Introduction
Diabetic nephropathy is one of the leading causes of
end-stage renal failure (1). The
inactivation of endothelial nitric oxide synthase (eNOS) has been
considered to be a recently discovered mechanism of diabetic
nephropathy (2,3), which severely impairs glomerular
endothelial cell nitric oxide (NO) production and causes glomerular
endothelial cell dysfunction, which are impairments that contribute
to the development of diabetic nephropathy (2,4).
However, the specific mechanism that causes eNOS inactivation
remains elusive.
The nuclear factor (NF)-κB signaling pathway is a
major pathway of chronic inflammation. Previously, it was
demonstrated that the NF-κB signaling pathway regulates the eNOS
activity of aortic endothelial cells (5). Studies have also revealed that NF-κB
signaling pathways in the diabetic kidney are abnormally activated
and NF-κB signaling pathway inhibitors may significantly reduce the
damage to the kidneys in diabetic rats (6,7).
Therefore, the NF-κB signaling pathway may participate in the
development of diabetic nephropathy by regulating the eNOS activity
of glomerular endothelial cells.
Human glucagon-like peptide-1 (GLP-1) analogues,
such as liraglutide, have been demonstrated to elicit protective
effects in diabetic vascular complications (4), thus suggesting that liraglutide
exhibits an endothelial cell function. Other investigations have
also demonstrated that the protective effect of liraglutide on
vascular systems involves the reduction of chronic inflammation
(7,8). These studies have indicated that
liraglutide may be involved in eNOS activity by alleviating chronic
inflammation.
In the present study, the effect of liraglutide on
diabetic nephropathy was examined. The correlation between eNOS
activity and the NF-κB chronic inflammation pathway was also
determined in rats with diabetic nephropathy.
Materials and methods
Animals
A total of 45 seven-week-old male Wistar rats were
purchased from Tianjin Radiology Institution (Tianjin, China) and
provided with a standard diet and water. The animals were kept
under natural circadian light/dark cycles under conditions of
18–22°C and 40–70% humidity. This study was conducted in strict
accordance with the recommendations in the Guide for the Care and
Use of Laboratory Animals of the National Institutes of Health. The
animal use protocol was reviewed and approved by the Institutional
Animal Care and Use Committee (IACUC) of Tianjin Medical
University. Diabetes was induced by injecting streptozotocin (STZ;
Sigma-Aldrich, St. Louis, MO, USA) in 0.1 mol/l citrate buffer pH
4.5 at a dose of 45 mg/kg body weight. The rats that fasted but
revealed blood glucose levels >16.7 mmol/l were considered
diabetic. The rats injected with citrate buffer alone were used as
non-diabetic control samples. One week following the induction of
diabetes, half of the diabetic rats were randomly selected and
subcutaneously injected with the GLP-1 analogue liraglutide (Novo
Nordisk Co, Copenhagen, Denmark) at a dose of 0.3 mg/kg/12 h for 12
weeks as previously reported (9,10).
The remaining diabetic rats were injected with phosphate buffer and
used as the control sample. The rats were housed in individual
metabolic cages for the 24-h urine collection on the day prior to
the administration of STZ and at the end of 12-week treatment.
Urine was centrifuged at 800 × g for 10 min at room temperature.
Whole urine was stored at −80°C until use. At the end of the
12-week treatment, the rats were sacrificed and blood was collected
from the left ventricle. Blood glucose (BG), total cholesterol
(TC), triglycerides (TG), serum creatinine (Scr) and blood urea
nitrogen (BUN) as well as microalbuminuria were determined using an
automatic biochemical analyzer (7070; Hitachi Ltd., Tokyo, Japan).
The kidneys were immediately excised and stored at −80°C until
use.
Histopathological examination
The rats from each group were sacrificed at the end
of the 12-week treatment by anaesthetising them with diethyl ether.
The kidneys were excised to evaluate the histopathological changes.
The procedures were performed as described previously (11). Briefly, the kidneys were carefully
excised and then washed with phosphate-buffered saline (PBS).
