Introduction
Renal cell carcinoma (RCC) is one of the most common
urological conditions (1);
however, due to high levels of resistance to chemotherapy and
radiotherapy, the treatment of RCC is confounded by problems of
tumor recurrence and metastasis (2). Since RCC is an immunogenic tumor,
treatment with immunological therapies is possible. Numerous RCC
patients have exhibited spontaneous, partial or complete remission,
and immunotherapies have increased the reactivity of the immune
system against RCC (3).
G250 protein, also known as carbonic anhydrase-9, is
an isomer of the carbonic anhydrase enzyme family (4). The G250 gene can induce a malignant
phenotype and is expressed in the majority of renal cell
carcinomas, however it is not expressed in normal tissues (3,5). The
high expression levels of G250 in RCC makes G250 a potential target
for immunotherapy (6). In previous
studies, the RCC-associated antigen G250 was shown to encode
naturally processed epitopes, which contain cytotoxic lymphocyte
(CTL), and T-helper cell recognition sites. Both of these epitopes
are found within the same region of the G250 protein; within amino
acids 249–268 (7,8). However the immunogenicity of G250 is
low because G250 produces a small, 20-kDa protein (9). In our previous study, the G250 gene
was inserted into the major immunodominant region of the Hepatitis
B core Antigen gene (HBcAg), resulting in the successful generation
of the fusion protein C-G250 (10).
DNA vaccine constructs have been evaluated and
tested in numerous human clinical trials for generating an immune
response to various diseases, including infectious, allergic and
autoimmune diseases, as well as cancers (11,12).
Unfortunately, the immunogenicity of plasmid DNA in humans has
proven to be modest as compared with the immunogenicity observed in
other species treated using microbial expression vectors. The
synthetic polycationic polymer polyethylenimine (PEI), which
consists of chains of ethylenimine
units-CH2CH2NH-, is considered to be the gold
standard technique for enhancing the in vivo expression of
administered genes (13,14). PEI is superior to other
non-microbial transfection agents because of its ability to protect
DNA from degradation (15), to
escape intracellular endosomal lysis, and to efficiently deliver
DNA into the cell nucleus (16).
Formulation with PEI polymers has been employed in DNA and siRNA
(small interfering RNA)-based cancer immunotherapies (17) and in vaccination studies targeting
infectious agents (18).
In the present study, pVAX1, a Food and Drug
Administration-approved plasmid vector, was used as the carrier of
G250 DNA, and combined with the synthetic PEI. The C-G250 protein
was added to the immunization strategy in order to produce a
prime-boost effect. The levels of humoral and cellular immune
responses against G250 were observed to determine whether the DNA
prime-G250 protein boost immunization strategy may provide a novel
treatment strategy for RCC.
Materials and methods
Animals
BALB/c female mice, aged between 6 and 8 weeks, were
housed in the Animal Experimental Center at the Medical College of
Xi’an Jiaotong University (Xi’an, China). The mice received access
to standard diet and water ad libitum and were housed under
pathogen-free conditions in the Animal Experimental Center at the
Medical College of Xi’an Jiatong University (Xi’an, China). All
animal care and experimental procedures were approved by the
Institutional Animal Care and Use Committee of the Xi’an Jiaotong
University.
Construction and identification of the
recombinant pVAX/C-G250 plasmid
The C-G250 gene was cloned from the pET28a(+)/C-G250
plasmid and inserted into the eukaryotic expression vector pVAX1
(Invitrogen Life Technologies, Carlsbad, CA, USA) with EcoRI
and XhoII restriction endonuclease sites. The recombinant
plasmid pVAX1/C-G250 with the inserted sequence was verified by DNA
sequencing. The plasmid was transformed into Escherichia
coli Top10 competent cells (Invitrogen Life Technologies) for
amplification purposes. Extraction and purification of the plasmid
was performed using a DNA Extraction and Purification kit
(Invitrogen Life Technologies). The recombinant plasmid was the
transfected into HEK293 human embryonic kidney cells with
Lipofectin® transfection reagent (Invitrogen Life
Technologies), according to the manufacturer’s instructions. The
transfected cells were harvested and analyzed by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
western blotting, to determine the mRNA and protein C-G250
expression levels, respectively.
