Introduction
Large leaf moss, the dried or fresh grass of
bryaceae, such as Rhodobryum roseum (Hedw.) Limpr. [Mnium
roseum Hedw.], R. giganteum (Schwaegr) par. and R.
giganteum par. (1–3), is a traditional herbal remedy widely
used in the Yunnan province of China. Large leaf moss is commonly
used to treat diseases, including heart palpitations, chest
tightness and neurasthenia.
Myocardial ischemic injury is associated with the
generation of reactive oxygen species (ROS); it has been
demonstrated that the production of ROS increased and antioxidant
enzyme activity decreased during myocardial ischemia (4). Oxidative stress resulting from oxygen
free radical production may exceed the capacity of endogenous free
radical scavenging mechanisms, resulting in lipid peroxidation and
DNA damage in mitochondria (5,6).
Data have indicated that cellular oxidative stress is important in
the pathogenesis of atrial fibrillation (7). Results from another study have
indicated that trying to prevent and avoid oxidative damage to
atrial cells may prevent and relieve the development of atrial
fibrillation to a certain extent (8).
In a study of monomeric compounds in Large leaf
moss, piperine and pepper acid methyl ester protected
cardiomyocytes from the injury induced by hydrogen peroxide
(H2O2), and piperine effectively protected
ventricular myocytes from the injury induced by oxygen radicals
(1). In the present study, an
oxidative injury model with low concentrations of
H2O2 was used to analyze the key steps in the
oxidative stress response and further investigate the protective
effect of piperine on primary atrial cells in the oxidative injury
model.
Materials and methods
Reagents
The superoxide dismutase (SOD), malondialdehyde
(MDA) and glutathione (GSH) kits were from Jiancheng Bioengineering
Corporation (Nanjing, China), and piperine and MTT were obtained
from Sigma-Aldrich (St. Louis, MO, USA). Dulbecco’s modified
Eagle’s medium (DMEM), 3% H2O2 and fetal
bovine serum were purchased from Gibco-BRL (Carlsbad, CA, USA). The
penicillin-streptomycin mixture and 0.25% trypsin-EDTA were
supplied by Beijing Solarbio Science & Technology Co., Ltd.
(Beijing, China). The primer probes and SYBR Green were purchased
from Shanghai ShineGene Molecular Biotech, Inc. (Shanghai, China).
Fluorochrome Fluo-2/acetoxymethyl ester (Fura-2 AM) was obtained
from Anaspec (Fremont, CA, USA).
Animals
Neonatal New Zealand rabbits (age, 3–5 days; weight,
150–200 g) were obtained from the Experimental Animal Center of
Military Medical Sciences (Beijing, China) and housed in specific
pathogen-free conditions. The protocols were approved by the
Institutional Animal Care and Use Committee of General Hospital of
People’s Liberation Army. All procedures were in accordance with
the Declaration of Helsinki of the World Medical Association.
Animal grouping and primary culture of
atrial muscle cells
A total of 18 animals were randomly divided into
three groups: Piperine, H2O2 and control
groups. Briefly, following sacrifice by injection of ~10 ml air
into the ear vein, the neonatal rabbit was fixed in a supine
position and the chest skin was disinfected. The chest wall was cut
and removed along the right edge of the sternum. The heart was
rapidly removed and washed in D-Hanks fluid (Baihao, Tianjin,
China) and the blood was removed. The left and right atria were
washed with serum-free medium under sterile conditions and cut into
small ~1-mm diameter sections with ophthalmic scissors. A volume of
5 ml 0.08% trypsin was added to the tissue sections, which were
incubated in a 37°C water bath for 5 min. Digestion was then
terminated by adding serum to the medium. A single cell suspension
culture was prepared and the concentration of cells was adjusted to
1×105/ml. To inhibit the growth of fibroblast cells, 1
ml of 0.1 mmol/l bromodeoxyuridine (Sigma-Aldrich) was added. The
cultures were incubated at 37°C in a humidified incubator with 5%
CO2.
The primary atrial myocytes in the piperine group
were pretreated with 7×10−6 mol/l piperine for 1 h, then
100 μmol/l H2O2 was added and the cells were
incubated for 2 h. The H2O2 and control
groups were treated with 100 μmol/l H2O2 or
phosphate-buffered saline (PBS) for 2 h, respectively.
Detection of cell viability
The cell concentrations were adjusted to
1×106 cells/ml in DMEM supplemented with 10% fetal calf
serum. Samples of the suspensions (100 μm) were dispensed into
96-well round-bottom culture plates (Costar, Cambridge, MA, USA)
and incubated for 72 h at 37°C in a 5% CO2 humid
incubator. Cell proliferation was then determined with the MTT
assay (9).