Following this, the kidneys were stored in 10% neutral buffered
formalin and embedded in paraffin. Four micron sections were cut
and stained with hematoxylin and eosin (H&E; Tianjin Runtai,
Co., Ltd., Tianjin, China) and periodic acid Schiff base (PAS;
Tianjin Runtai, Co., Ltd.) for histopathological observations using
a HMIAS-2000 Image Analysis system (Guangzhou Longest Technology,
Guangzhou, China). The sections were then analyzed for the degree
of tubular and glomerular damage. Glomerular damage index (GDI) was
calculated from 0 to 4 on the basis of the degree of
glomerulosclerosis, mesangiolysis and mesangial expansion.
Approximately 80–100 glomerular cells from the renal cortex were
observed for each section. GDI was obtained by averaging the scores
from the counted glomerular cells.
Determination of inflammatory cytokine
levels in rat kidneys
Renal tissue was homogenized in 10 mM Tris-HCl
buffer solution (pH 7.4) containing 2 M NaCl, 1 mM EDTA, 0.01%
Tween-80 and 1 mM phenylmethanesulfonyl fluoride (PMSF), and
centrifuged at 9,000 × g for 30 min at 4°C. The resulting
supernatant was used for cytokine determination. A Cytometric Bead
array (CBA; BD Biosciences, Franklin Lakes, NJ, USA) mouse
inflammation kit was used to determine the levels of interleukin
(IL)-6, IL-10, IL-12p70, interferon-γ (IFN-γ), tumor necrosis
factor-α (TNF-α) and monocyte chemotactic protein 1 (MCP-1)
according to the manufacturer’s instructions (12). The data were acquired on a
FACSCalibur flow cytometer (BD Biosciences) and analyzed using FCAP
Array software version 3.0 (BD Biosciences).
Quantitative polymerase chain reaction
(qPCR)
Total RNA from rat kidneys was extracted using
TRIzol reagent (Life Technologies Co., Carlsbad, CA, USA) according
to the manufacturer’s instructions. Approximately 2 μg of RNA was
reverse transcribed into cDNA by using Moloney murine leukemia
virus reverse transcriptase (Invitrogen Life Technologies). The
primers were designed using Primer 5.0 software (Premier Biosoft
International, Palo Alto, CA, USA) and primers were as follows:
NF-κB, forward 5′-GTATGGCTTCCCGCACTATGG-3′ and reverse
5′-TCGTCACTCTTGGCACAATCTC-3′; eNOS forward
5′-GACATTGAGAGCAAAGGGCTGC-3′ and reverse 5′-CGGCTTGTCACCTCCTGG-3′;
β-actin forward 5′-TGGAGAAGAGCTATGAGCTGCCTG-3′ and reverse
5′-GTGCCACCAGACAGCACTGTGTTG-3′. The reaction conditions were set as
follows: 95°C for 2 min, 40 cycles at 95°C for 30 sec, then 56°C
for 30 sec and 72°C for 30 sec. Melting curve analysis was
performed from 55–95°C by monitoring fluorescence at a 0.5°C
increase with 30 sec intervals. The sample measurements were
performed in triplicate. For each experimental sample, the target
mRNA levels were normalized to β-actin mRNA.
Western blot analysis
Total protein from rat kidneys was prepared by
lysing buffer containing protease inhibitors (20 mM Tris, pH 7.4;
150 mM NaCl; 1 mM EDTA; 1 mM PMSF; 1 mM orthovanadate; 1 μg/ml
leupeptin; 10 μg/ml aprotinin). Following homogenization and
centrifugation, the amount of protein in the supernatant was
determined with a bicinchoninic acid protein assay kit (Pierce
Biotechnology, Inc., Chicago, IL, USA). The aliquots of extract (35
μg of protein/lane) from each sample were loaded on SDS-PAGE using
a 10% Tris-glycine gel, and then transferred onto a polyvinylidene
difluoride (PVDF) membrane at 30 V for 2 h. The membrane was
blocked for 1 h at room temperature with 5% skimmed milk and
incubated for 1 h at room temperature with the following primary
antibodies: Anti-NF-κB (Cell Signaling Technology, Inc., Danvers,
MA, USA), anti-p65 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA), anti-p-eNOS (Ser 1177; Cell Signaling Technology, Inc.) and
anti-eNOS (Cell Signaling Technology, Inc.). The membranes were
washed and then the secondary antibody was added and incubated for
1 h at room temperature. Peroxidase activity on the PVDF membranes
was visualized on an X-ray film with an ultraviolet transmission
analyzer. Protein levels were normalized to β-actin.
