (including microRNAs and long
non-coding RNAs) also have critical roles in the regulation of the
EMT (20–22).
Although it has been demonstrated that scinderin
expression is low or absent in the human adult stomach, no studies,
to the best of our knowledge, have reported the biological effect
of scinderin on human gastric cancer. In the present study, the
effects of scinderin knockdown on cell cycle, proliferation and
migration of the highly tumorigenic and metastatic human gastric
cancer cell line SGC-7901 (scinderin is highly expressed) were
investigated. Furthermore, the changes in E-cadherin, N-cadherin
and β-catenin expression following scinderin knockdown were
examined and the role of scinderin in the tumor EMT process was
analyzed.
Materials and methods
SGC-7901 cell line
The human gastric cancer cell line SGC-7901 was
purchased from Chinese Academy of Sciences (Shanghai, China). The
cells were cultured in RPMI-1640 medium (Invitrogen Life
Technologies, Carlsbad, CA, USA) with 10% fetal bovine serum (FBS)
in a humidified incubator containing 5% CO2 at 37°C.
Construction of scinderin-small hairpin
(sh)RNA lentiviral plasmid
The target shRNA against the scinderin gene
(Gene Bank accession NM_033128) was designed as follows: 5′-CGA GAT
GAG CTG ACA ACA T-3′. Oligonucleotides encoding shRNA sequences
were synthesized (Genechem Corporation, Shanghai, China) and
annealed into double strands. Double-stranded DNAs were inserted
into HpaI/XhoI restriction sites of lentiviral frame
plasmid (Genechem Corporation), encoding green fluorescent protein
(GFP). They were then transfected into Escherichia coli and
positive recombinant lentiviral plasmids were selected by
polymerase chain reaction (PCR), using the primers 5′-GCC CCG GTT
AAT TTG CAT AT-3′ and 5′-GAG GCC AGA TCT TGG GTG-3′. The conditions
for PCR were denaturation at 94°C for 30 sec, then 94°C for 30 sec,
55°C for 30 sec and 72°C for 30 sec, for 30 cycles and extension at
72°C for 6 min. The products were then verified by electrophoretic
analysis on a 1.5% agarose gel containing ethidium bromide and DNA
sequencing. The lentiviral vectors expressing GFP alone were used
as the control.
Packaging and titration of lentiviral
vectors
The recombinant lentiviral plasmid was
co-transfected into 293T cells with packaging plasmids (pHelper 1.0
including gag/pol and pHelper 2.0 including VSVG; Genechem
Corporation) by Lipofectamine 2000 (Invitrogen Life Technologies)
to produce target lentivirus. Following 48 h, the virus in the
supernatant was collected and the virus titer was measured
following the dilution method: i) a total of 4×104
cells/well of the 293T cells were seeded in a 96-well plate in
Dulbecco’s modified Eagle’s medium (Gibco Life Technologies,
Carlsbad, CA, USA) supplemented with 10% FBS and cultured
overnight; ii) the serial diluted vectors were added to the
cultured cells and continued to culture for 48 h; iii) the
GFP-positive cells were counted. The titer was represented as
transduction unit (TU) per milliliter concentrated vector (TU/ml):
Titer = GFP-positive cell number/dilution × 103.
Establishment of the stable
scinderin-silenced cell line SGC-7901
A preliminary experiment identified that the best
multiplicity of infection (MOI) of SGC-7901 cells was 10 (MOIs of
1, 10 and 20 were tested). Under these best transfection
conditions, scinderin-shRNA lentivirus was transfected into
the SGC-7901 cells. The efficiency of transfection was observed
under a fluorescence microscope (MicroPublisher 3.3 RTV; Olympus,
Tokyo, Japan). The effects of gene silencing were confirmed by
quantitative (q)PCR and western blot analyses. The cells were
divided into three groups: The CON group (uninfected), the NC group
(transfected with negative control lentivirus) and the KD group
(transfected with scinderin-shRNA lentivirus).
Cell migration assay
Cell migration was assessed using a Transwell assay
(Corning Incorporated Life Sciences, Lowell, MA, USA). Transwell
insert chambers were firstly rehydrated with 100 μl serum-free
medium at 37°C for 1 h. Following removing the medium from the
chambers, 1×105 cells in 100 μl serum-free medium were
added to the upper chamber, with 600 μl medium containing 30% FBS
in the lower chamber, and then cultured for 24 h. Subsequently, the
cells were removed from the top side of the membrane using a
cotton-tipped swab, and the invaded cells attached to the bottom of
the membrane were fixed with 10% glutaraldehyde, stained with 0.1%
crystal violet (Shanghai Genebase Gene-Tech Co., Ltd, Shanghai,
China) and photographed under an inverted microscope
(MicroPublisher 3.3 RTV; Olympus). Next, the cells were destained
with 10% acetic acid, and the optical density (OD) of the solution
was measured at 570 nm. The OD value was directly proportional to
the cell quantity and indirectly represented the cellular migration
ability.
