Introduction
Bacterial biofilms are a polymeric matrix produced
by the bacterial cells embedded within it. The biofilm and cells
together constitute a structured community that is a common cause
of numerous persistent infections (1). Furthermore, such a community enables
certain bacteria to control their individual gene expression via
quorum sensing (QS), by detecting autoinducers secreted by
themselves or their neighbors (2).
Protected within the biofilm, the bacteria are more
resistant to antibiotics than their planktonic forms (3). An important example is Escherichia
(E.) coli, a commonly encountered pathogen that is often
responsible for community- and hospital-acquired infections.
Isolates of E. coli that are harbored within the biofilm are
often resistant to antibiotic treatment (4).
Emerging evidence strongly suggests that antibiotics
at sub-minimum inhibitory concentrations (MICs) may nonetheless
interfere with bacterial functions. These effects may have clinical
relevance as bacteria are commonly exposed to sub-MICs of
antibiotics at a certain period, particularly at the beginning and
end of the treatment (5).
Third-generation cephalosporins, a class of β-lactam
antibiotics, are widely used in the treatment of bacterial
infections caused by gram-negative bacteria, such as E.
coli. Examples of cephalosporins include ceftazidime (CAZ),
cefoperazone (CFP), ceftriaxone and cefotaxime. Several studies
have demonstrated that CAZ at sub-MICs inhibited QS in
Pseudomonas aeruginosa and Burkholderia pseudomallei,
thereby decreasing the synthesis of a range of QS-regulated
virulence factors (6,7).
The present study aimed to investigate the effects
that sub-MICs of cephalosporins may have on the biofilm production
of E. coli isolates. To investigate these effects, the
biofilm production of 52 E. coli reference strains and
clinical isolates following treatment with 1/4 MICs of
third-generation cephalosporins were observed. In one E.
coli clinical isolate, CFP and CAZ exerted opposite effects on
biofilm formation. The mechanisms of these effects were then
examined in that isolate.
Materials and methods
Bacterial strains and growth
conditions
In order to investigate the effects of sub-MICs of
third-generation cephalosporins on the biofilm formation of E.
coli, at the beginning of the study each of the three E.
coli reference strains (ATCC700926, ATCC35218 and DH5α) and 49
clinical isolates were treated separately with four
third-generation cephalosporins [Ceftazidime (CAZ; Sigma-Aldrich,
Shanghai, China), ceftriaxone, cefotaxime or cefoperazone (CFP)
(all from National Institutes for Food and Drug Control, Beijing,
China)] at 1/4 MICs. The study was approved by the Ethics Committee
of Southwest Hospital, Third Military Medical University,
(Chonqing, China).
The ATCC700926, ATCC35218, DH5α and BAA1117
(Vibrio harveyi BB170) strains were purchased from the
American Type Culture Collection (Manassas, VA, USA). A total of 49
E. coli isolates collected from Southwest Hospital
(Chongqing, China) between January 2009 and February 2009 were used
in this study. Written informed consent from the patient/patient’s
family was obtained prior to the study.
Among the E. coli examined, E42, isolated
from the pus of a surgical patient who had undergone a curative
resection of a colorectal carcinoma, was noteworthy as formation of
its biofilm was suppressed by 1/4 MIC CAZ, while it was enhanced by
1/4 MIC CFP. To examine the underlying mechanisms controlling these
opposite effects, E42 was selected for further investigation in the
subsequent experiments.
E. coli isolates were grown at 37°C in
Luria-Bertani (LB) broth, and BAA1117 was grown at 30°C in marine
broth (BD, 2216). In addition, the bacterial growth was determined
using LB broth containing 1/4 MIC of CAZ or CFP with rapid shaking
at 37°C. For the growth curve experiments, 50 μl of the culture
sample was collected every 4 h for 24 h to measure the optical
density at 600 nm with a Thermo Multiskan Spectrum (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) (8).
Determination of MICs for E. coli
strains
The MICs of the four cephalosporins against the
E. coli strains were determined in accordance with the
Clinical and Laboratory Standards Institute guidelines (9). Cultures were adjusted to a turbidity
equivalent to 0.5 MacFarlane standard suspension prior to being
inoculated on Mueller Hinton agar (Oxoid, Basingstoke, UK) in the
presence of CAZ, ceftriaxone, cefotaxime or CFP at concentrations
ranging from 256 to 0.0625 μg/ml (12 doubling-dilution drug
concentrations). Cultures were incubated for 20 h at 37°C under
aerobic conditions. The lowest drug concentration that could
prevent growth was recorded as the MIC.
Biofilm formation assay
Biofilm formation was assayed by crystal violet
staining of adherent cells as described previously (10), with a few modifications. The
bacterial cultures that were adjusted to 1×107 cfu/l
were inoculated in LB broth on 96-well polystyrene plates in the
presence of CAZ or CFP at sub-MICs. Following incubation at 37°C
for 6–24 h, the plates were rinsed twice with phosphate-buffered
saline and dried in an inverted position. The adherent cells were
stained with 1% crystal violet (Sigma-Aldrich) for 10 min, and the
wells were rinsed three times with sterile water. The dye was
dissolved in 30% acetic acid, and the absorbance of the solubilized
dye at 590 nm was then determined using the Thermo Multiskan
Spectrum. Each treatment was assayed in 5-wells per plate and the
experiments were repeated three times.