C-G250 expression and purification
The pET28a(+)/C-G250 plasmid was transformed into
BL21 (DE3) E. coli cells and gene expression was induced
with 1 mM isopropyl β-D-1-thiogalactopyranoside. The expressed
proteins were purified using a nickel-nitrilotriacetic acid
(Ni-NTA) purification system (Invitrogen Life Technologies) and
analyzed by SDS-PAGE and western blotting. The bacterial lysates
were resolved by Bis-Tris SDS-PAGE, and transferred to
polyvinylidene fluoride (PVDF) membranes (Invitrolon™, 0.45 mm;
Invitrogen Life Technologies). The western blots were probed with
anti-G250 (American Research Products, Inc., Belmont, CA, USA) and
anti-His antibodies (AbD Serotec, Raleigh, NC,USA) at 1:1,000
dilutions, followed by an incubation with alkaline phosphatase
(AP)-conjugated rabbit antimouse immunoglobulin G (IgG) (H+L) at a
1:2,500 dilution. AP detection was performed using a picoBLUE™
Immunoscreening kit (Stratagene, La Jolla, CA, USA).
RT-qPCR
For qPCR, total RNA was extracted from transfected
cells using TRIzol reagent (Invitrogen Life Technologies) according
to manufacturer’s instructions. Reverse transcription was performed
on 2 μg total RNA with the Superscript III First-Strand Synthesis
System for RT-PCR kit (Invitrogen Life Technologies) using a
mixture of oligo (dT)20 and random hexamer primers. The sequences
of the primers for PCR were as follows: Sense:
5′-ATGATTACGCCAAGCTTGGG-3′ and antisense:
5′-TCACGGAAGTGTTGATAGGA-3′. The PCR conditions were as follows:
Denaturation at 94°C for 30 sec, annealing at 62°C for 30 sec, and
extension at 72°C for 30 sec, which was repeated for 35 cycles. The
PCR amplicons were then separated on 2.0% agarose gel by
electrophoresis. The gel was stained with ethidium bromide,
digitally photographed and scanned with the UVI Gel Analyzing
system (UVI Tech, Cambridge, England).
Animal assay
BALB/c mice were divided into four groups (n=5). The
mice in the DNA group were injected with pVAX1/C-G250, the DNA-PEI
complex group were injected with DNA-PEI complex at a ratio of 5:1.
The injections were made into the quadriceps femoris muscles. The
mice of the DNA and DNA-PEI groups were immunized four times over a
10-day period. The mice were immunized with 100 μl of pVAX1/C-G250
(2 μg/μl) each. The following three immunizations, consisted of 50
μl of pVAX1/C-G250. The mice of the DNA-PEI + protein group were
immunized with 100 μl and 50 μl of DNA-PEI complex for the first
two injections respectively, and then immunized with 50 μl of
DNA-PEI complex combined with 50 μg of purified C-G250 protein for
the final two injections. The blank group were injected with
phosphate-buffered saline (PBS) at the same time points. Blood was
collected 10 days after the last vaccination, incubated at 37°C for
30 min, centrifuged at 800 × g for 10 min, and then stored at
−80°C, until further use.
ELISA to determine the antibodies raised
against G250
96-well plates were coated with purified C-G250 (10
μg/ml). The wells were blocked and 100 μl of test serum (pooled
from the immunized mice), diluted at 1:100, was added to each well
followed by a 1 h incubation at 37°C. The bound antibody was probed
with goat anti-mouse IgG (1:2,000, DAKO, Carpinteria, CA, USA),
followed by an incubation with tetramethylbenzidine for 15–30 min.
The optical density (OD) of the plates was read at A450.
Lymphocyte proliferation assay
Mice were sacrificed 10 days after the last
immunization. Splenocytes were isolated from the mice and
resuspended in RPMI-1640 medium supplemented with 10% fetal calf
serum (Gibco-BRL, Carlsbad, CA, USA). A total of 2×104
splenocytes were seeded in triplicate in 96-well flat-bottom plates
and incubated with 2 μg/ml purified C-G250 for 3 days. Following
this, 0.5 mg/ml MTT was added to the culture media and incubated at
37°C for 4 h. Dimethyl sulfoxide was added to the culture media and
the absorbance was then measured at A570. The splenic cell
stimulation index (SI) was calculated by the formula: SI=stimulated
group OD value/control group OD value.