RT-qPCR
Total RNA was extracted from cells using TRIzol
(Invitrogen Life Technologies), according to manufacturer’s
instructions. Quantitative RNA expression was measured by the
SmartSpecTM Plus Spectrophotometer (Bio-Rad, Hercules,
CA, USA). Reverse transcription in a 20 μl system was performed
using the GoTaq® 2-step RT-qPCR System kit (Promega
Corporation, Madison, WI, USA) following the manufacturer’s
instructions. qPCR was performed using the MX3005P qPCR System
(Stratagene, La Jolla, CA, USA). Primers for qPCR were
scinderin, forward 5′-TCT GCG TTC CTG ACT GTT C-3′ and
reverse 5′-GAC CTC CTT TCT TTG ATG TTC C-3′; GAPDH, forward
5′-TGA CTT CAA CAG CGA CAC CCA-3′ and reverse 5′-CAC CCT GTT GCT
GTA GCC AAA-3′; E-cadherin, forward 5′-CCA TCG CTT ACA CCA
TCC T-3′ and reverse 5′-GCT GTT GCT GTT GTG CTT-3′;
N-cadherin, forward 5′-CTC CTA TGA GTG GAA CAG GAA CG-3′ and
reverse 5′-TTG GAT CAA TGT CAT AAT CAA GTG CTG TA-3′;
β-actin, forward 5′-TCG TGC GTG ACA TTA AGG-3′ and reverse
5′-AAG GAA GGC TGG AAG AGT-3′. The relative mRNA expression was
calculated with the 2−ΔΔCT method. GAPDH or
β-actin were used as normalizers for each sample. All of the
experiments were repeated in biological duplicate.
Western blot analysis
The cells were lysed with radioimmunoprecipitation
assay (RIPA) lysis buffer (Bioteke Corporation, Beijing, China),
and the protein concentration was measured by bicinchoninic acid
assay (Beyotime Corporation, Nantong, China). The total cell
proteins were separated by 10% SDS-PAGE and transferred to
nitrocellulose filter (NC) membranes (Millipore Corporation,
Bedford, MA, USA). The NC membranes were firstly blocked by 5%
skimmed milk for 1 h and then incubated overnight at 4°C with
primary antibodies, including scinderin rabbit mAb (1:1,000, Santa
Cruz Biotechnology, Dallas, TX, USA), GAPDH mouse mAb (1:1,000,
Santa Cruz Biotechnology), E-cadherin rabbit mAb (1:1,000, Cell
Signaling Technology, Danvers, MA, USA), N-cadherin rabbit pAb
(1:1,000, Cell Signaling Technology), β-catenin rabbit mAb
(1:1,000, Cell Signaling Technology) and β-actin rabbit mAb
(1:1,000, Cell Signaling Technology). Thereafter, blots were
stained with fluorescent secondary antibodies IRDye 800CW Goat
anti-Rabbit IgG (H+L) (1:10,000, LI-COR Bioscience, Lincoln, NE,
USA) or IRDye 800CW Goat anti-Mouse IgG (H+L) (1:10,000, LI-COR
Bioscience) and protein bands were visualized using an Odyssey
Imaging system (LI-COR Bioscience). GAPDH or β-actin was used as
the internal control.
Real-time cell proliferation assay
(RTCA)
The real-time cell proliferation experiment was
performed with a RTCA single plate (SP) instrument (Roche Applied
Science, Mannheim, Germany), which was placed in a 37°C incubator
with 5% CO2. Based on the manufacturer’s instructions,
the background signal of the culture medium was first measured by
adding 100 μl medium to 96-well plates (E-plate 96, Roche Applied
Science) containing gold microelectrodes on its bottom. Next, the
cells were seeded in the special 96-well plates at density of 8,000
cells/well. Following 30 min of incubation at room temperature, the
cells were placed in the RTCA SP and monitored every 15 min for 160
h. Data analysis was performed using RTCA software 1.2 supplied
with the instrument. The RTCA software comprised the xCELLigence
system, which converts impedance values into cell index (CI) values
corresponding to each well. The CI value is directly proportional
to the quantity of cells and was used as an indirect measure for
cellular proliferation capability.
Cell cycle analysis
SGC-7901 cells stably transfected with
scinderin-shRNA or empty lentiviral vector were firstly
cultured in serum-free medium for 24 h for synchronization in G0/G1
phase, and then cultured in RPMI-1640 medium with 10% FBS for
another 24 h. The collected cells were washed twice with
phosphate-buffered saline (PBS) and fixed in ice-cold 70% ethanol
overnight at −20°C. Next, the cell pellets were washed twice with
ice-cold PBS and stained with propidium iodide solution (BD
Biosciences, San Jose, CA, USA) for 20 min in the dark. Finally,
the cells were analyzed using the BD FACSCalibur Flow Cytometer (BD
Biosciences) for their DNA content. The percentages of cells in the
different phases of the cell cycle were determined with Cell Quest
Pro software 3.1 (BD Biosciences).