Detection of oxidative stress markers
SOD, MDA and GSH
Supernatant (50 μl) was extracted from the
myocardial cell cultures. SOD expression levels were determined
with the xanthine oxidase method, MDA expression levels were
measured by thiobarbituric acid assay and GSH expression levels
were determined by a colorimetric method. All assays were performed
according to the manufacturer’s instructions of the respective
kits.
Measurement of intracellular
Ca2+
Intracellular Ca2+ levels were determined
with Ca2+-sensitive Fluo-2/acetooxymethyl ester by a
Becton Dickinson FACS Calibur flow cytometer (Becton-Dickinson,
Franklin Lakes, NJ, USA) or a Perkin-Elmer LS 55 fluorescence
spectrophotometer (Perkin-Elmer, Waltham, MA, USA) (10,11).
The cells were incubated with 3 μM Fluo-2/AM at 37°C for 30 min in
the dark following treatment with salidroside and
H2O2 as described. The cells were then gently
rinsed three times with D-Hanks solution and the fluorescence was
analyzed by flow cytometry. Fluo-2/AM bound to cytoplasmic free
calcium generates a strong fluorescence at an excitation wavelength
of 330–350 nm, which is reduced at the 380 nm excitation
wavelength. Thus, the fluorescence ratio of 340 to 380 nm was used
to detect the intracellular calcium ion concentrations.
Quantitative analysis of mitochondrial
mRNA in atrial myocytes
Total RNA was isolated with an RNApure kit (Bioteke,
Beijing, China) and retrotranscribed with MLV-reverse transcriptase
(Invitrogen Life Technologies, Carlsbad, CA, USA) with random
primers. Reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) was performed by real-time PCR ABI Prism 7500
(Applied Biosystems China, Beijing, China), using 40 cycles of 95°C
for 12 sec and 60°C for 1 min with SYBR Green. The primers were
designed using Primer Premier, version 5 (www.premierbiosoft.com and OLIGO Primer Analysis
Software, version 6 (oligo.net). The sequences of the primers were
5′-AGGATGGTAGCAAGGAGGAAG-3′ and 5′-ACCCTGGAGCGATGTGGA-3′. The
length of p22-phox (Oryctolagus cuniculus) was 137 bp.
Statistical analysis
Data were analyzed using SPSS software (SPSS Inc.,
Chicago, IL, USA). All data are expressed as the mean ± standard
deviation or standard error of the mean. P<0.05 was considered
to indicate a statistically significant difference.
Results
Primary culture of atrial myocytes and
morphological observation
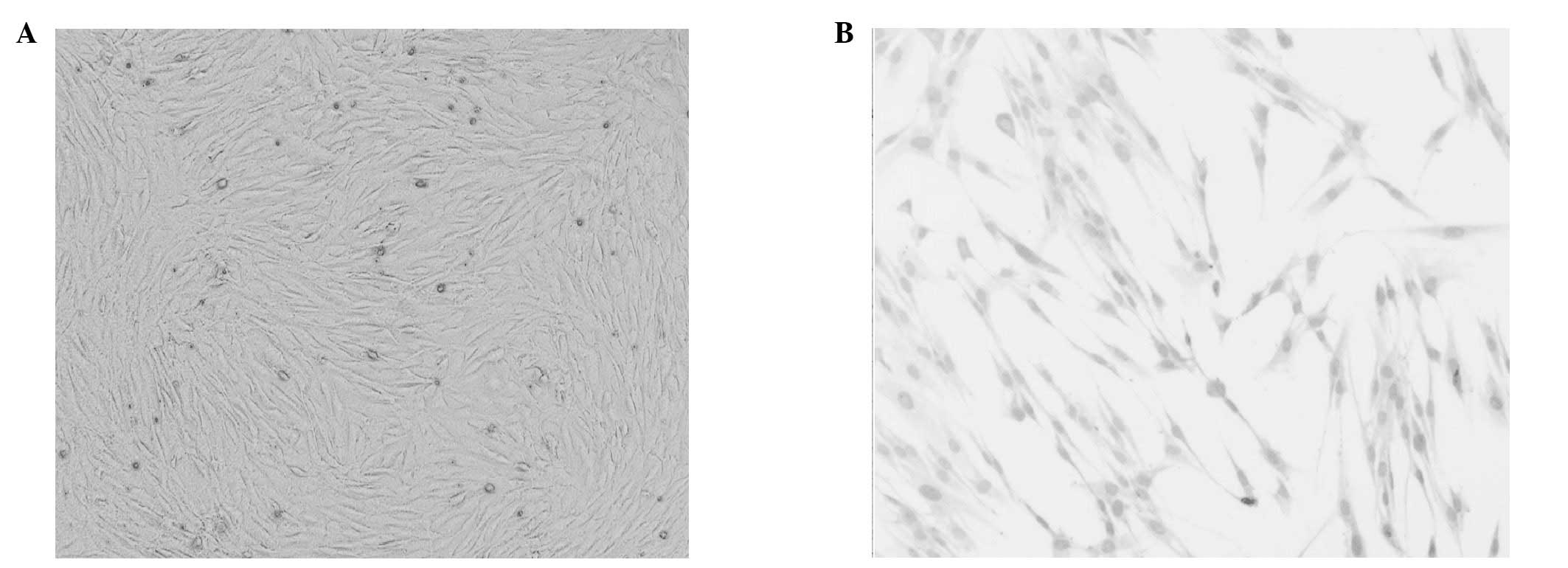
After 72 h culture, cells with a variety of shapes
were visible under an inverted microscope, although the cell
morphology was dominated by spindle, triangular and irregular
shapes (Fig. 1A). Hemotoxylin and
eosin staining of the cells is presented in Fig. 1B. Certain adherent cardiomyocytes
formed multicellular beating cell clusters within 24 h.