Enzyme-linked immunosorbent spot
(ELISPOT) analysis
Five days following the second of two weekly
vaccinations, splenocytes were collected, depleted of red cells and
CD8+ T cells were purified using the CD8a (Ly-2) Microbead kit
(Miltenyi Biotech, Bergisch Gladbach, Germany) on an AutoMACS
device (Miltenyi Biotech). ELISPOT analysis was performed to
enumerate the number of CD8+ interferon-γ (IFN-γ)-producing cells,
using the BD™ ELISPOT Mouse IFN-γ kit (Becton Dickinson, Franklin
Lakes, NJ, USA) and 96-well PVDF membrane plates (Millipore,
Bedford, MA, USA), according to the manufacturer’s instructions.
Briefly, peritoneal exudate cells (PECs) were used as
antigen-presenting cells; ~1×105 PECs from naïve BALB/c
mice were placed in the ELISPOT wells, which were then loaded with
25 μg/ml of recombinant enhanced green fluorescent protein (EGFP)
or G250 peptide in 100 μl of media, for 4 h at 37°C. CD8+ T cells
(1×105 cells/100 ml) were added to each well for 18 h to
test for antigen recognition. The spots were counted using an
automated Immunospot 3 reader (Cellular Technology, Ltd.,
Cleveland, OH, USA).
Statistical analysis
The data were processed using SPSS version 17.0 for
Windows (SPSS, Inc., Chicago, IL, USA). All the results are
expressed as the means ± standard deviation. One-way analysis of
variance was employed to determine the differences amongst the
groups. A P<0.05 was considered to indicate a statistically
significant difference.
Results
Construction and identification of the
recombinant plasmids pVAX1/C-G250 expressing C-G250
The C-G250 gene was cloned and inserted into the
eukaryotic expression vector pVAX. As shown in Fig. 1A, a band was present at ~500 bp,
when the recombinant pVAX1/C-G250 was digested with EcoRI
and XhoI. The DNA sequencing data confirmed that the C-G250
gene had been cloned into the pVAX1 expression vector successfully
(data not shown). The qPCR and western blotting data showed that