Measurement of mRNA changes of the genes
encoding biofilm-modulating proteins
Reverse transcription-polymerase chain reaction
(RT-PCR) and quantitative (q)PCR were used to investigate the
levels of the mRNA products of the biofilm-modulating genes of this
isolate. The cultures were inoculated in 20 ml LB broth containing
1/4 MIC of CAZ or CFP and then cultivated at 37°C with shaking in
an environmental chamber (Yuejin, Shanghai, China) for 0.5–10 h.
RNA isolation was performed in accordance with the manufacturer’s
instructions provided for the TRIzol reagent (Invitrogen, Carlsbad,
CA, USA). RT using a Reverse Transcription system (Promega
Corporation, Madison, WI, USA) was conducted as described
previously (11). The primers are
listed in Table I. RT-PCR and qPCR
amplifications were performed. The latter were performed in
quadruplet using MyiQ Color Fluorescence Real-Time Quantitative PCR
apparatus (Bio-Rad Laboratories, Hercules, CA, USA). The reaction
conditions are listed in Table
II. For qPCR, 40 cycles were completed and the annealing
temperature was 60°C. The fold changes of each transcript were
calculated using the 2−ΔΔCT method (12) and were expressed as values relative
to the control group.
 | Table IOligonucleotides used in reverse
transcription-polymerase chain reaction. |
Table I
Oligonucleotides used in reverse
transcription-polymerase chain reaction.
| Gene | Accession no. | Primer | Sequence
(5′-3′) | Product size
(bp) |
|---|
|
16s-rRNA | NC_000913 | 16s-rRNA-F |
ggaggaaggtggggatgacg | 638 |
| | 16s-rRNA-R |
atggtgtgacgggcggtgtg | |
| pfs | NC_000913 | pfs-F |
cctggcaccaacgttgaaag | 347 |
| | pfs-R |
tggcgcgtacgacaacaaac | |
| luxS | NC_000913 | luxS-F |
tgccgaacaaagaagtgatgc | 348 |
| | luxS-R |
cttcgttgctgttgatgcgtac | |
| ariR | NC_000913 | ariR-F |
tcagcagtgttagggcaggc | 156 |
| | ariR-R |
tcgcaacacgatttccagtg | |
| hha | NC_000913 | hha-F |
aatgcgtttacgtcgttgcc | 135 |
| | hha-R |
ttcatggtcaattcggcgag | |
| tnaA | NC_000913 | tnaA-F |
aactgttgccgcatatcccg | 248 |
| | tnaA-R |
attcgccgcgttctctttca | |
| tomB | NC_000913 | tomB-F |
caatcatggctgggtaaacga | 265 |
| | tomB-R |
cgcaggattctctttcgtcg | |
| mcbR | NC_000913 | mcbR-F |
cgctttctgtcgcacctgca | 191 |
| | mcbR-R |
gcccttttcttgcgcctgct | |
| mqsR | NC_000913 | mqsR-F |
caatgccgggcaagttcgta | 186 |
| | mqsR-R |
tggcctgtaacaagcctggg | |
| QseB | NC_000913 | qseB-F |
cgtcagggaaaagaggcgct | 258 |
| | qseB-R |
ggttcggcgcatcagagctt | |
| QseC | NC_000913 | qseC-F |
actcgcgccgctgaacaaac | 273 |
| | qseC-R |
agtgcttttttccgcgcctg | |
 | Table IIPolymerase chain reaction
protocols. |
Table II
Polymerase chain reaction
protocols.
| Reaction
conditions |
|---|
|
|
|---|
| Gene | Denaturation | Annealing | Extension | Final
extension | No. of cycles |
|---|
|
16s-rRNA | 94°C for 30
sec | | 72°C for 30
sec | 72°C for 10
min | 30 |
| pfs | 94°C for 30
sec | 53°C for 30
sec | 72°C for 30
sec | 72°C for 10
min | 30 |
| luxS | 94°C for 30
sec | 57°C for 30
sec | 72°C for 30
sec | 72°C for 10
min | 30 |
| airR | 94°C for 30
sec | 52°C for 30
sec | 72°C for 30
sec | 72°C for 10
min | 30 |
| hha | 94°C for 30
sec | 52°C for 30
sec | 72°C for 30
sec | 72°C for 10
min | 30 |
| tnaA | 94°C for 30
sec | 54°C for 30
sec | 72°C for 30
sec | 72°C for 10
min | 30 |
| tomB | 94°C for 30
sec | 51°C for 30
sec | 72°C for 30
sec | 72°C for 10
min | 30 |
| mcbR | 94°C for 30
sec | 57°C for 30
sec | 72°C for 30
sec | 72°C for 10
min | 30 |
| mqsR | 94°C for 30
sec | 55°C for 30
sec | 72°C for 30
sec | 72°C for 10
min | 30 |
| qseB | 94°C for 30
sec | 55°C for 30
sec | 72°C for 30
sec | 72°C for 10
min | 30 |
| qseC | 94°C for 30
sec | 56°C for 30
sec | 72°C for 30
sec | 72°C for 10
min | 30 |
Autoinducer-2 (AI-2) bioassay
The AI-2 bioassay was performed to determine whether
AI-2 production in the E42 isolate was affected by CAZ or CFP and
to verify whether the gene expression had been inhibited. The
bioassays were performed in accordance with the method previously
described (13,14). The strains treated with a 1/4 MIC
of CAZ or CFP were pelleted by centrifugation at 12,000 × g for 10
min, and then the supernatant was filtered through a 0.22-μm filter
(Millipore, Bedford, MA, USA). V. harveyi BB170 was diluted
1:5,000 in fresh Difco Marine Broth 2216 (BD Biosciences, Franklin
Lakes, NJ, USA) and then 180 μl of culture was mixed with 20 μl of
the supernatant sample. The luminescence values of the mixtures
were measured with a luminometer (Turner Biosystems, Sunnyvale, CA,
USA) after incubating at 30°C for 3 h. A positive control was
obtained from the overnight cultures of BB170 and sterile medium
served as a negative control. AI-2 activity was expressed as the
difference in relative light units compared with the level of
luminescence induced by the negative control. For each experiment,
the bioassay was performed in triplicate for each sample.