Measurement of glomerular eNOS activity
and NO levels
An eNOS activity classifying kit and an NO test kit
(Nanjing Jiancheng Bioengineering Institute, Nanjing, China) were
used to detect the eNOS and NO levels in the glomeruli,
respectively. The procedures were performed as described previously
(4).
Statistical analysis
Statistical analyses were conducted using SPSS 15.0
software (SPSS, Inc., Chicago, IL, USA). All values are expressed
as the mean ± standard deviation. The significance of differences
among the experimental groups was determined by analysis of
variance. P<0.05 was considered to indicate a statistically
significant difference.
Results
Rats’ body weights, blood pressure and
biochemical parameters
Rats’ body weights, blood pressure and biochemical
parameters were evaluated prior to and following 12 weeks of
administration of liraglutide (0.3 mg/kg/12 h). The body weight,
blood pressure and biochemical parameters of rats are summarized in
Table I. No differences were
observed in body weight, systolic blood pressure, BG, TG and TC in
each group at baseline (P>0.05). Following liraglutide treatment
for 12 weeks, systolic blood pressure, TG and TC of both diabetic
groups were significantly higher than those of the normal control
group. Liraglutide was able to significantly reduce TC (P<0.05),
and may decrease systolic blood pressure (P=0.089) and TG
(P=0.065). Compared with the NC group, the body weights were
decreased in the diabetes and liraglutide groups (P<0.01), while
being increased in liraglutide treatment group as compared with the
DM group (P<0.05). No significant difference was observed in the
BG between the DM and liraglutide groups (P>0.05).
 | Table IBody weight, blood pressure and
biochemical parameters of the experimental rats. |
Table I
Body weight, blood pressure and
biochemical parameters of the experimental rats.
| Baseline | 12 weeks |
|---|
|
|
|
|---|
| Body weight (g) | Systolic blood
pressure (mmHg) | Blood glucose
(mmol/l) | TG (mmol/l) | TC (mmol/l) | Body weight (g) | Systolic blood
pressure (mmHg) | Blood glucose
(mmol/l) | TG (mmol/l) | TC (mmol/l) |
|---|
| NC | 254.3±6.04 | 101.6±5.69 | 6.15±0.39 | 1.06±0.13 | 1.22±0.16 | 466.2±11.94 | 103.7±3.54 | 6.435±0.20 | 1.09±0.14 | 1.32±0.14 |
| DM | 256.8±9.62 | 95.4±4.82 | 6.05±0.32 | 0.91±0.07 | 1.21±0.16 | 243.3±6.33b | 128.9±7.97a | 27.39±2.25b | 2.32±0.24b | 2.83±0.25b |
| Lira | 249.5±7.9 | 102.8±5.54 | 6.06±0.28 | 0.99±0.104 | 1.17±0.15 | 330.3±20.3b,c | 117±5.4a | 22.45±1.74a | 1.75±0.15b | 2.15±0.14a,c |
| F-value | 0.2171 | 0.5479 | 0.02699 | 0.5625 | 0.03425 | 61.90 | 4.531 | 44.4 | 11.86 | 16.07 |
Parameters associated with the kidney
function of rats
The parameters associated with kidney function,
including kidney weight index, urine volume, microalbuminuria
(UmA), BUN and Scr, were examined prior to and following
liraglutide treatment for 12 weeks. No significant difference was
observed in all of the parameters in each group at the baseline.
Kidney weight index, urine volume, UmA, BUN and Scr significantly
increased in the diabetic rats with or without liraglutide compared
with those of the normal control rats (P<0.05 or P<0.01).