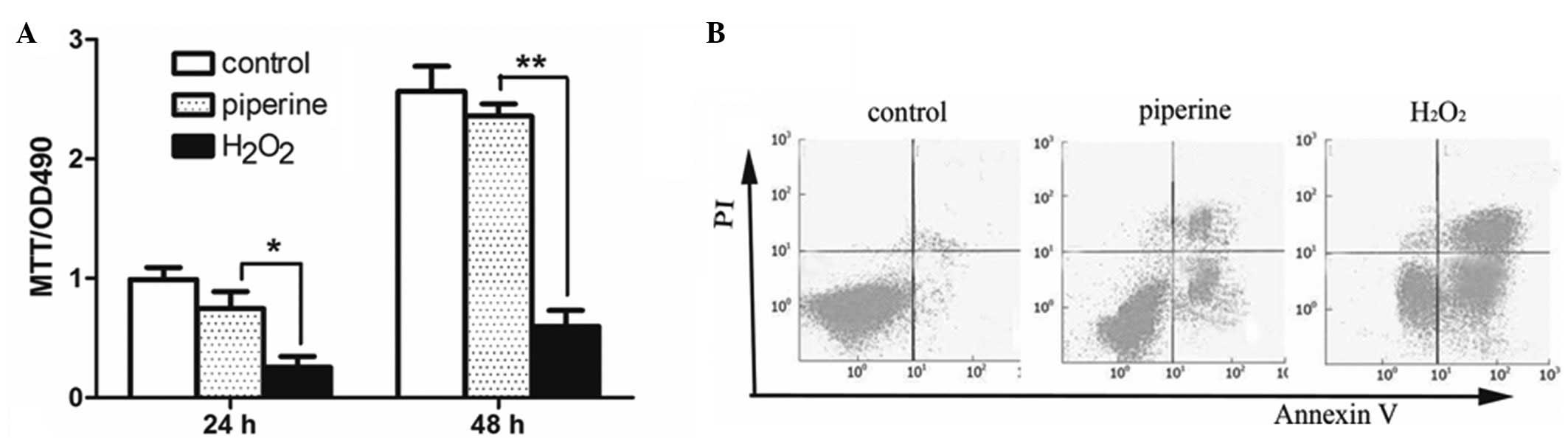
Cell viability of primary atrial myocytes
increases following piperine treatment
In order to determine the role of piperine on the
cell viability of primary atrial myocytes, an MTT assay was
performed. The results demonstrate that the cell viability of the
primary atrial myocytes significantly increased following treatment
with piperine compared with the cells only treated with
H2O2. Furthermore, the cell viability
increased in a time-dependent manner, as analyzed by MTT (Fig. 2A). The results detected by MTT were
consistent with those observed with FACS analysis (Fig. 2B).
Detection of oxidative stress markers
SOD, MDA and GSH
The levels of oxidative stress markers were detected
in different groups. As shown in Fig.