Transduction of antisense
oligonucleotides (AS-ODNs)
AS-ODNs targeting S-ribosylhomocysteine lyase
(luxS) of E. coli were designed and used to inhibit
gene expression (15,16). Firstly, four AS-ODNs were designed
(5′-GGTAATGGTGTAGAGATTATCGATA-3′, 5′-CAATGGAAGACGTGCTGAAAGTGCA-3′,
5′-GAACGTCTACCAGTGTGGCACTTAC-3′ and
5′-CGAAAGAGAAGTTGCAGGAACTGCA-3′; available at https://rnaidesigner.invitrogen.com/rnaiexpress/)
and the one with the highest blocking efficiency was selected for
the subsequent experiments. Briefly, 4 μl (20 μg) of the
transfection reagent Sofast (5 mg/ml; Sunmabio, Xiamen, China) was
diluted in 20 μl LB broth. Following incubation at room temperature
for 20 min, 20 μl of AS-ODN was added to the diluted transfection
reagent. A total of 40 μl of the mixture was added to 160 μl of the
competent bacterial suspension with a final concentration of 10 μM
AS-ODN. Following culturing at 37°C for 1 h, the transfected
bacteria were adjusted to a turbidity equivalent of a 0.5
MacFarlane standard suspension and cultivated at 37°C with shaking
in an environmental chamber for 4 h.
Statistical analysis
The significance of the intergroup differences in
terms of biofilm formation, growth or AI-2 production was analyzed
using an independent samples t-test. The significance of
differences in RT-PCR results was determined using a paired samples
t-test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Biofilm formation by E. coli
clinical isolate E42 is inhibited by CAZ but induced by CFP
Firstly, the effects of four third-generation
cephalosporins (CAZ, ceftriaxone, cefotaxime and CFP) at 1/4 MICs
on the biofilm formation of 52 E. coli isolates were
measured. It was identified that CAZ inhibited biofilm formation in
seven strains, while CFP enhanced biofilm formation in 18 strains.
The results from the E42 isolate were noteworthy as biofilm
formation was suppressed by CAZ at 1/4 MIC, whereas it was enhanced
by CFP at 1/4 MIC. Therefore, E42 was selected for investigation in
subsequent experiments.
CAZ at sub-MICs suppressed biofilm formation by E42
in a dose-dependent manner, and CFP enhanced its biofilm formation
in a dose-dependent manner (Fig.
1A). In addition, 1/4 MIC CAZ exerted maximal effects on E42
biofilm formation at 18 h and the effect of CFP at 1/4 MIC reaches
a maximum at 24 h (Fig. 1B).
Effects of CAZ and CFP at 1/4 MICs on the
mRNA expression of biofilm regulator genes
To determine which regulator gene is involved in the
effects of CAZ and CFP on E42 biofilm formation, the changes in the
mRNA levels of important regulator genes (pfs, luxS,
ariR, tnaA, hha and tomb) in the
bacterial cultures 3 h following inoculation were examined. Among
these six genes, the levels of luxS mRNA were associated
with CAZ or CFP treatment, whereas mRNA levels of the five other
genes were not changed (Fig. 2).
The present data suggested that luxS/AI-2 QS may be involved
in the inhibitory effects of CAZ and the inductive functions of CFP
on E. coli biofilm formation. Therefore, the subsequent
experiments focused on luxS/AI-2 QS.