C-G250 was expressed in the HEK293 cells which were transfected
with pVAX1/C-G250 at a concentration of 2 μg/ml. The bands of the
qPCR and western blot analysis (Fig.
1B and C) identified that C-G250 expression 48 h after
transfection was increased as compared with the expression levels
24 h after transfection.
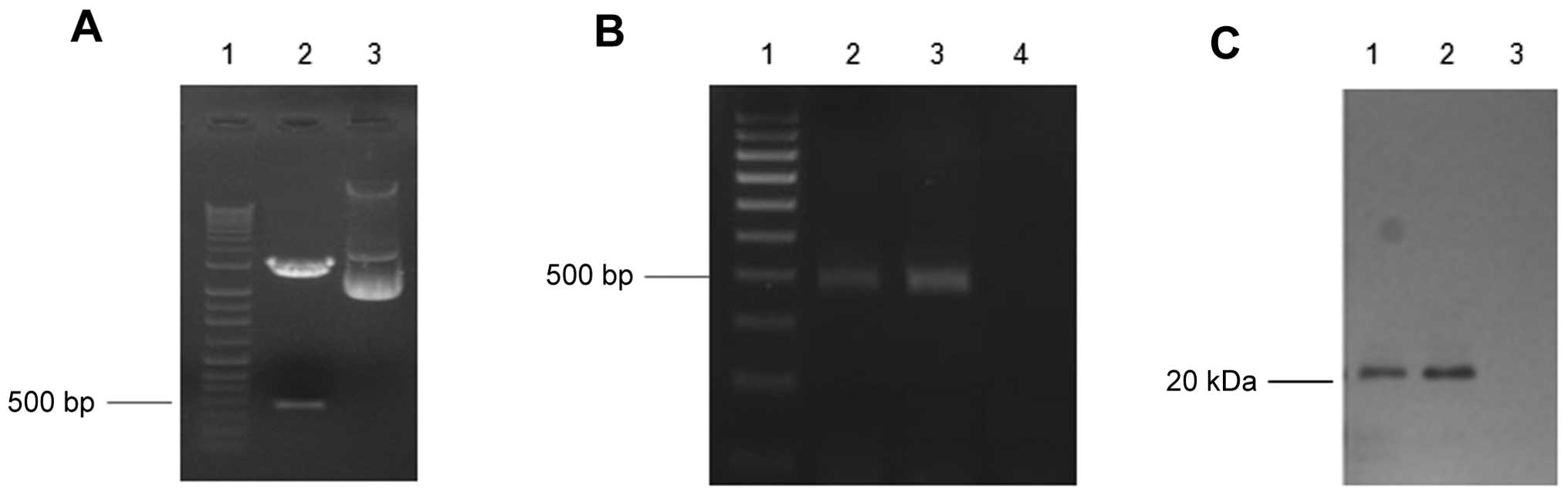 | Figure 1Construction and expression of C-G250
in human embryonic kidney (HEK) 293 cells. (A) The DNA band of
C-G250 (508 bp) was observed ~500 bp. Lane 1, DNA molecular marker;
Lane 2, pVAX1/C-250 plasmid digested with EcoRI and
XhoII. Lane 3, pVAX1/C-250 plasmid. (B) Quantitative
polymerase chain reaction analysis of C-G250 expression in HEK293
cells. Lane 1, DNA molecular marker; Lane 2, cell lysates of HEK293
cells transfected with pVAX1/C-250 (24 h); Lane 3, polymerase chain
reaction product of HEK293 cells transfected with pVAX1/C-250 (48
h); Lane 4, polymerase chain reaction product of HEK293 cells
transfected with pVAX1 (48 h). (C) Western blot analysis of C-G250
expression in HEK293 cells. Lane 1, cell lysates of HEK293 cells
transfected with pVAX1/C-250 (24 h); Lane 2, cell lysates of HEK293
cells transfected with pVAX1/C-G250 (48 h); Lane 3, cell lysates of
HEK293 cells transfected with pVAX1 (48 h). Bp, base pairs; kDa,
kilodaltons. |
C-G250 expression and purification from
pET28a(+)/C-G250
Recombinant C-G250 was expressed in the pET28a
bacterial vector, encoding an N-terminal polyhistidine-tag (His6)
sequence. The proteins containing the His6 tag were purified from
the bacterial lysates using a Ni-NTA column. SDS-PAGE analysis
detected a new band ~20 kDa produced in bacteria that were
transformed with pET28a(+)/C-G250 (Fig. 2A and B). The purified protein was
also ~20 kDa and was recognized by both the anti-His and anti-G250
antibodies (Fig. 2C).
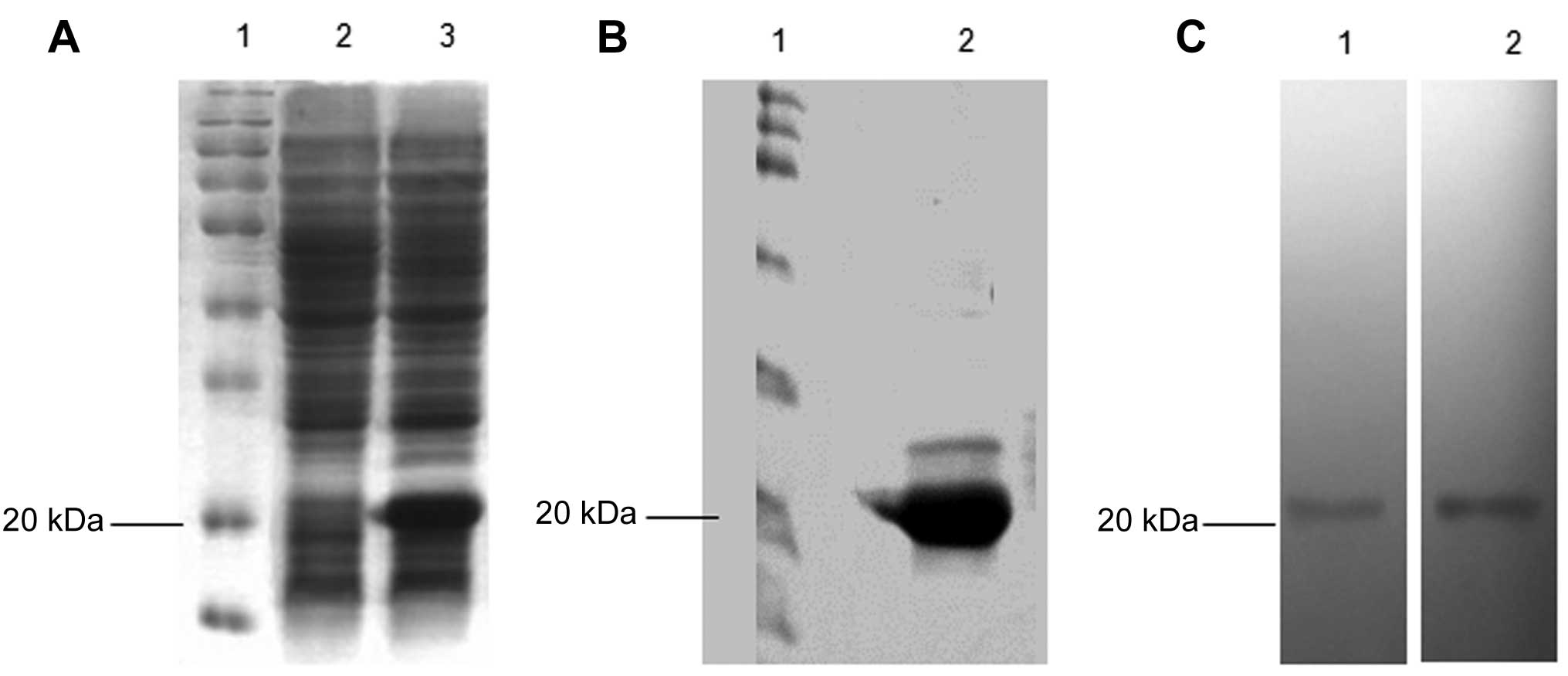 | Figure 2Expression and purification of C-G250.