3 and Table I, the mean SOD
production in the piperine group was significantly higher than that
in the H2O2 group (P<0.05) and the
expression levels of MDA and GSH in the culture supernatant of
primary myocardial cells in the piperine group were significantly
lower than those in the H2O2 group
(P<0.01). Untreated cells served as negative controls.
 | Table IEffects of piperine on oxidative
stress markers SOD, MDA and GSH. |
Table I
Effects of piperine on oxidative
stress markers SOD, MDA and GSH.
| Group | SOD (U/l) | MDA (U/l) | GSH (μM/ml) |
|---|
| Control |
1706.666±13.278 | 0.333±0.101 | 0.189±0.047 |
|
H2O2 | 202.423±65.498 | 12.593±0.201 | 3.869±0.192 |
| Piperine |
407.272±59.598a | 4.965±0.335a | 2.485±0.117a |
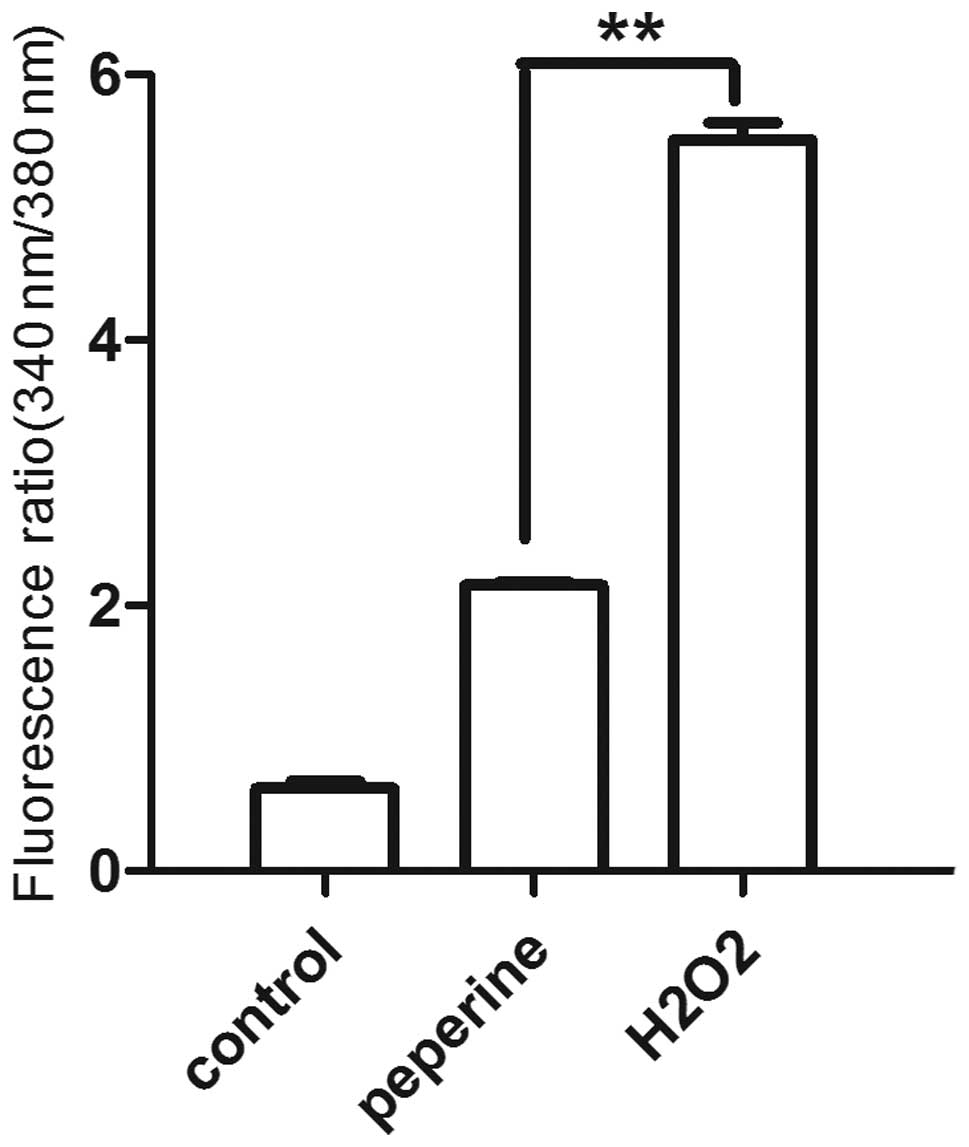
Detection of the intracellular calcium
concentration
Impaired oxidative phosphorylation may result in
lowered ATP synthase function and reduce the ability of the
mitochondria to synthesize ATP. In addition, oxidative stress may
reduce the activity of ATP-dependent ion pumps in the cell membrane
and intracellular calcium levels may become elevated. Thus, the
intracellular calcium levels in the different groups were examined.
As shown in Fig. 4, the
intracellular calcium concentration was significantly lower in the
group treated with piperine than in those treated with
H2O2 alone (P<0.01).