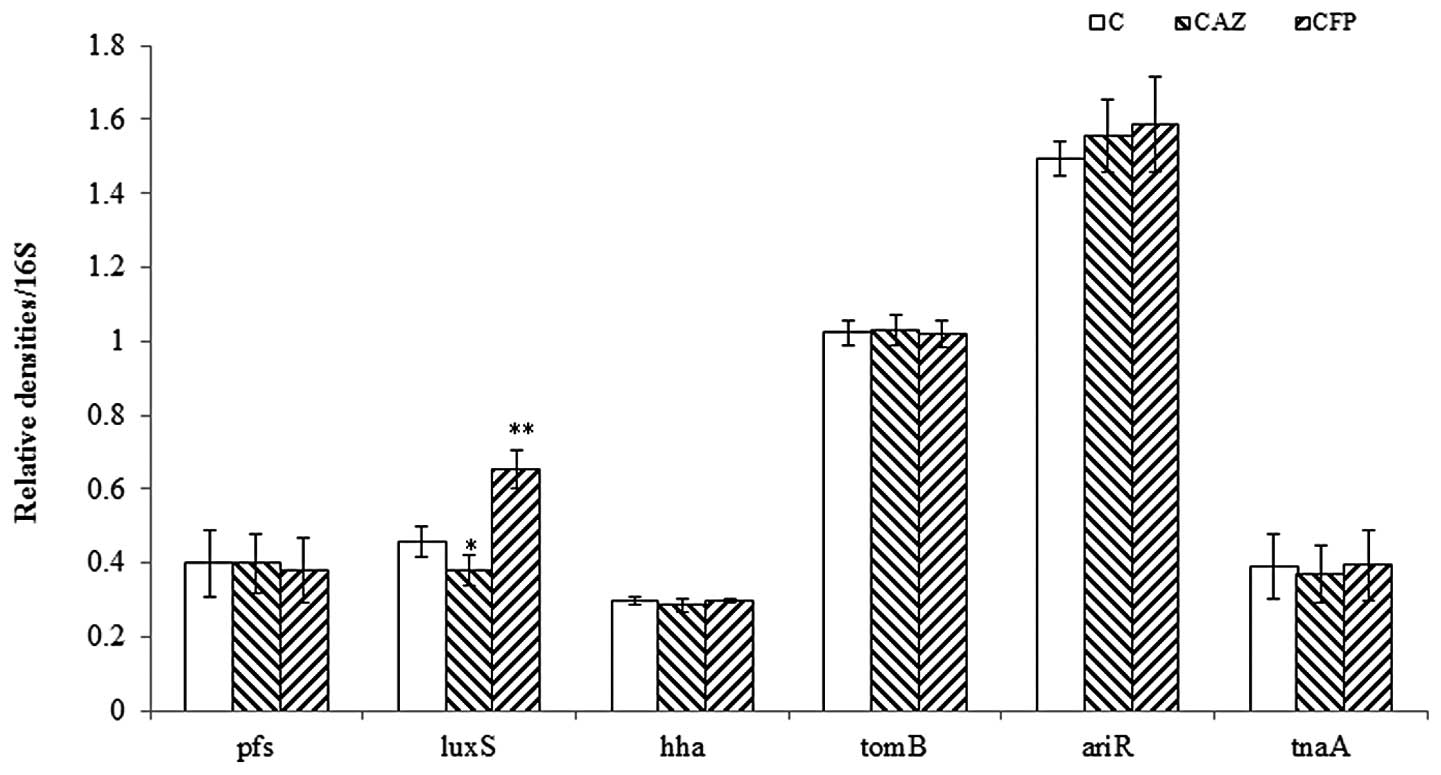 | Figure 2Expression of pfs, luxS, hha,
tomB, ariR and tnaA in the cultures grown for 3 h in the
presence of 1/4 MIC of CAZ or CFP. A 16S rRNA was used as an
internal standard. For all experiments, n=3. *P<0.05,
compared with C. CAZ, ceftazidime; CFP, cefoperazone; MICs, minimum
inhibitory concentrations; C, control; luxS,
S-ribosylhomocysteine lyase. |
CAZ exerts effects opposite to that of
CFP on the mRNA levels of luxS and AI-2 production in E. Coli
isolates
QS with luxS/AI-2 is known to be important
for E. coli biofilm formation (17). Therefore, the mRNA levels of
luxS and AI-2 production in the clinical isolate E42 in the
presence of 1/4 MICs of CAZ or CFP were further quantified.
Considering that AI-2 production is primarily determined by the
density of bacterial inoculates, the growth of bacterial cells was
examined simultaneously. CAZ and CFP at 1/4 MICs exerted effects on
the mRNA levels of luxS and AI-2 production, without any
effect on the growth of the isolate (Fig. 3). In E42, the mRNA levels of
luxS was significantly reduced following growth in the LB
medium with 1/4 MIC CAZ incubated for 30 min or 1 h, compared with
the controls in which CAZ was absent. However, these levels were
higher following exposure to 1/4 MIC CFP for 0.5–6 h, compared with
the controls without CFP (Fig.
3A). Based on the AI-2 bioassay, changes in bioluminescence
occurred following treatment for 4 h (Fig. 3B). These results were concurrent
with those of the mRNA levels of luxS.