Statistical analysis
Data are presented as the mean ± standard deviation.
The Student’s t-test was used in quantitative data analysis,
P<0.05 was considered to indicate a statistically significant
difference. All statistical analysis was performed with SPSS 20.0
software (SPSS, Inc., Chicago, IL, USA).
Results
Construction of scinderin-shRNA
lentiviral plasmid
The double-stranded DNAs encoding shRNA sequences
were inserted into HpaI/XhoI restriction sites of the
lentiviral frame plasmids. The electrophoresed PCR products of the
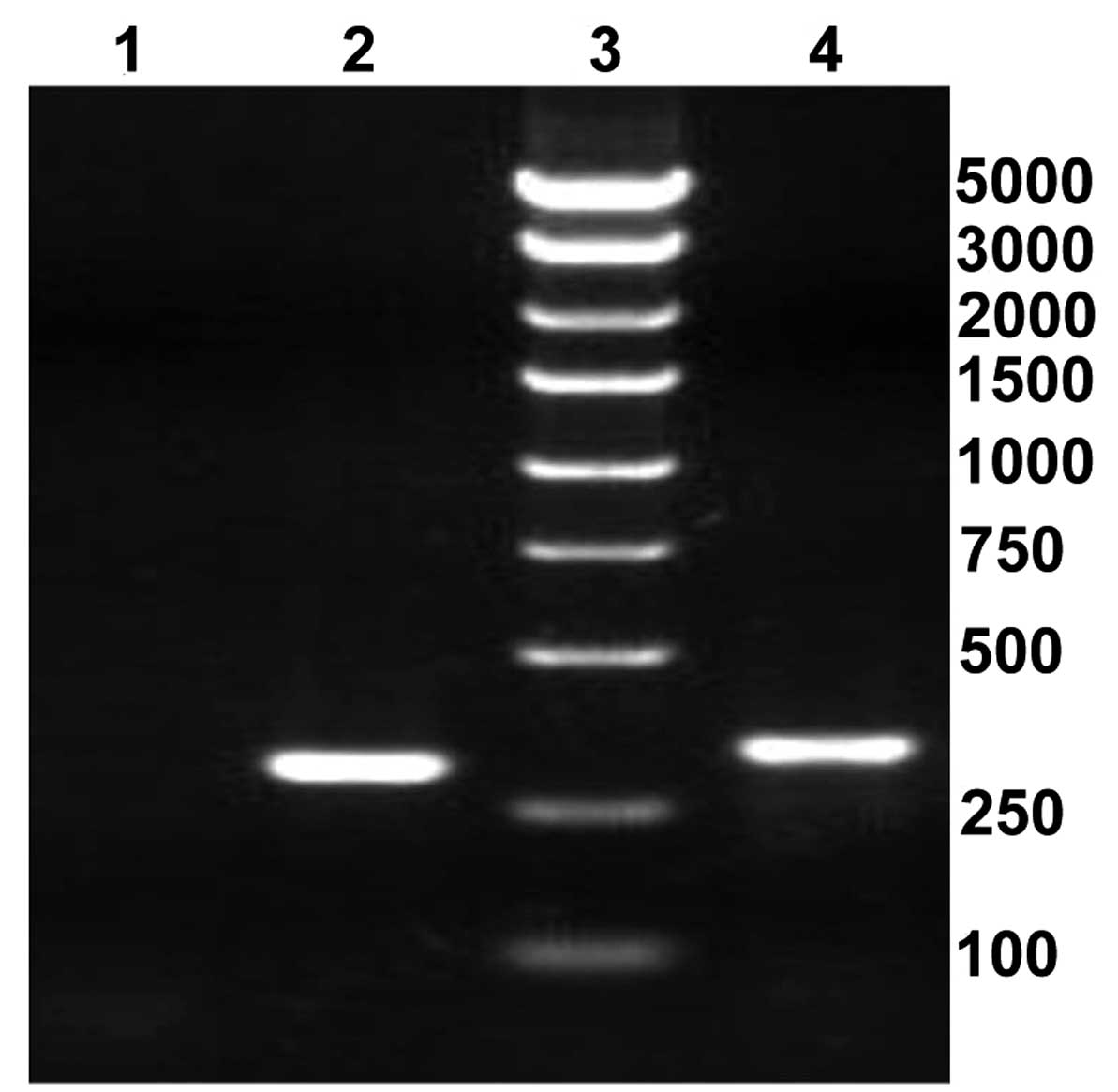
recombinant lentiviral plasmids were as follows: The length of
positive clones containing shRNA was 343 bp and the length of blank
clones was 299 bp (Fig. 1).