(A) C-G250 was expressed by the pET28a(+) bacterial vector in BL21
(DE3) Escherichia coli cells. A distinct band of 20 kDa,
corresponding to C-G250, was resolved by SDS-PAGE. Lane 1, protein
molecular marker; Lane 2, bacteria transformed with empty pET28a(+)
vector; Lane 3, bacteria transformed with C-G250 recombinant
pET28a(+) vector. (B) Recombinant C-G250 purified with a
nickel-nitriloacetic acid column. Lane 1, protein molecular marker;
Lane 2, purified protein from bacteria transformed with C-G250
recombinant pET28a(+) vector. (C) Western blot analysis of the
purified C-G250. Lane 1, proteins probed with anti-His antibody;
Lane 2, proteins probed with anti-G250 antibody. kDa,
kilodaltons. |
G250-specific antibodies are detected in
C-G250-immunized mice
BALB/c mice were divided into four groups: Blank,
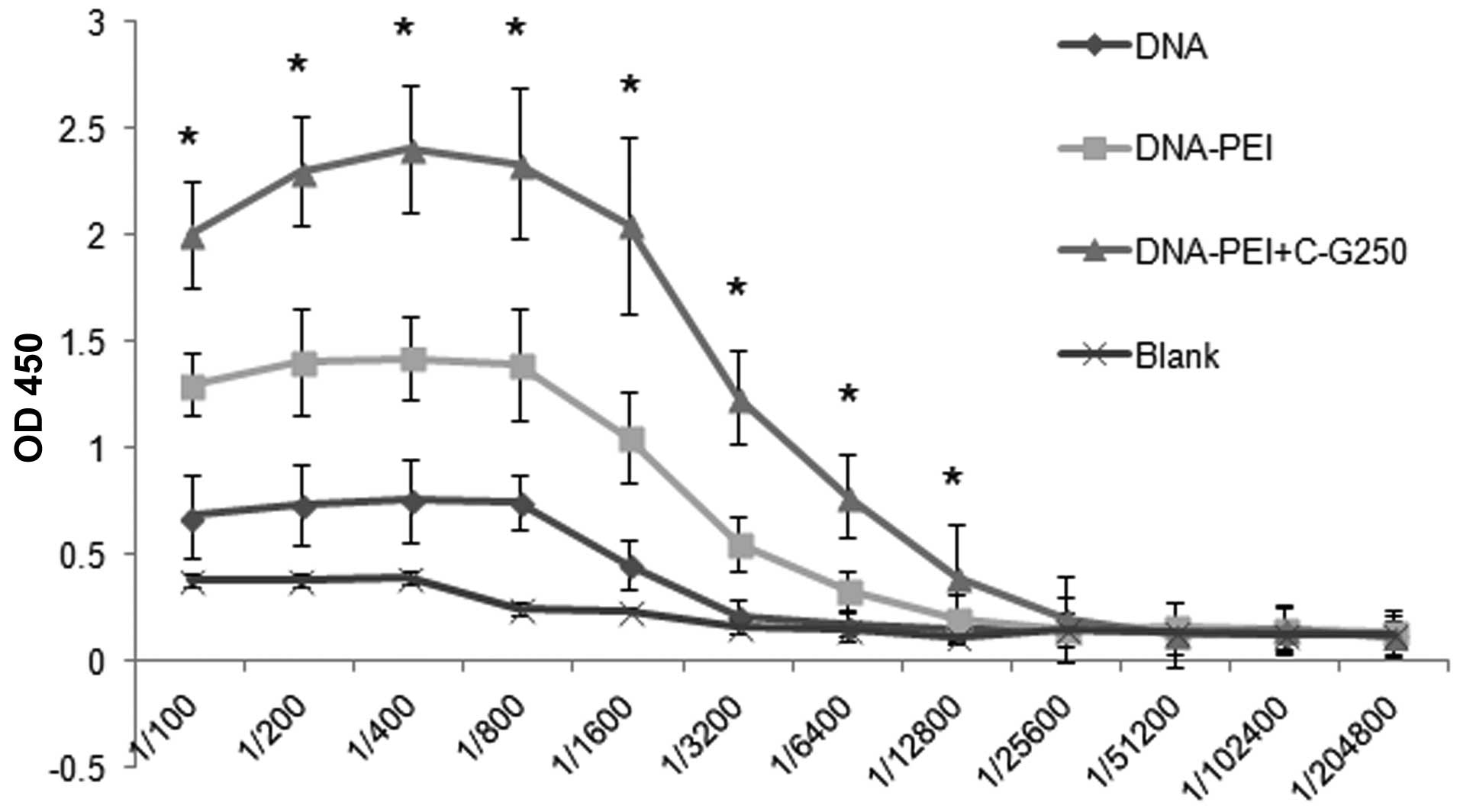
DNA, DNA-PEI and DNA-PEI + protein groups. The serum IgG levels of
the mice from the groups was determined by ELISA. As shown in
Fig. 3, G250-specific IgG
antibodies were detected in the sera of the DNA, DNA-PEI and
DNA-PEI + protein groups following the final immunizations;
however, they were not detected in the blank group.
The IgG level of the DNA-PEI group was significantly
higher as compared with the DNA group. The highest antibody titer
was found when the DNA-PEI complex + C-G250 protein was used to
immunize the mice. This difference was significant at the 1:6,400
dilution as compared with the other groups (Fig. 3).
Antigen-specific responses of
splenocytes
Ten days after the final immunization, splenocytes
were isolated and stimulated with G250 peptide to analyze the cell
proliferation. As shown in Fig.
4A, splenocytes from all of the immunized groups responded to
G250 stimulation, and the SI of the DNA-PEI+C-G250 group was
significantly higher as compared with the DNA and DNA-PEI groups
(P<0.05). CD8+ T-cell response against G250 was also detected
using an IFN-γ ELISPOT assay, in which PECs were used as the