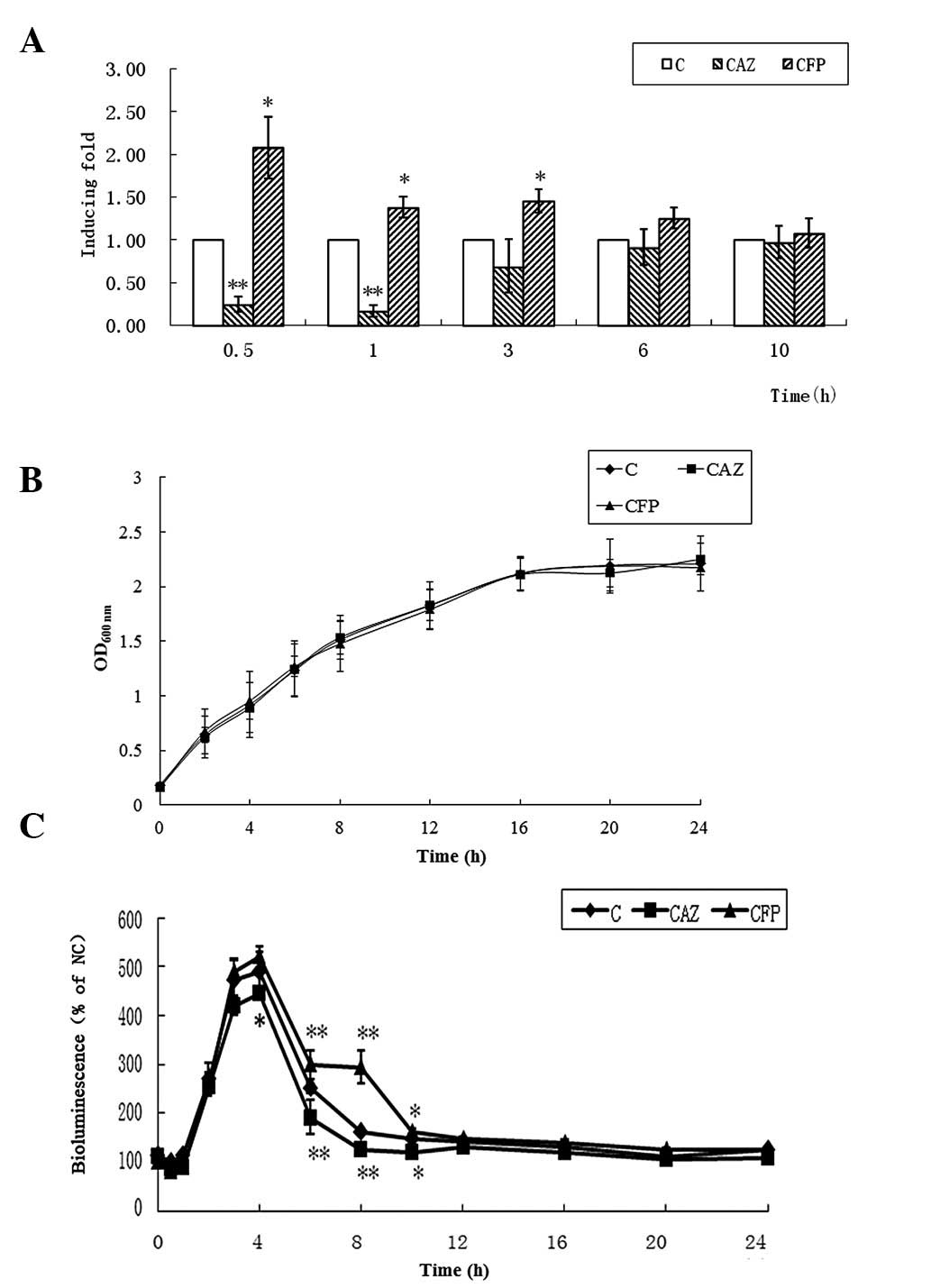 | Figure 3Effects of CAZ or CFP at 1/4 MIC on
luxS/AI-2 QS. (A) mRNA levels of luxS determined by qPCR, (B)
production of AI-2 and (C) growth of E42 in the presence of 1/4 MIC
of CAZ or CFP. The values are expressed as the mean ± standard
deviation. For the qPCR experiments, n=4; for the AI-2 bioassays,
n=3. **P<0.01 and *P<0.05, compared
with the C. CAZ, ceftazidime; CFP, cefoperazone; MICs, minimum
inhibitory concentrations; C, control; qPCR, quantitative
polymerase chain reaction; luxS, S-ribosylhomocysteine
lyase.. |
Effects of CAZ and CFP at 1/4 MICs on the
mRNA levels of biofilm-modulating factors that are regulated by
AI-2
During biofilm formation, AI-2 stimulates biofilm
formation and changes its architecture by stimulating flagella
motility via MqsR, QseBC and McbR (18). The mRNA levels of key downstream
genes that are regulated by AI-2 in the cultures incubated with CAZ
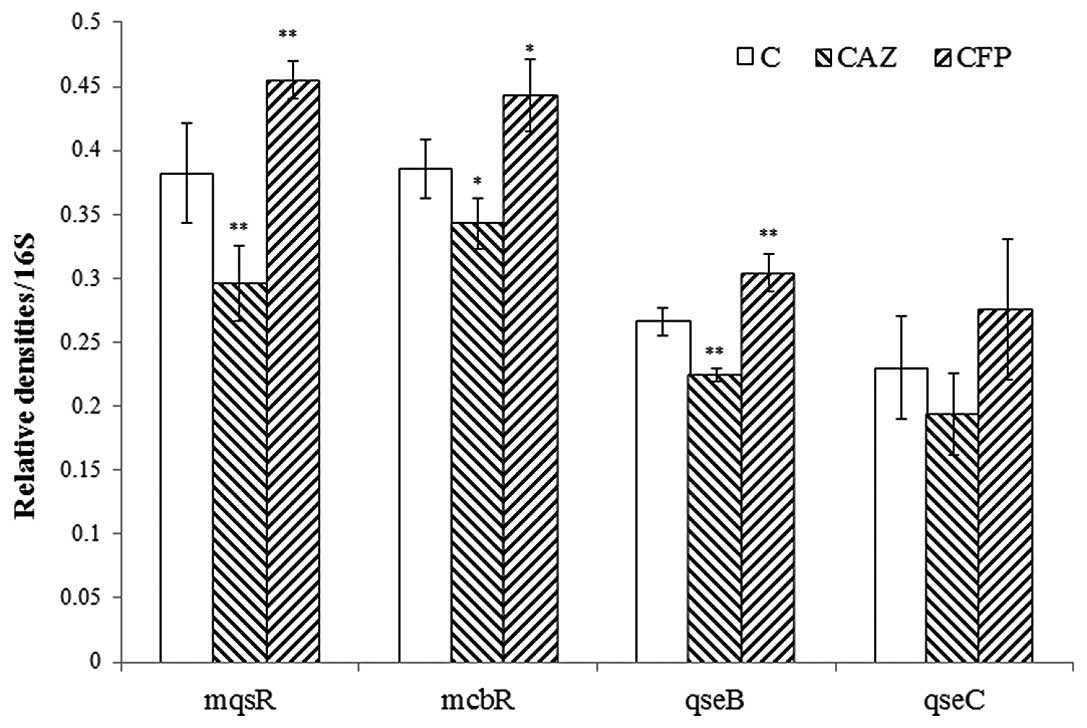
or CFP at 1/4 MICs for 12 h. The expression levels of mqsR,
qseB, qseC and mcbR in E42 were significantly
decreased in the presence of 1/4 MIC of CAZ, whereas CFP exerted
the opposite effect on these genes (Fig. 4).
AS-ODNs targeting luxS modulate the
effects of CAZ and CFP on E. coli biofilm formation
To confirm the role of luxS in the response
to CAZ and CFP, the AS-ODNs targeting luxS were employed.
AI-2 production was measured to verify whether luxS
expression was blocked. As AS-ODNs do not pass freely through cell
membranes due to their negative charge, the cationic polymer
reagent Sofast was used to transduce the AS-ODNs, as previously
described (15,16). The results demonstrated that the
transduction of AS-ODNs targeting luxS decreased AI-2
production significantly, indicating that luxS expression
was successfully blocked (Fig.
5A). As AS-ODN-4 (5′-CGAAAGAGAAGTTGCAGGAACTGCA-3′) was the most
efficient at blocking luxS expression, it was selected for