Furthermore, DNA sequencing demonstrated that the RNA interference
sequence targeting scinderin was coincident with the
anticipative results (data not shown). This suggested that the
shRNA sequence targeting scinderin was successfully inserted
into the lentiviral plasmid.
Packaging and titration of
lentivirus
Recombinant lentivirus were produced in 293T cells
co-transfected with the recombinant lentiviral plasmid and
packaging plasmids. The viral titer indicated that the recombinant
lentivirus was packaged successfully and the viral titer was
≤6~8×108 TU/ml (Fig.
2).
Transfection efficiency of lentivirus in
gastric cancer cell line SGC-7901
The preliminary experiment found that the optimal
MOI of SGC-7901 cells was 10. Following transfection under these
optimal conditions for 96 h, the transfection efficiency was
determined to be >90% by counting GFP-positive cells under the
fluorescent microscope. The result demonstrated that
scinderin-shRNA lentivirus was transfected into SGC-7901
cells successfully.
Interference efficiency of scinderin in
SGC-7901 cells
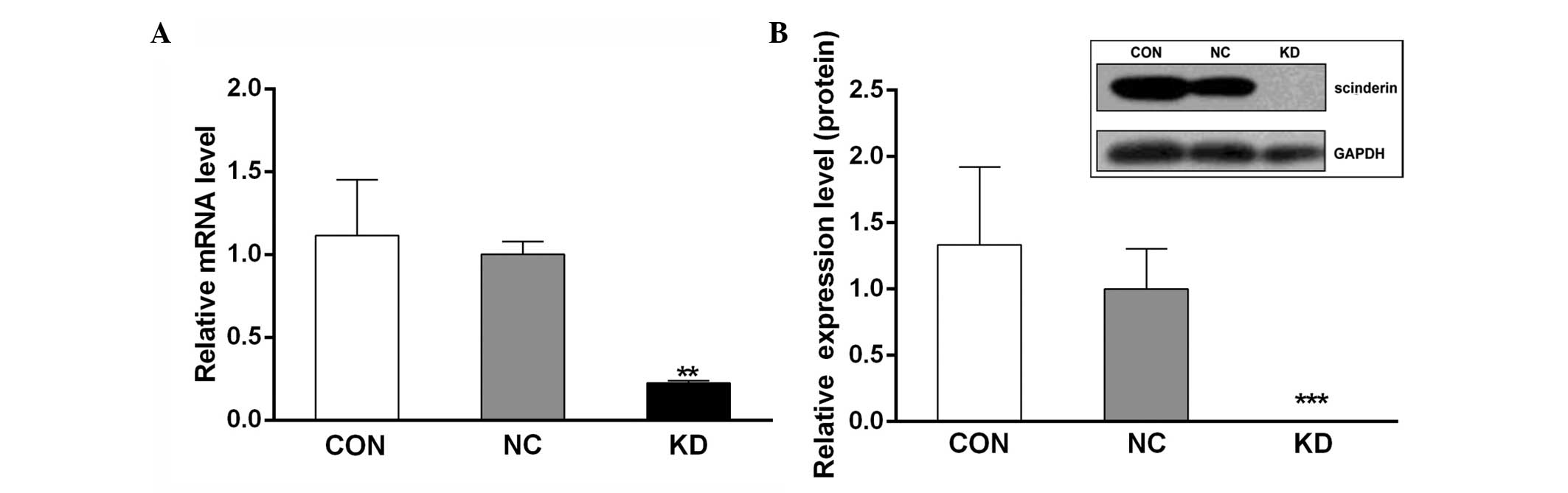
The silencing of scinderin was confirmed by
qPCR and western blot analyses. The results demonstrated that
following transfection into the SGC-7901 cells, both the mRNA and
protein levels of scinderin were significantly decreased in the KD
group compared with the CON and NC groups (Fig. 3). This suggested that stable
scinderin-silenced cancer cells were established and
appropriate for use in subsequent experiments.