Liraglutide significantly decreased the urine volume and levels of
UmA, BUN and Scr. Kidney weight index in the liraglutide group was
higher than that in the DM group (Table II).
 | Table IIParameters associated with rat kidney
function in each group. |
Table II
Parameters associated with rat kidney
function in each group.
| Baseline | 12 weeks |
|---|
|
|
|
|---|
| Kidney weight index
(mg/g) | Urine (ml) | UmA (μg/24 h) | BUN (mmol/l) | Scr (μmol/l) | Kidney weight index
(mg/g) | Urine (ml) | UmA (μg/24 h) | BUN (mmol/l) | Scr (μmol/l) |
|---|
| NC | 2.72±0.18 | 14.7±1.48 | 39.7±1.89 | 5.54±0.17 | 53.83±3.03 | 2.40±0.14 | 10.47±0.92 | 43.55±3.15 | 5.68±0.26 | 53.9±2.82c |
| DM | 2.74±0.15 | 10.7±1.28 | 41.5±2.14 | 5.51±0.196 | 52.91±2.07 | 3.36±0.20b | 79.48±3.75 | 1092±101.1 | 10.31±0.52b | 77.33±2.21c |
| Lira | 2.82±0.14 | 10.5±1.18 | 39.3±0.94 | 5.578±0.195 | 52.94±2.54 | 3.91±0.21b | 76.27±4.37 | 663.1±60.8 | 6.38±0.37b,d | 66.21±2.81c |
| F value | 0.1484 | 0.1208 | 0.2916 | 0.03151 | 0.04096 | 16.98 | 133.8 | 60.15 | 39.37 | 21.92 |
Histological changes in diabetic rat
kidneys
Following liraglutide was administered for 12 weeks,
the histological changes in the kidneys were evaluated by H&E
and PAS staining, respectively. Fig.
1 illustrates the changes in the kidney of the rats in all of
the groups. The diabetic rats demonstrated several changes,
including inflammatory cell infiltration, mesangial expansion and