the subsequent experiments. The E. coli biofilm formation in
the presence of 1/4 MIC of CAZ or CFP was then quantified.
Following AS-ODN transduction, the effects of CAZ and CFP on E.
coli biofilm formation were diminished (Fig. 5B), suggesting that CAZ and CFP were
unable to affect biofilm formation once the luxS gene was
blocked.
 | Figure 5(A) AS-ODNs targeting luxS
decrease AI-2 production and (B) the effects of CAZ and CFP on
biofilm formation by Escherichia coli isolates. For the AI-2
bioassay experiments, n=3; **P<0.01 and
*P<0.05, compared with E42. For the biofilm formation
experiments, n=5; **P<0.01 and *P<0.05,
compared with C. AS-ODNs, antisense oligonucleotides; CAZ,
ceftazidime; CFP, cefoperazone; MICs, minimum inhibitory
concentrations; AI-2, autoinducer-2; C, control. |
Discussion
To the best of our knowledge, this is the first
study to demonstrate that two antibiotics, CAZ and CFP, sharing the
same antimicrobial mechanism have opposite effects on biofilm
formation of an E. coli clinical isolate at their respective
sub-MICs.
In previous studies, drugs with similar chemical
structures and antimicrobial activities have been demonstrated to
have varied effects on the biofilm formation of different species
of bacteria. For example, azithromycin was demonstrated to inhibit
biofilm formation by Pseudomonas aeruginosa (19) and Porphyromonas gingivalis
(20), but enhanced biofilm
formation by Staphylococcus epidermidis (21). However, for a given bacterial
species, it is rarely reported that drugs with similar
antimicrobial activities may cause opposite changes in biofilm
formation. Only one study demonstrated a similar effect, showing
that clarithromycin induced biofilm formation in isolates of
Pseudomonas aeruginosa (22), while azithromycin did the opposite
(19). However, these effects took
place with different strains. In the present study, it was reported
that CAZ and CFP had opposite effects on biofilm formation in the
same isolate of E. coli.