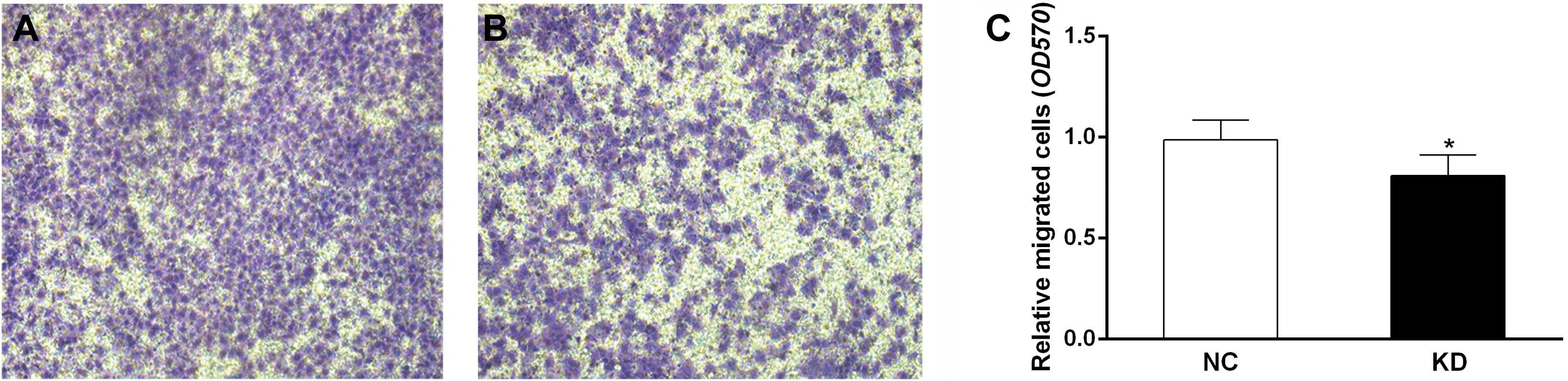
Scinderin knockdown suppresses migration
of SGC-7901 cells
The migration assay demonstrated that the number of
cells penetrating the membrane of chambers was significantly lower
in the KD group than that in the NC group (P<0.05, Fig. 4). This result indicated that
scinderin knockdown may attenuate migration of SGC-7901 cells.
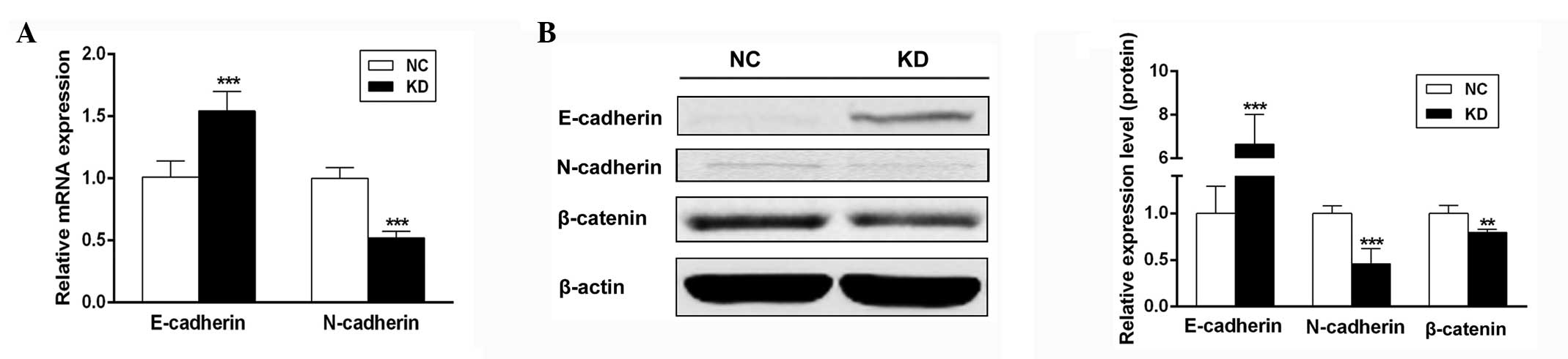
Scinderin mediates EMT marker expression
in SGC-7901 cells
Considering scinderin knockdown inhibits the
migration of the metastatic human gastric cancer cell line
SGC-7901, it was investigated whether scinderin mediates EMT, a
critical event in tumor metastases. qPCR and western blot analyses
revealed that concomitant with an evident decrease in N-cadherin
expression, E-cadherin expression in the KD group was significantly
upregulated compared with that in the NC group (Fig. 5). Furthermore, it was observed that
scinderin knockdown decreased the expression of β-catenin protein
(Fig. 5B), an important regulatory
molecule in EMT. Therefore, these results illustrated that
scinderin knockdown may inhibit the EMT process in SGC-7901
cells.
Scinderin knockdown suppresses
proliferation and arrests cell cycle of SGC-7901 cells
Furthermore, the effect of scinderin knockdown on
cell proliferation was also investigated. The results of RTCA
proliferation (Fig. 6A)
demonstrated that there were no significant differences in the CI
values between the NC and KD groups at the initial stage
(P>0.05). Following 24 h, however, the proliferation of SGC-7901
cells in the KD group was significantly lower than that in the NC
group (P<0.01 or P<0.001) and entered the stationary stage in
advance. These data clearly indicated that scinderin knockdown
effectively suppressed the proliferation of SGC-7901 gastric cancer
cells.
The cell cycle distribution based on DNA content was
examined by flow cytometric analysis. It was identified that there
was a 1.03% increase in the number cells in G2/M-phase in the KD
group compared with the NC group (P<0.01, Fig. 6B). These results suggested that
scinderin knockdown in SGC-7901 cells may arrest the cell cycle at
G2/M phase.
Discussion
A study by Haifu Wu demonstrated that
scinderin is the most differentially expressed gene in colon
cancer patients with and without liver metastasis by gene-chip
technology and qPCR assay (23).