proliferation in the glomeruli. Prominent nodular
glomerulosclerosis with glomerular basement membrane thickening was
also observed. However, minimal changes were observed in the
diabetic rats that were supplemented with liraglutide. The
semiquantitative analysis demonstrated that liraglutide
significantly decreased the GDI of diabetic rats.
Effects of liraglutide on NF-κB (p65) and
eNOS expression
The mRNA expression of NF-κB and eNOS were measured
following the 12-week treatment with or without liraglutide. The
results demonstrated that the mRNA levels in diabetic kidneys
increased (P<0.01). Liraglutide inhibited this increase
(P<0.05; Fig. 2A). No
significant difference was observed in the mRNA levels of eNOS
between each group (P>0.05; Fig.
2B).
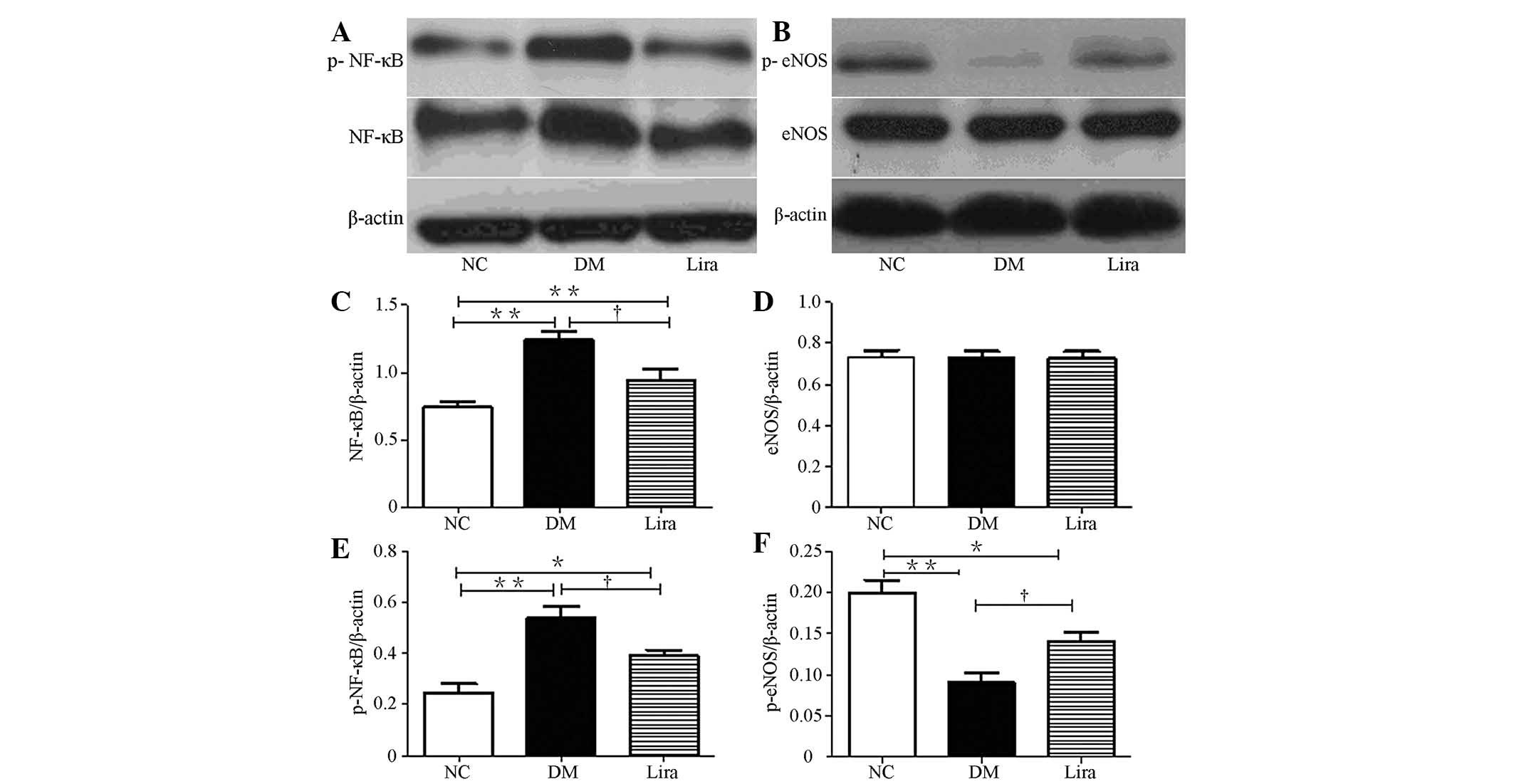
The effects of liraglutide on the protein levels of
NF-κB and eNOS in the diabetic rat kidneys was also examined. The
expression levels of NF-κB and p-NF-κB (p65) in diabetic groups
were higher than those in the normal control group
(P<0.01). Liraglutide reduced this increase
(P<0.01; Figs. 3A, C and
E). No significant difference was observed between each group
in terms of the expression levels of eNOS, but eNOS (ser 1177)
phosphorylation was reduced in both diabetic groups (Figs. 3B and D). Liraglutide significantly
restored eNOS (ser 1177) phosphorylation (Figs. 3B, D and F).