antigen presenting cells. CD8+ T cells were enriched from each
group by immune magnetic selection. Control cultures, containing
either T cells plus PBS or EGFP-loaded PECs, had low numbers of
IFN-γ producing cells. In contrast, significant anti-G250
reactivity was found in T cells of all the immunized groups, with
the highest anti-G250 reactivity in the DNA-PEI + C-G250 group
(Fig. 4B).
Discussion
RCC belongs to a small group of immunogenic tumors.
In patients with RCC, partial or complete remission has been
observed in response to immunotherapy, and continued immunotherapy
treatment can increase the immune reaction against RCC (3). The presence of CD4+ T-lymphocytes and
CD8+ T-lymphocytes in RCC supports the hypothesis that the immune
system is triggered in these tumors (7). Only a small number of RCC-associated
antigens have been characterized that are recognized by CD8+ CTLs,
including advanced glycosylation end product-specific receptor,
receptor tyrosine-protein kinase erbB-2, G250 and squamous cell
carcinoma antigen recognized by T-cells 3 (8,19–20).
The present study aimed at improving the efficacy of
the heterologous DNA prime-protein boost vaccination strategy
against the RCC-associated antigen G250, in inducing both cellular
and humoral immune responses. Previous research has demonstrated
the capability of a DNA prime-protein boost regimen, which can
elicit both an antigen-specific antibody and cytotoxic T-cell
response (22–24). Problems, including the low
transfection efficiency of the DNA vector, and the low
immunogenicity of the antigens, remain to be overcome. In the
present study, low molecular weight PEI was used as a DNA vector
carrier in order to enhance the transfection efficiency, and the
G250 gene was fused with the major immunodominant region (MIR) of
the HBcAg gene (C-G250) to increase the immunogenicity of the G250
antigen. Following boosting the vaccine with the fusion protein
C-G250, both the CD8+ T-cell response and the CD4+ T-cell response
were significantly enhanced.