The study also identified, consistent with the results of the
present study, that scinderin knockdown in colorectal cancer cell
lines SW480 and DLD-1 evidently inhibited cell proliferation and
migration. These results suggested that scinderin has important
roles in the development and progression of cancer.
The present analyses of scinderin knockdown in
highly metastatic SGC-7901 cells suggested that scinderin is
associated with cell migration. It is well-established that the
actin cytoskeleton contributes to maintaining the distinctive
structures and functions of epithelial cells by mediating
interactions with the cellular basement membranes and cell-cell
contacts (24). Aberrance of the
actin cytoskeleton is considered as one fundamental characteristic
of the majority of malignant and metastatic cells. Studies
performed in epithelial cells have demonstrated that gelsolin and
villin, two proteins associated with the scinderin family, regulate
cell migration by altering actin filament dynamics (25,26).
Scinderin is an important actin-capping, -severing and -nucleating
protein. Since it can depolymerize actin filaments and bind actin
monomers, it is hypothesized that the loss of scinderin in
epithelial cells may increase the cellular levels of F-actin, thus
modifying actin filament dynamics. Another role of scinderin may
serve to regulate the proper distribution of F-actin filaments in
cells.
Furthermore, scinderin may also regulate the EMT
process to affect cell migration. The present study indicated that
scinderin knockdown in SGC-7901 cells resulted in a high increase
of E-cadherin and reduced the expression of N-cadherin. E-cadherin
as an important Ca2+-dependent adhesion molecule has key
roles in the cell adhesion of solid tissues. It is anchored to the
actin cytoskeleton via α-, γ- and β-catenin, providing the
physical structure for both cell-cell attachment and the
recruitment of signaling complexes (27). Loss or inactivation of E-cadherin
is generally regarded as the main trigger for the disruption of
tight epithelial cell-cell contacts, causing migratory or invasive
states of malignant cells. In several carcinoma types, a deficiency
of E-cadherin expression is most commonly accompanied by the gain
of N-cadherin, which has been established as the cadherin switch in
the EMT process. Although the functional implication of this
cadherin switch for tumor progression has remained unknown, the
overexpression of N-cadherin may be equally necessary and
sufficient to overcome E-cadherin-mediated cell-cell adhesion and
to promote therioma development. Of note, gelsolin has also been
found to regulate EMT in human mammary epithelial cells. However,
this is inconsistent with the present results that gelsolin
knockdown by siRNA in MCF10A cells induces EMT, controlling
E-cadherin and N-cadherin conversion via Snail (28).
Notably, the present study identified that the loss
of scinderin reduced the expression of β-catenin protein, a key
nuclear effector of canonical Wnt signaling in the nucleus. The
Wnt/β-catenin pathway is one of the fundamental signaling pathways
controlling EMT, which may facilitate the expression of key
transcriptional repressors that target E-cadherin, including
Snail2, ZEB1 and Twist (29–31).
Based on present knowledge, it is suggested that overexpression of
scinderin may enhance free cytoplasmic levels of β-catenin by
altering the actin cytoskeleton or other mechanisms. β-catenin,
escaping cytoplasmic degradation, translocates into the cell
nucleus and triggers transcription of Wnt-specific genes (32). As a result, the expression of
E-cadherin, a key hallmark of EMT, is highly aberrant in epithelial
cells. Cadherins appear to directly affect the function of each
other. N-cadherin, one of the mesenchymal cadherins, is highly
expressed at the same time and facilitates the acquisition of a
migratory phenotype. Whether this conjecture is valid remains to be
investigated in the future. The physiological function of scinderin
is remains limited to actin filaments. Thus far, it has been
confirmed that changes of megakaryoblastic cells brought about by
scinderin expression are mediated through the activation of rho
family of guanosine triphosphatase/p21 activated
kinase/mitogen-activated protein kinase kinase kinase.
Mitogen-activated protein kinase kinase 4/c-Jun N-terminal
kinase/c-jun, c-fos and Raf/mitogen-activated protein
kinase kinase/extracellular signal-regulated kinase pathways, the
only molecular mechanisms reported that scinderin participates in
(10). However, the present study
indicated that scinderin may act as a potential positive regulator
in the EMT process of gastric cancer cells.
In addition, the present study identified that the
cell cycle was arrested in G2/M phase (based on DNA content), which
may lead to impairment of proliferation of SGC-7901 cells with low
scinderin expression. However, a recent study showed that
scinderin silencing reduced the proliferation and colony
formation of prostate cancer cell line PC3 by arresting the cell
cycle at the G0/G1 phase (33).