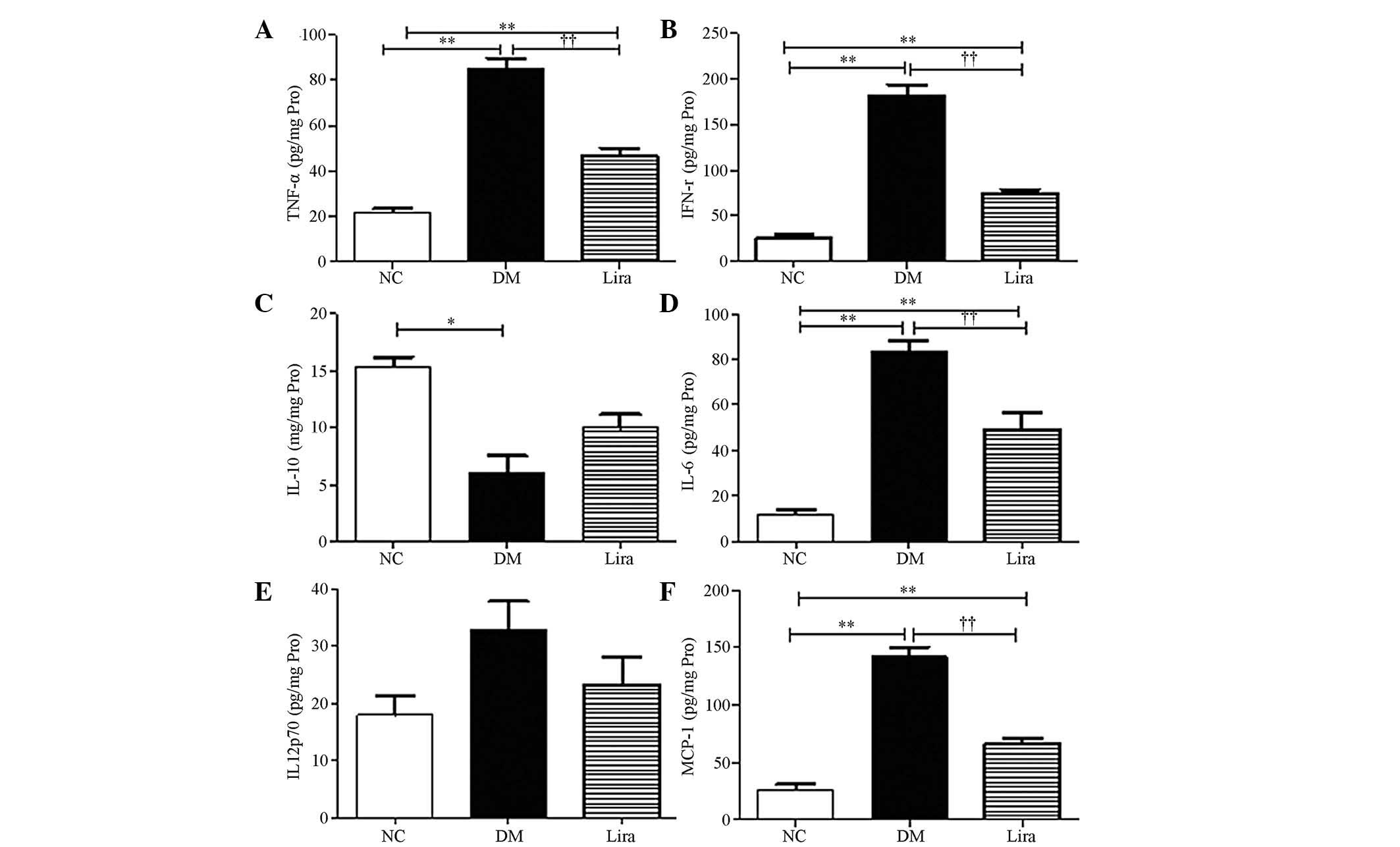
Effect of liraglutide on the levels of
inflammatory cytokines
NF-κB could regulate inflammatory cytokine
expression. In the present study, the levels of inflammatory
cytokines of diabetic kidneys were further examined using the CBA
flow cytometry kit. The results demonstrated that TFN-α, IFN-γ,
IL-6 and MCP-1 increased significantly in the diabetic kidneys
(P<0.01 or P<0.05) and liraglutide was able to inhibit these
effects (P<0.01 or P<0.05; Figs.
4A, B, D and F). A decrease in IL-10, which is an
anti-inflammatory cytokine in diabetic kidneys, was noted. An
increasing trend was observed in the liraglutide treatment group,
but this increase was not statistically significant (Fig. 4C). No significant difference was
noted in the levels of IL-12p70 between the groups (P>0.05;
Fig. 4E).