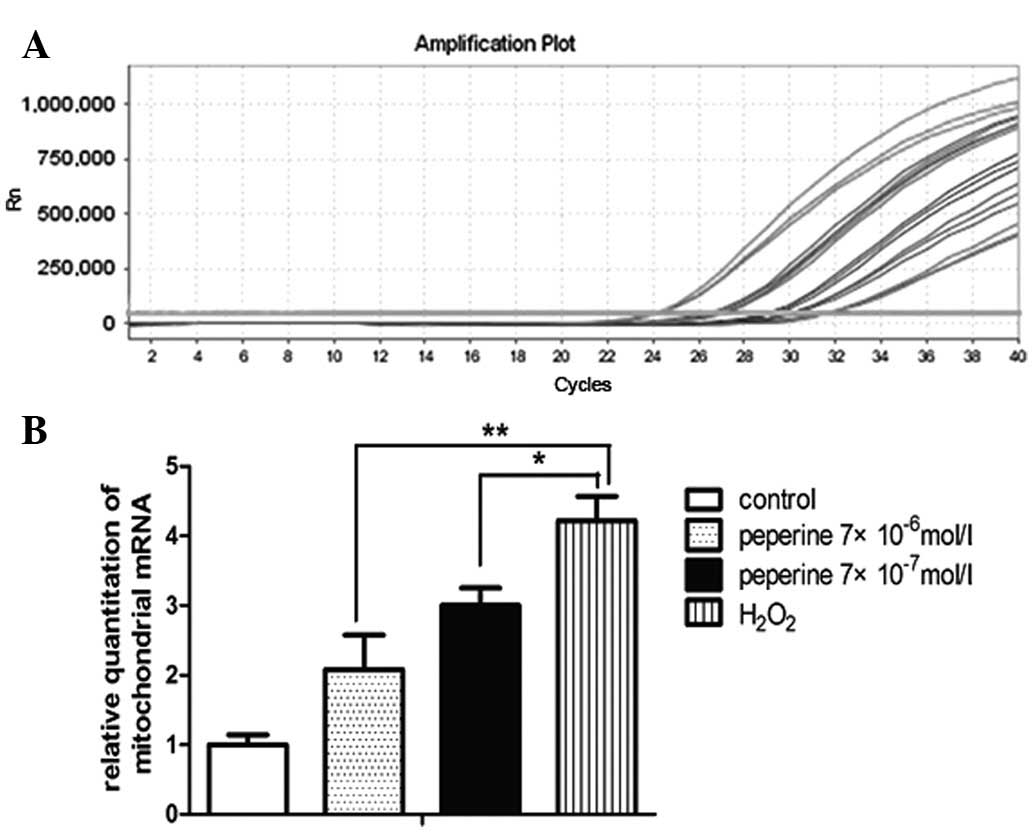
RT-qPCR in atrial myocytes
Quantitative analysis of mitochondrial mRNA
expression levels in atrial myocytes was performed. The results
demonstrated that the mitochondrial mRNA expression levels were
significantly reduced in the groups treated with piperine compared
with those treated with H2O2 alone
(P<0.01) (Fig. 5). Piperine
significantly reduced the mitochondrial mRNA levels in a
dose-dependent manner following H2O2
stimulation as analyzed by RT-qPCR.
Discussion
Oxidative injury/oxidative stress refers to cell
damage resulting from excessive oxidation, when the body produces
excessive ROS and/or the antioxidant capacity is reduced (12). The reactive oxygen species (ROS)
and secondary ROS, such as peroxynitrite, are mainly generated in
the mitochondrial respiratory chain reaction and by monoamine
oxidase (13,14). ROS are involved in metabolism and
signal transduction, which are important in normal physiology.
Under normal circumstances, the body removes reactive oxygen free
radicals to maintain ROS production and clearance in a dynamic
equilibrium. Certain antioxidants, such as vitamin E, vitamin C,
trace elements (including selenium and zinc), SOD and catalase are
utilized for ROS clearance (15,16).
SOD is an active protease containing metallic
elements, which is present in the cytosol and mitochondria of
eukaryotic and prokaryotic cells (17,18).
SOD is an important in vivo antioxidant enzyme, which
catalyzes the disproportionation reaction of superoxide anion to
H2O2 and oxygen. To a certain extent, SOD
activity may reflect the degree of oxidative damage to tissue cells
and the body’s ability to scavenge free radicals.
GSH is the main source of the sulfhydryl group in
the majority of living cells and is important in maintaining the
thiol redox state of the sulfhydryl group in proteins (19). In addition, GSH is a key
antioxidant in animal cells. Several studies have found that a
reduction in GSH levels is an early signal of apoptosis (20,21).
In addition to directly scavenging oxygen free radicals, GSH also
participates in the myeloperoxidase-derived oxidase reaction to
reduce the generation of ROS.
In the present study, SOD activity was significantly
increased in the group treated with piperine compared with the
group treated with H2O2 (P<0.05).