The mRNA level of cell cycle-related molecules was also assessed by
RT-qPCR analysis, indicating that scinderin silencing
induces upregulation of p21waf1/cip1 and p16 and downregulation of
cyclin A2 in PC3 cells. In view of the selectivity of gene
expression in different tissues, these two studies perhaps do not
appear to be consistent: SGC-7901 cells are derived from lymph node
metastasis of gastric cancer, and PC3 cells are from bone
metastasis in prostate cancer, both of which are in different
microenvironment for tumor growth and survival. Flow cytometry is
unnable to distinguish between G2 and M phase of the cell cycle,
which may explain why the present study demonstrated that scinderin
knockdown significantly suppressed the proliferation of SGC-7901
cells but the changes in cell-cycle distribution were not evident.
As is well-established, microfilaments have a number of important
roles in the telophase of cell division. The function of scinderin
in regulating actin dynamics has already been clearly demonstrated.
It was therefore suggested that scinderin may effect cell
proliferation by regulating F-actin; however, further studies are
required to confirm this hypothesis.
The metastases of carcinomas are formed following a
complex succession of cell-biological events including local
invasion and metastatic colonization (34). As mentioned previously, scinderin
expression is low or absent in the human adult stomach. The results
of the present study suggested that: i) scinderin knockdown is able
to reverse EMT process and effectively prevent migration of highly
metastatic SGC-7901 cells, which also can be understood to weaken
the local invasion in primary gastric cancer; ii) scinderin
knockdown is able to inhibit the proliferation of SGC-7901 cells,
greatly impairing the proliferation of cancer cells at metastatic
sites and the formation of metastatic colonization. If human
gastric cancer tissues provide similar results to those of SGC-7901
cells highly expressing scinderin, which is closely associated with
carcinoma metastasis, scinderin may be a useful prognostic
biomarker to distinguish the progression of gastric cancer and
guide personalized therapy for patients.
In conclusion, the present study demonstrated that
suppression of scinderin impaired the proliferation and migration
of gastric cancer SGC-7901 cells and attenuated their EMT process.
Therefore, scinderin may be a potential target for tumor EMT and
therapy against gastric cancer.
Acknowledgements
The present study was supported by the Natural
Science Foundation of Zhejiang Province (grant no. Y2110961) and
Natural Science Foundation of Ningbo (grant no. 2011A610051).
Abbreviations:
|
F-actin
|
filamentous actin
|
|
EMT
|
epithelial-mesenchymal transition
|
|
RTCA
|
real-time cell analyzer
|
|
RT-qPCR
|
real-time quantitative polymerase
chain reaction
|
References
|
1
|
Marcu MG, Zhang L, Elzagallaai A and
Trifaró JM: Localization by segmental deletion analysis and
functional characterization of a third actin-binding site in domain
5 of scinderin. J Biol Chem. 273:3661–3668. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lejen T, Pene TD, Rosé SD and Trifaró JM:
The role of different Scinderin domains in the control of F-actin
cytoskeleton during exocytosis. Ann NY Acad Sci. 971:248–250. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dumitrescu Pene T, Rosé SD, Lejen T, Marcu
MG and Trifaró JM: Expression of various scinderin domains in
chromaffin cells indicates that this protein acts as a molecular
switch in the control of actin filament dynamics and exocytosis. J
Neurochem. 92:780–789. 2005.
|
|
4
|
Chumnarnsilpa S, Lee ML, Nag S, et al: The
crystal structure of the C-terminus of adseverin reveals the
actin-binding interface. Proc Natl Acad Sci USA. 106:13719–13724.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lueck A, Brown D and Kwiatkowski DJ: The
actin-binding proteins adseverin and gelsolin are both highly
expressed but differentially localized in kidney and intestine. J
Cell Sci. 111:3633–3643. 1998.PubMed/NCBI
|
|
6
|
Trifaró JM, Vitale ML and Rodríguez Del
Castillo A: Scinderin and chromaffin cell actin network dynamics
during neurotransmitter release. J Physiol Paris. 87:89–106.
1993.PubMed/NCBI
|
|
7
|
Trifaró JM, Rosé SD and Marcu MG:
Scinderin, a Ca2+-dependent actin filament severing
protein that controls cortical actin network dynamics during
secretion. Neurochem Res. 25:133–144. 2000.
|
|
8
|
Trifaró JM, Gasman S and Gutiérrez LM:
Cytoskeletal control of vesicle transport and exocytosis in
chromaffin cells. Acta Physiol (Oxf). 192:165–172. 2008.PubMed/NCBI
|
|
9
|
Ehre C, Rossi AH, Abdullah LH, et al:
Barrier role of actin filaments in regulated mucin secretion from
airway goblet cells. Am J Physiol Cell Physiol. 288:C46–C56.
2005.PubMed/NCBI
|
|
10
|
Zunino R, Li Q, Rosé SD, et al: Expression
of scinderin in megakaryoblastic leukemia cells induces
differentiation, maturation, and apoptosis with release of
plateletlike particles and inhibits proliferation and
tumorigenesis. Blood. 98:2210–2219. 2001. View Article : Google Scholar
|
|
11
|
Bush WS, McCauley JL, DeJager PL, et al: A
knowledge-driven interaction analysis reveals potential
neurodegenerative mechanism of multiple sclerosis susceptibility.