PEI has been previously used in gene therapy since
its initial introduction as a gene delivery vehicle (13). The PEI/DNA complex can prolong DNA
retention, and enhance the transfection of the target gene
(25,26). Furthermore, PEI has previously been
used in a heterologous prime-boost vaccination strategy (13). Li et al (22) explored a heterologous approach
using DNA prime-adenovirus boost vaccination with PEI as a DNA
vaccine adjuvant. The results indicated that PEI could be used as a
competent carrier for the delivery of a DNA vaccine. This idea was
supported by Wegmann et al (27), who showed that PEI was a safe and
efficient DNA vaccine adjuvant that induced a stronger immune
response.
In the present study, PEI was used as a DNA carrier
in order to improve and retain a high transfection efficiency. The
DNA-PEI complex group demonstrated an improved T-cell immune
response and higher antibody levels as compared with the DNA group.
These results indicate that PEI has a strong adjuvant effect. Thus,
it can be inferred from these results that PEI is a potent DNA
vaccine carrier, which can induce a strong immune response.
The boost vaccination of C-G250 significantly
enhanced the immune responses as compared with the DNA vaccination
alone. In the DNA-PEI+protein group, antigen-specific antibody IgG,
and antigen-specific CTL levels were measured. From the results, it
can be observed that following protein boosting, the IgG titer of
the DNA-PEI+protein group was significantly higher as compared with
the DNA and DNA-PEI groups (Fig.
3). A significant anti-G250 reactivity was found in CD8+ T
cells of all of the immunized groups, especially in the
DNA-PEI+C-G250 group, indicating that the cellular and humoral
immune responses, were enhanced by the DNA prime-protein boost
regimen.
In conclusion, PEI was used as a DNA vaccine carrier
and the effects of a heterologous DNA priming plus protein boosting
vaccination strategy were evaluated in mice. The results indicated
that the DNA-PEI+protein regimen significantly enhanced the
anti-G250 immune response, as compared with the DNA and DNA-PEI
groups. This resulted in higher CD8+ and CD4+ T-cell responses and
cytokine production levels, which make this strategy a potential
vaccine candidate against RCC. Future research may focus on the
mechanism of vaccination, and evaluate its efficacy on an animal
model of RCC.
Acknowledgements
This study was supported by grants from the
Technology Research Project of Shaanxi Province (nos. 2012SF2-21
and 2012K16-09-02), and the National Natural Science Foundation of
China (nos. 81200545 and 81270545).
References
|
1
|
Awakura Y, Ito N, Nakamura E, et al:
Matrix metalloproteinase-9 polymorphisms and renal cell carcinoma
in a Japanese population. Cancer Lett. 241:59–63. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dutcher JP: Recent developments in the
treatment of renal cell carcinoma. Ther Adv Urol. 5:338–353. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tostain J, Li G, Gentil-Perret A and
Gigante M: Carbonic anhydrase 9 in clear cell renal cell carcinoma:
a marker for diagnosis, prognosis and treatment. Eur J Cancer.
46:3141–3148. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stadick H, Stockmeyer B, Kühn R, et al:
Epidermal growth factor receptor and g250: useful target antigens
for antibody mediated cellular cytotoxicity against renal cell
carcinoma? J Urol. 167:707–712. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li G, Feng G, Gentil-Perret A, Genin C and
Tostain J: CA9 gene expression in conventional renal cell
carcinoma: a potential marker for prediction of early metastasis
after nephrectomy. Clin Exp Metastasis. 24:149–155. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Muselaers S, Mulders P, Oosterwijk E, Oyen
W and Boerman O: Molecular imaging and carbonic anhydrase
IX-targeted radioimmunotherapy in clear cell renal cell carcinoma.
Immunotherapy. 5:489–495. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vissers JL, De Vries IJ, Engelen LP, et
al: Renal cell carcinoma-associated antigen G250 encodes a
naturally processed epitope presented by human leukocyte antigen-DR
molecules to CD4(+) T lymphocytes. Int J Cancer. 100:441–444. 2002.