Furthermore, the GSH levels were reduced in the piperine group
compared with the H2O2 group (P<0.05).
Thus, piperine may increase the activity of SOD and enhance the
activity of antioxidant enzymes to reduce oxidative stress damage
to cells.
MDA is a lipid peroxide, which is produced by
peroxidation of polyunsaturated fatty acids and induced cell damage
(22). In oxidative injury
reactions, ROS and macromolecules in the biofilm, including the
side chains of polyunsaturated fatty acids and nucleic acids, react
to form lipidperoxidation products by lipid peroxidation. The
decomposition products of lipid peroxidation may result in cell
damage, therefore MDA levels may reflect the extent of lipid
peroxidation in the body (23).
Simultaneously, MDA levels may also indicate the degree of
oxidative damage to cells. The results suggest that piperine
effectively reduces oxygen radicals and decreases lipid
peroxidation by reducing the attack of polyunsaturated fatty acids
by oxygen free radicals (24).
Mitochondria are intracellular organelles where
oxidative phosphorylation and ATP synthesis are performed.
Mitochondria are semi-autonomous organelles that contain their own
genetic machinery and their own genome. Mitochondrial DNA (mtDNA)
has a closed circular double-strand structure. Mitochondria are
important producers of oxygen free radicals, and mtDNA is
vulnerable to oxidative damage and susceptible to mutations as it
lacks histone protection and does not have effective repair
mechanisms (25) in an environment
with high levels of ROS. In addition, when oxidative injury occurs,
lipid peroxidation occurs. ROS and polyunsaturated fatty acids
react to produce lipid peroxides. The fluidity and permeability of
the membrane may change, eventually resulting in changes in cell
structure and function. When cells are damaged by
H2O2, mitochondrial mtDNA levels undergo a
compensatory increase. Piperine can enhance antioxidant enzyme
activity, reduce the generation of oxygen free radicals and the
oxidative damage of lipid peroxidation to the mitochondrial
membrane. Thus, the function of mitochondrial oxidative
phosphorylation is protected during the process of oxidative
damage.
When oxidative damage was observed to occur,
coordination activity between mitochondria-regulating genes and
mtDNA was revealed to be impaired, and the oxidative
phosphorylation and mitochondrial ATP synthesis abilities were
damaged (26). The mtDNA
compensatorily increased resulting in the upregulation of
mitochondrial mRNA levels (27,28).
Additionally, accumulated ROS exert a direct effect on
mitochondria, which reduces the membrane potential. When the
mitochondrial membrane potential reaches below a threshold value,
cell apoptosis is induced (29).
In the present study, mitochondrial mtDNA in primary cells was
quantitatively analyzed, and the results demonstrated that mRNA
expression levels were significantly reduced in the piperine group
and the normal group, compared with the H2O2
group (P<0.05). However, no significant differences were
identified between the piperine group and the control group
(P>0.05). The results revealed that when cells were damaged by
H2O2, mitochondrial copies of mtDNA increased
due to functional compensation. Piperine was found to enhance the
activity of antioxidant enzymes, inhibit the production of oxygen
free radicals, reduce the lipid peroxidation damage on the
mitochondrial membrane and partially protect from mitochondrial
oxidative phosphorylation.
Ca2+ is a key second messenger in
regulating signaling pathways, which is also involved in the
activation of enzymes that decompose a variety of proteins,
phospholipids and nucleic acids (30). In normal circumstances,
Ca2+ enters cells through intracellular calcium
channels, proteins located and embedded in the cell membrane.
Calcium homeostasis is co-manipulated by
Ca2+-translocating enzymes in the plasma membrane and
intracellular calcium pool. However, when cells are damaged by
oxidative injury, a large quantity of oxygen free radicals are
generated (31,32). Lipid peroxidation is induced by
oxygen radicals and membrane polyunsaturated fatty acids, which may
disrupt the structure of cell membranes. In addition, ATP synthesis
is impaired by oxygen free radicals due to mitochondrial damage. In
the presence of ROS, activities of the ATP-dependent Na+
and Ca2+ pumps in the cell membrane were found to be
reduced, the concentration of intracellular Na+ was
increased, the rate of Na+-Ca2+ exchanges was
enhanced, and Ca2+ influx was increased (33). In the present study, the
concentration of intracellular calcium ions in the
H2O2 group was significantly higher than in
the piperine or the normal groups (P<0.01), which demonstrated
that the membrane structures of primary atrial cells were damaged
by oxidative injury, and that the accumulation of uncleared oxygen
free radicals results in abnormalities of
Na+-Ca2+ exchange in the cell membrane. Thus,
intracellular calcium became elevated.