Genes Immun. 12:335–340. 2011. View Article : Google Scholar
|
|
12
|
Abouzahr S, Bismuth G, Gaudin C, et al:
Identification of target actin content and polymerization status as
a mechanism of tumor resistance after cytolytic T lymphocyte
pressure. Proc Natl Acad Sci USA. 103:1428–1433. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Miura N, Takemori N, Kikugawa T, et al:
Adseverin: a novel cisplatin-resistant marker in the human bladder
cancer cell line HT1376 identified by quantitative proteomic
analysis. Mol Oncol. 6:311–322. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zavadil J, Haley J, Kalluri R, Muthuswamy
SK and Thompson E: Epithelial-mesenchymal transition. Cancer Res.
68:9574–9577. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Scanlon CS, Van Tubergen EA, Inglehart RC
and D’Silva NJ: Biomarkers of epithelial-mesenchymal transition in
squamous cell carcinoma. J Dent Res. 92:114–121. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Turley EA, Veiseh M, Radisky DC and
Bissell MJ: Mechanisms of disease: epithelial-mesenchymal
transition-does cellular plasticity fuel neoplastic progression.
Nat Clin Pract Oncol. 5:280–290. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
De Craene B and Berx G: Regulatory
networks defining EMT during cancer initiation and progression. Nat
Rev Cancer. 13:97–110. 2013.PubMed/NCBI
|
|
19
|
Zheng H and Kang Y: Multilayer control of
the EMT master regulators. Oncogene. 33:1755–1763. 2013. View Article : Google Scholar
|
|
20
|
Iorio MV and Croce CM: MicroRNA
dysregulation in cancer: diagnostics, monitoring and therapeutics.
A comprehensive review. EMBO Mol Med. 4:143–159. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Beltran M, Puig l, Peña C, et al: A
natural antisense transcript regulates Zeb2/Sip1 gene expression
during Snail1-induced epithelial-mesenchymal transition. Genes Dev.
22:756–769. 2008. View Article : Google Scholar
|
|
22
|
Luo M, Li Z, Wang W, et al: Long
non-coding RNA H19 increases bladder cancer metastasis by
associating with EZH2 and inhibiting E-cadherin expression. Cancer
Lett. 333:213–221. 2013. View Article : Google Scholar
|
|
23
|
Haifu Wu: Correlated function study of
scinderin gene in liver metastasis of colorectal cancer
(unpublished PhD thesis). Fudan University; 2010
|
|
24
|
Khurana S and George SP: Regulation of
cell structure and function by actin-binding proteins: villin’s
perspective. FEBS Lett. 582:2128–2139. 2008.PubMed/NCBI
|
|
25
|
Tomar A, Wang Y, Kumar N, et al:
Regulation of cell motility by tyrosine phosphorylated villin. Mol
Biol Cell. 15:4807–4817. 2004. View Article : Google Scholar
|
|
26
|
Li GH, Arora PD, Chen Y, McCulloch CA and
Liu P: Multifunctional roles of gelsolin in health and diseases.
Med Res Rev. 32:999–1025. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Valenta T, Hausmann G and Basler K: The
many faces and functions of β-catenin. EMBO J. 31:2714–2736.
2012.
|
|
28
|
Tanaka H, Shirkoohi R, Nakagawa K, et al:
siRNA gelsolin knockdown induces epithelial-mesenchymal transition
with a cadherin switch in human mammary epithelial cells. Int J
Cancer. 118:1680–1691. 2006. View Article : Google Scholar
|
|
29
|
Conacci-Sorrell M, Simcha I, Ben-Yedidia
T, et al: Autoregulation of E-cadherin expression by
cadherin-cadherin interactions: the roles of beta-catenin
signaling, Slug, and MAPK. J Cell Biol. 163:847–857. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Onder TT, Gupta PB, Mani SA, et al: Loss
of E-cadherin promotes metastasis via multiple downstream
transcriptional pathways. Cancer Res. 68:3645–3654. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gradl D, Kühl M and Wedlich D: The Wnt/Wg
signal transducer beta-catenin controls fibronectin expression. Mol
Cell Biol. 19:5576–5587. 1999.PubMed/NCBI
|
|
32
|
Anastas JN and Moon RT: WNT signalling
pathways as therapeutic targets in cancer. Nat Rev Cancer.
13:11–26. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang D, Sun SQ, Yu YH, et al: Suppression
of SCIN inhibits human prostate cancer cell proliferation and
induces G0/G1 phase arrest. Int J Oncol. 44:161–166.
2014.PubMed/NCBI
|
|
34
|
Valastyan S and Weinberg RA: Tumor
metastasis: molecular insights and evolving paradigms. Cell.
147:275–292. 2011. View Article : Google Scholar : PubMed/NCBI
|