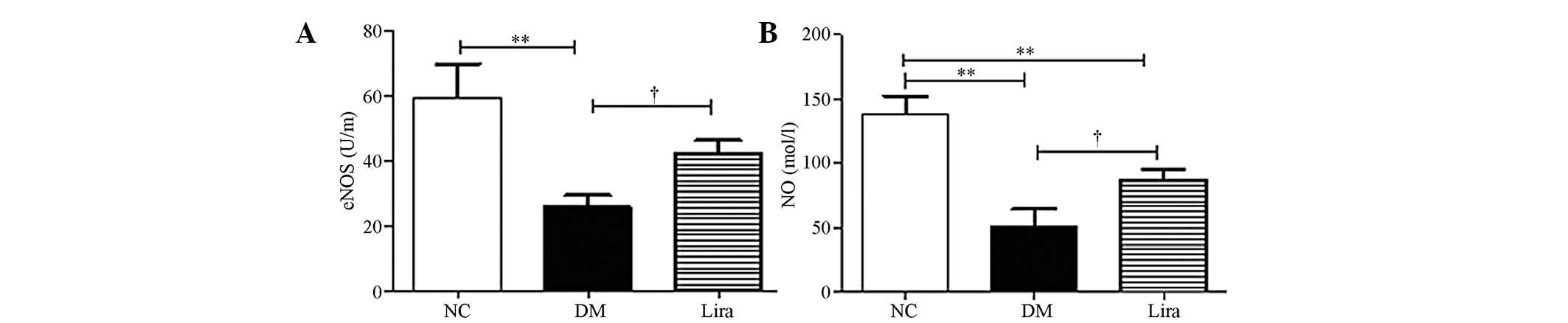
Liraglutide improves eNOS activity and NO
production
eNOS activity and glomerular NO synthesis were
examined. Fig. 5 demonstrated that
eNOS activity and NO production were decreased significantly in
both diabetic groups. Liraglutide restored the eNOS activity
(P<0.01 or P<0.05) and NO production (P<0.01; Fig. 5A and B).
Discussion
Liraglutide, a GLP-1 analogue, is a novel
prospective medication for Type 2 diabetes mellitus. Liraglutide
also facilitates the maintenance of glucose homeostasis by
stimulating insulin secretion and inhibiting glucagon release in a
glucose-dependent manner (13).
Accumulating evidence indicates that GLP-1 analogues ameliorate
diabetic nephropathy in mice and humans (6,9,14);
however, the underlying mechanism has remained elusive. In the
present study, liraglutide activated eNOS activity in diabetic
kidneys via downregulation of the NF-κB signaling pathway and
reduction of chronic inflammation in diabetic rats. Therefore,
liraglutide exhibits a protective function in diabetic kidneys. The
results of the present study revealed that liraglutide
significantly decreased inflammatory cell infiltration in the
intestines, alleviated the damage of the glomerular filter barrier
in diabetic rats and reduced urine volume, BUN, Scr and 24-h urine
microalbumin levels. This result indicated that liraglutide elicits
a protective effect on tissues against diabetic nephropathy in
rats. The present study also identified that liraglutide
significantly delayed hyperfiltration as indicated by the kidney
weight index and reduced urinary protein excretion in diabetic rats
(Table II). However, no
significant difference was observed in the BG levels among the
groups, which is consistent with results of previous studies
(9,10). The results further revealed that
the protective effect of liraglutide against diabetic nephropathy
in rats was independent of the glucose-lowering effect, but may be
associated with the anti-inflammatory effect.
To identify the potential mechanism, the effect of
liraglutide on the expression and activity of eNOS in the kidneys
of diabetic rats was further assessed. No significant decrease was
observed in the eNOS expression in mRNA and protein levels in the
kidneys of diabetic rats. By comparison, eNOS phosphorylation on
serine 1177 was increased, which is a process that is the key
phosphorylation involved in eNOS activation (15). The decreased activity of eNOS in
glomerular endothelial cells is the leading cause of NO secretion
disorder (2). The present study
also determined the NO production and the results demonstrated that
liraglutide restored NO production. These results indicated that
the dysfunction of eNOS in DN mainly occurred due to decreases in
eNOS phosphorylation, further confirming the conclusions presented
in previous studies (2,3). Liraglutide also contributed to the
pathogenesis of DN by increasing eNOS phosphorylation on serine
1177 and promoting NO production. Therefore, eNOS is a potentially
new target for the treatment of diabetic nephropathy. Further
studies are required to determine the mechanism by which eNOS
activity is enhanced.
The present study further investigated the potential
mechanism by which liraglutide improves eNOS activity. In this type
of activity, chronic inflammation is important in the process of
DN. Previous studies demonstrated that liraglutide suppressed
inflammatory cytokine expression in diabetic patients (7,16).