CAZ and CFP are third-generation cephalosporins.
They have similar antimicrobial activities (23), based on their identical β-lactam
structure. However, in the present study they induced completely
opposite effects on biofilm formation in a clinical isolate of
E. coli, indicating that these activities are likely
independent of their antimicrobial roles. Furthermore, at
concentrations that influenced biofilm production, these
antibiotics were unable to change bacterial growth, which also
suggests the independent nature of these two activities.
To examine which regulator gene is involved in the
effects of CAZ and CFP on E. coli biofilm formation, the
changes in the mRNA levels of the important regulator genes were
examined, including pfs (encoding the
5′-methylthioadenosine/S-adenosylhomocysteine nucleosidase enzyme),
luxS (encoding synthetases of AI-2), hha/tomb
(a toxin-antitoxin pair), tnaA (encoding tryptophanase to
synthesize indole) and ariR (a regulator of acid resistance
influenced by indole) in 3-h bacterial cultures (18). The present results indicated that
luxS/AI-2 QS may be associated with the effects of CAZ and
CFP on E. coli biofilm formation. QS has been implicated in
the control of a number of bacterial functions, including the
secretion of virulence factors, biofilm formation, bioluminescence
production, conjugation and swarming motility (2). luxS/AI-2 QS has been
demonstrated to regulate E. coli biofilm formation (24). In the present study, CAZ at 1/4 MIC
reduced luxS mRNA levels and AI-2 production in E.
coli isolates, whereas CFP at 1/4 MIC had the opposite
effect.
The designed AS-ODNs were effective at
downregulating the expression of the targeted gene; AS-ODNs
targeting luxS decreased the inhibitory effects of CAZ and
the enhancer role of CFP in biofilm formation by an E. coli
isolate. These results provide more evidence that the roles of
these two compounds on biofilm formation depend on changes in QS
activity.
The similar antimicrobial activities of CAZ and CFP
are due to the similarity of their basic β-lactam structure
(25). It was speculated that
their opposite effects on biofilm formation were due to different
side chains (26). To determine
whether this hypothesis is reasonable, the studies published in the
past three years regarding the effects of antibiotics on the
biofilm formation of various bacteria were reviewed. However, there
was little evidence indicating an association between
biofilm-influencing activity and the structures of the antibiotics.
Therefore, this hypothesis requires further investigation.
In the present study, the concentrations tested were
carefully selected so that the antibiotics did not effect the
bacterial growth. Nevertheless, the biofilm formation of the E.
coli isolate E42 was affected by these low CAZ and CFP doses.
Previous studies have demonstrated that the effects of certain
antibiotics on the growth and virulence-gene expression of bacteria
at higher concentrations than MICs may act via QS (6,17).
The results indicate that the response of this isolate to CAZ and
CFP at 1/4 MIC may also be due to QS.
In the present study, the biofilm formation of 52
E. coli isolates was measured, but only the production of
biofilm by E42 responded to CAZ in an opposite manner to that of
CFP. This result appears to indicate an individual case.
Differences in the reactions among E. coli isolates appear
to be due to individual differences in isolates. The QS of
luxS/AI-2, as an important intermediate, may be involved in
the responses to CAZ and CFP, while the underlying mechanisms
require further study.
In conclusion, the present study indicates that the
effects of antibiotics at sub-MICs may differ from their effects at
high doses. The present study does not fully explain the potential
effects of these drugs, and therefore extensive and careful
investigation is required to determine the clinical application of
this data.
Acknowledgements
This study was supported by a grant from the
Sci-Tech of Chongqing Drug Innovation Incubation Base (grant no.
2010ZX09401-306-1-3) and the National Natural Science Foundation of
China (grant no. 81373451).
References
|
1
|
Costerton JW, Stewart PS and Greenberg EP:
Bacterial biofilms: a common cause of persistent infections.
Science. 284:1318–1322. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Galloway WR, Hodgkinson JT, Bowden SD,
Welch M and Spring DR: Quorum sensing in Gram-negative bacteria:
small-molecule modulation of AHL and AI-2 quorum sensing pathways.
Chem Rev. 111:28–67. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nickel J, Ruseska I, Wright J and
Costerton J: Tobramycin resistance of Pseudomonas aeruginosa
cells growing as a biofilm on urinary catheter material. Antimicrob
Agents Chemother. 27:619–624. 1985.
|
|
4
|
Joly V, Pangon B, Vallois JM, et al: Value
of antibiotic levels in serum and cardiac vegetations for
predicting antibacterial effect of ceftriaxone in experimental
Escherichia coli endocarditis. Antimicrob Agents Chemother.
31:1632–1639. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kaplan JB: Antibiotic-induced biofilm
formation. Int J Artif Organs. 34:737–751. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kanthawong S, Bolscher JG, Veerman EC, et
al: Antimicrobial and antibiofilm activity of LL-37 and its
truncated variants against Burkholderia pseudomallei. Int J
Antimicrob Agents. 39:39–44. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Skindersoe ME, Alhede M, Phipps R, et al:
Effects of antibiotics on quorum sensing in Pseudomonas
aeruginosa. Antimicrob Agents Chemother. 52:3648–3663. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Song Z, Kong K, Wu H, et al: Panax ginseng
has anti-infective activity against opportunistic pathogen
Pseudomonas aeruginosa by inhibiting quorum sensing, a
bacterial communication process critical for establishing
infection. Phytomedicine. 17:1040–1046. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Clinical Laboratory Standards Institute.