View Article : Google Scholar
|
|
8
|
Vissers JL, De Vries IJ, Schreurs MW, et
al: The renal cell carcinoma-associated antigen G250 encodes a
human leukocyte antigen (HLA)-A2.1-restricted epitope recognized by
cytotoxic T lymphocytes. Cancer Res. 59:5554–5559. 1999.PubMed/NCBI
|
|
9
|
Xiao Y, Gao J, Gao K, et al: Prokaryotic
expression, purification and antigenicity identification of human
renal cell carcinoma-associated antigen G250. Xi Bao Yu Fen Zi Mian
Yi Xue Za Zhi. 29:269–272. 2013.(In Chinese).
|
|
10
|
Liu B, Sun Z, Liu Q, et al: Construction
and prokaryotic expression of recombinant gene G250 antigenic
peptide-HBcAg and the immunogenicity analysis of the fusion
protein. Journal of Xi’an Jiaotong University (Medical Sciences).
1:6–11. 2014.
|
|
11
|
Manoj S, Babiuk LA and van Drunen
Littel-van den Hurk S: Approaches to enhance the efficacy of DNA
vaccines. Crit Rev Clin Lab Sci. 41:1–39. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ada G: Vaccines and vaccination. N Engl J
Med. 345:1042–1053. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Grant EV, Thomas M, Fortune J, Klibanov AM
and Letvin NL: Enhancement of plasmid DNA immunogenicity with
linear polyethylenimine. Eur J Immunol. 42:2937–2948. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Krishnamachari Y, Geary SM, Lemke CD and
Salem AK: Nanoparticle delivery systems in cancer vaccines. Pharm
Res. 28:215–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Demeneix B and Behr JP: Polyethylenimine
(PEI). Adv Genet. 53PA:215–230. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kichler A, Leborgne C, Coeytaux E and
Danos O: Polyethylenimine-mediated gene delivery: a mechanistic
study. J Gene Med. 3:135–144. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ma YF and Yang YW: Delivery of DNA-based
cancer vaccine with polyethylenimine. Eur J Pharm Sci. 40:75–83.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bivas-Benita M, Bar L, Gillard GO, et al:
Efficient generation of mucosal and systemic antigen-specific CD8+
T-cell responses following pulmonary DNA immunization. J Virol.
84:5764–5774. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gaugler B, Brouwenstijn N, Vantomme V, et
al: A new gene coding for an antigen recognized by autologous
cytolytic T lymphocytes on a human renal carcinoma. Immunogenetics.
44:323–330. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Brossart P, Stuhler G, Flad T, et al:
Her-2/neu-derived peptides are tumor-associated antigens expressed
by human renal cell and colon carcinoma lines and are recognized by
in vitro induced specific cytotoxic T lymphocytes. Cancer Res.
58:732–736. 1998.
|
|
21
|
Kawagoe N, Shintaku I, Yutani S, et al:
Expression of the SART3 tumor rejection antigen in renal cell
carcinoma. J Urol. 164:2090–2095. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li K, Gao H, Gao L, et al: Enhancement of
humoral and cellular immunity in chickens against
reticuloendotheliosis virus by DNA prime-protein boost vaccination.
Vaccine. 31:1944–1949. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li M, Jiang Y, Xu C, Zhang Z and Sun X:
Enhanced immune response against HIV-1 induced by a heterologous
DNA prime-adenovirus boost vaccination using mannosylated
polyethyleneimine as DNA vaccine adjuvant. Int J Nanomedicine.
8:1843–1854. 2013.
|
|
24
|
Lan J, Gao Z, Xiong H, et al: Generation
of protective immune responses against coxsackievirus B3 challenge
by DNA prime-protein boost vaccination. Vaccine. 29:6894–6902.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang X, Xu J, Qiu C, et al: Mucosal
priming with PEI/DNA complex and systemic boosting with recombinant
TianTan vaccinia stimulate vigorous mucosal and systemic immune
responses. Vaccine. 25:2620–2629. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Goula D, Becker N, Lemkine GF, et al:
Rapid crossing of the pulmonary endothelial barrier by
polyethylenimine/DNA complexes. Gene Ther. 7:499–504. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wegmann F, Gartlan KH, Harandi AM, et al:
Polyethyleneimine is a potent mucosal adjuvant for viral
glycoprotein antigens. Nat Biotechnol. 30:883–888. 2012. View Article : Google Scholar : PubMed/NCBI
|