H2O2-induced oxidative damage
may increase the production of oxygen free radicals, reduce the
activity of antioxidant enzymes, and promote the peroxidation of
polyunsaturated lipid acids and oxidative phosphorylation in
mitochondria in primary atrial cells. In the present study, the
results demonstrated that piperine extract from Large leaf moss,
effectively reduced intracellular accumulation of ROS, decreased
damage to cells resulting from lipid peroxidation, protected
against mitochondrial oxidative phosphorylation and reduced
oxidative injury to mitochondria. Piperine effectively enhanced the
activity of antioxidant enzymes, reduced oxygen free radicals,
enhanced oxygen radical scavenging and reduced calcium overload
damage to cells. Piperine also reduced the effect of oxidative
injury on cell viability. In conclusion, piperine exerted a
protective function to relieve the oxidative damage to primary
atrial myocytes, and has the potential to be used in future to
create therapies for oxidative stress injuries.
Acknowledgements
This study was supported by the National Natural
Science Fund (project no. 30772886).
References
|
1
|
Hu Y, Guo DH, Liu P, et al: Antioxidant
effects of a Rhodobryum roseum extract and its active
components in isoproterenol-induced myocardial injury in rats and
cardiac myocytes against oxidative stress-triggered damage.
Pharmazie. 64:53–57. 2009.
|
|
2
|
Wang B, Liu P, Shen YM and Dai C: Studies
on the chemical constituents from herb of Rhodobryum roseum.
Zhongguo Zhong Yao Za Zhi. 30:895–897. 2005.(In Chinese).
|
|
3
|
Dai C, Liu P, Liu C, Wang B and Chen RY:
Studies on chemical constituents from moss Rhodobryum roseum
II. Zhongguo Zhong Yao Za Zhi. 31:1080–1082. 2006.(In Chinese).
|
|
4
|
Pinho RA, Araujo MC, Ghisi GL and Benetti
M: Coronary heart disease, physical exercise and oxidative stress.
Arq Bras Cardiol. 94:549–555. 2010.(In Portuguese).
|
|
5
|
Piloni NE, Fermandez V, Videla LA and
Puntarulo S: Acute iron overload and oxidative stress in brain.
Toxicology. 314:174–182. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mancuso C, Barone E, Guido P, et al:
Inhibition of lipid peroxidation and protein oxidation by
endogenous and exogenous antioxidants in rat brain microsomes in
vitro. Neurosci Lett. 518:101–105. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Van Wagoner DR: Oxidative stress and
inflammation in atrial fibrillation: role in pathogenesis and
potential as a therapeutic target. J Cardiovasc Pharmacol.
52:306–313. 2008.PubMed/NCBI
|
|
8
|
Kalpana KB, Srinivasan M and Menon VP:
Evaluation of antioxidant activity of hesperidin and its protective
effect on H2O2 induced oxidative damage on
pBR322 DNA and RBC cellular membrane. Mol Cell Biochem. 323:21–29.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival: application to proliferation and
cytotoxicity assays. J Immunol Methods. 65:55–63. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang XL, Ye D, Peterson TE, et al:
Caveolae targeting and regulation of large conductance
Ca(2+)-activated K+ channels in vascular endothelial
cells. J Biol Chem. 280:11656–11664. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nagai H, Minatoguchi S, Chen XH, et al:
Cilnidipine, an N+L-type dihydropyridine Ca channel blocker,
suppresses the occurrence of ischemia/reperfusion arrhythmia in a
rabbit model of myocardial infarction. Hypertens Res. 28:361–368.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang XP, Liu XD and Deng CQ: Effects of
the combination of active component extracts from Astragalus
membranaceus and Panax notoginseng on apoptosis, reactive oxygen
species and mitochondrial membrane potential of PC12 cells with
oxidative injury. Zhong Xi Yi Jie He Xue Bao. 10:1127–1134.
2012.(In Chinese).
|
|
13
|
Sato H, Takahashi T, Sumitani K, Takatsu H
and Urano S: Glucocorticoid generates ROS to induce oxidative
injury in the hippocampus, leading to impairment of cognitive
function of rats. J Clin Biochem Nutr. 47:224–232. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim J, Seok YM, Jung KJ and Park KM:
Reactive oxygen species/oxidative stress contributes to progression
of kidney fibrosis following transient ischemic injury in mice. Am
J Physiol Renal Physiol. 297:F461–F470. 2009. View Article : Google Scholar
|
|
15
|
Xu J, Duan X, Yang J, Beeching JR and
Zhang P: Enhanced reactive oxygen species scavenging by
overproduction of superoxide dismutase and catalase delays
postharvest physiological deterioration of cassava storage roots.