Performance standards for antimicrobial susceptibility testing;
20th informational supplement. CLSI document M100-S20. Clinical
Laboratory Standards Institute; Wayne, PA, USA: 2010
|
|
10
|
Kim YH, Lee Y, Kim S, et al: The role of
periplasmic antioxidant enzymes (superoxide dismutase and thiol
peroxidase) of the Shiga toxin-producing Escherichia coli
O157: H7 in the formation of biofilms. Proteomics. 6:6181–6193.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li Y, Li Q, Du Y, et al: Prevalence of
plasmid-mediated AmpC beta-lactamases in a Chinese university
hospital from 2003 to 2005: first report of CMY-2-Type AmpC
beta-lactamase resistance in China. J Clin Microbiol. 46:1317–1321.
2008. View Article : Google Scholar
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
|
|
13
|
Surette MG, Miller MB and Bassler BL:
Quorum sensing in Escherichia coli, Salmonella typhimurium,
and Vibrio harveyi: a new family of genes responsible for
autoinducer production. Proc Natl Acad Sci USA. 96:1639–1644.
1999.PubMed/NCBI
|
|
14
|
Wang Y, Zhang W, Wu Z, Zhu X and Lu C:
Functional analysis of luxS in Streptococcus suis reveals a key
role in biofilm formation and virulence. Vet Microbiol.
152:151–160. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li B, Yao Q, Pan XC, et al: Artesunate
enhances the antibacterial effect of β-lactam antibiotics against
Escherichia coli by increasing antibiotic accumulation via
inhibition of the multidrug efflux pump system AcrAB-TolC. J
Antimicrob Chemother. 66:769–777. 2011.PubMed/NCBI
|
|
16
|
Meng J, Wang H, Hou Z, et al: Novel anion
liposome-encapsulated antisense oligonucleotide restores
susceptibility of methicillin-resistant Staphylococcus
aureus and rescues mice from lethal sepsis by targeting
mecA. Antimicrob Agents Chemother. 53:2871–2878. 2009.
View Article : Google Scholar
|
|
17
|
Jones MB, Jani R, Ren D, Wood TK and
Blaser MJ: Inhibition of Bacillus anthracis growth and
virulence-gene expression by inhibitors of quorum-sensing. J Infect
Dis. 191:1881–1888. 2005.PubMed/NCBI
|
|
18
|
Wood TK: Insights on Escherichia
coli biofilm formation and inhibition from whole-transcriptome
profiling. Environ Microbiol. 11:1–15. 2009.
|
|
19
|
Bala A, Kumar R and Harjai K: Inhibition
of quorum sensing in Pseudomonas aeruginosa by azithromycin
and its effectiveness in urinary tract infections. J Med Microbiol.
60:300–306. 2011.PubMed/NCBI
|
|
20
|
Maezono H, Noiri Y, Asahi Y, et al:
Antibiofilm effects of azithromycin and erythromycin on
Porphyromonas gingivalis. Antimicrob Agents Chemother.
55:5887–5892. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Q, Sun FJ, Liu Y, et al: Enhancement
of biofilm formation by subinhibitory concentrations of macrolides
in icaADBC-positive and -negative clinical isolates of
Staphylococcus epidermidis. Antimicrob Agents Chemother.
54:2707–2711. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Garey KW, Vo QP, Lewis RE, et al:
Increased bacterial adherence and biomass in Pseudomonas
aeruginosa bacteria exposed to clarithromycin. Diagn Microbiol
Infect Dis. 63:81–86. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bijie H, Kulpradist S, Manalaysay M and
Soebandrio A: In vitro activity, pharmacokinetics, clinical
efficacy, safety and pharmacoeconomics of ceftriaxone compared with
third and fourth generation cephalosporins: review. J Chemother.
17:3–24. 2005.
|
|
24
|
González Barrios FG, Zuo R, Hashimoto Y,
et al: Autoinducer 2 controls biofilm formation in Escherichia
coli through a novel motility quorum-sensing regulator (MqsR,
B3022). J Bacteriol. 188:305–316. 2006.PubMed/NCBI
|
|
25
|
Mandell G and Sande M: Antimicrobial
agents: penicillins, cephalosporins, and other beta-lactam
antibiotics. Goodman and Gilman’s The Pharmacologic Basis of
Therapeutics. Gilman AG, Hardman JG, Limbird L and Ral TR: 9th
edition. McGraw-Hill; New York, NY: pp. 1090–1091. 1996
|
|
26
|
Arndt PA and Garratty G: Cross-reactivity
of cefotetan and ceftriaxone antibodies, associated with hemolytic
anemia, with other: cephalosporins and penicillin. Am J Clin
Pathol. 118:256–262. 2002. View Article : Google Scholar
|