Plant Physiol. 161:1517–1528. 2013. View Article : Google Scholar
|
|
16
|
Chen WJ, Huang YT, Wu ML, et al: Induction
of apoptosis by vitamin D2, ergocalciferol, via reactive oxygen
species generation, glutathione depletion, and caspase activation
in human leukemia cells. J Agric Food Chem. 56:2996–3005. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ueda Y, Uehara N, Sasaki H, Kobayashi K
and Yamakawa T: Impacts of acute ozone stress on superoxide
dismutase (SOD) expression and reactive oxygen species (ROS)
formation in rice leaves. Plant Physiol Biochem. 70:396–402. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gac M, Bigda J and Vahlenkamp TW:
Increased mitochondrial superoxide dismutase expression and lowered
production of reactive oxygen species during rotavirus infection.
Virology. 404:293–303. 2010. View Article : Google Scholar
|
|
19
|
Quintana-Cabrera R, Fernandez-Fernandez S,
Bobo-Jimenez V, et al: Gamma-glutamylcysteine detoxifies reactive
oxygen species by acting as glutathione peroxidase-1 cofactor. Nat
Commun. 3:7182012. View Article : Google Scholar
|
|
20
|
Park SH and Yu IJ: Effect of dibutyryl
cyclic adenosine monophosphate on reactive oxygen species and
glutathione of porcine oocytes, apoptosis of cumulus cells, and
embryonic development. Zygote. 21:305–313. 2013. View Article : Google Scholar
|
|
21
|
Franco R, Panayiotidis MI and Cidlowski
JA: Glutathione depletion is necessary for apoptosis in lymphoid
cells independent of reactive oxygen species formation. J Biol
Chem. 282:30452–30465. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cheng J, Wang F, Yu DF, Wu PF and Chen JG:
The cytotoxic mechanism of malondialdehyde and protective effect of
carnosine via protein cross-linking/mitochondrial
dysfunction/reactive oxygen species/MAPK pathway in neurons. Eur J
Pharmacol. 650:184–194. 2011. View Article : Google Scholar
|
|
23
|
Spirlandeli AL, Deminice R and Jordao AA:
Plasma malondialdehyde as biomarker of lipid peroxidation: effects
of acute exercise. Int J Sports Med. 35:14–18. 2013. View Article : Google Scholar
|
|
24
|
Feng Z, Hu W, Marnett LJ and Tang MS:
Malondialdehyde, a major endogenous lipid peroxidation product,
sensitizes human cells to UV- and BPDE-induced killing and
mutagenesis through inhibition of nucleotide excision repair. Mutat
Res. 601:125–136. 2006. View Article : Google Scholar
|
|
25
|
Pan JS, He SZ, Xu HZ, et al: Oxidative
stress disturbs energy metabolism of mitochondria in
ethanol-induced gastric mucosa injury. World J Gastroenterol.
14:5857–5867. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kang D and Hamasaki N: Mitochondrial
oxidative stress and mitochondrial DNA. Clin Chem Lan Med.
41:1281–1288. 2003.PubMed/NCBI
|
|
27
|
Kuwahara H, Shimazaki M, Kadoya Y, et al:
Immunohistochemical localization of two types of carbonic anhydrase
isozymes in oncocytoma and oncocytic epithelial cells. Osaka City
Med J. 35:121–136. 1989.
|
|
28
|
Richardson TE, Yu AE, Wen Y, Yang SH and
Simpkins JW: Estrogen prevents oxidative damage to the mitochondria
in Friedreich’s ataxia skin fibroblasts. PLoS One.
7:e346002012.PubMed/NCBI
|
|
29
|
Higuchi Y: Glutathione depletion-induced
chromosomal DNA fragmentation associated with apoptosis and
necrosis. J Cell Mol Med. 8:455–464. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jin C, Wu J, Watanabe M, Okada T and
Iesaki T: Mitochondrial K+ channels are involved in
ischemic postconditioning in rat hearts. J Physiol Sci. 62:325–332.
2012.
|
|
31
|
Belyaeva EA, Korotkov SM and Saris NE:
In vitro modulation of heavy metal-induced rat liver
mitochondria dysfunction: a comparison of copper and mercury with
cadmium. J Trace Elem Med Biol. 25(Suppl 1): S63–S73. 2011.
View Article : Google Scholar
|
|
32
|
Jomova K and Valko M: Advances in
metal-induced oxidative stress and human disease. Toxicology.
283:65–87. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Badziuk OB, Mazur I and Kosterin SO:
Regulation of the mitochondrial ATP-sensitive potassium channel in
rat uterus cells by ROS. Ukr Biokhim Zh. 83:48–57. 2011.(In
Ukrainian).
|