This evidently indicated that liraglutide activates eNOS by
downregulating chronic inflammatory pathways. NF-κB is one of the
key molecules that regulate chronic inflammation. The activation of
NF-κB and the transcription of certain pro-inflammatory chemokines,
including MCP-1, TNF-α and intracellular adhesion molecule 1, are
the markers of progressive diabetic nephropathy in patients with
type 2 diabetes (17). By
comparison, the inhibition of NF-κB activity reduces the damage of
diabetic nephropathy (6). These
studies have markedly suggested that NF-κB participates in the
pathogenesis of diabetic nephropathy. The results also demonstrated
that p65 expression and phosphorylation on serine 276 were
increased in the kidneys of diabetic rats, which is an increase
that is essential for NF-κB p65-dependent cellular responses
(18). The detection of NF-κB and
the phosphorylated p65 subunit of NF-κB is an effective method for
examining NF-κB activation (19,20).
The levels of inflammatory cytokines downstream of NF-κB were
further investigated in the present study. The results revealed
that MCP-1, TNF-α and IFN-γ were overexpressed in the kidneys of
diabetic rats, which is a result that is consistent with that
demonstrated in previous studies (17,21).
Liraglutide inhibited the increases in inflammatory cytokines,
including MCP-1, TNF-α, IFN-γ and IL-6. Furthermore, it also
restored the anti-inflammatory cytokine IL-10 levels; however, this
difference was not statistically significant. The results indicated
that the NF-κB signaling pathway is activated in diabetic
nephropathy and liraglutide is able to suppress p65 expression and
phosphorylation on serine 276 of NF-κB. Liraglutide may further
decrease the expression of inflammatory cytokines of kidneys in
diabetic rats. Therefore, it is concluded that liraglutide elicits
a protective effect on diabetic nephropathy by downregulating the
NF-κB signaling pathway.
To the best of our knowledge, the present study was
the first to identify that liraglutide activates eNOS activity via
the NF-κB inflammatory pathway and elicits a protective effect on
diabetic kidneys. However, the mechanism by which the NF-κB pathway
regulates eNOS activity in diabetic nephropathy requires further
study.
In conclusion, liraglutide may have a direct
beneficial effect on eNOS activity and diabetic nephropathy by
downregulating NF-κB inflammatory pathway without eliciting a
glucose-lowering effect.
References
|
1
|
Nakagawa T: Is endothelial dysfunction
more deleterious than podocyte injury in diabetic nephropathy?
Kidney Int. 83:1202–1203. 2013. View Article : Google Scholar
|
|
2
|
Nakagawa T, Sato W, Sautin YY, et al:
Uncoupling of vascular endothelial growth factor with nitric oxide
as a mechanism for diabetic vasculopathy. J Am Soc Nephrol.
17:736–745. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nakagawa T, Sato W, Glushakova O, et al:
Diabetic endothelial nitric oxide synthase knockout mice develop
advanced diabetic nephropathy. J Am Soc Nephrol. 18:539–550. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tian S, Gan Y, Li J, et al: Imbalance of
glomerular VEGF-NO axis in diabetic rats: prevention by chronic
therapy with propyl gallate. J Nephrol. 24:499–506. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chiu WC, Chiou TJ and Chiang AN:
β-Glycoprotein I inhibits endothelial cell migration through the
nuclear factor κB signalling pathway and endothelial nitric oxide
synthase activation. Biochem J. 445:125–133. 2012.
|
|
6
|
Kim JE, Lee MH, Nam DH, et al: Celastrol,
an NF-κB inhibitor, improves insulin resistance and attenuates
renal injury in db/db mice. PLoS One. 8:e620682013.
|
|
7
|
Shiraki A, Oyama J, Komoda H, et al: The
glucagon-like peptide 1 analog liraglutide reduces TNF-α-induced
oxidative stress and inflammation in endothelial cells.
Atherosclerosis. 221:375–382. 2012.PubMed/NCBI
|
|
8
|
Parthsarathy V and Hölscher C: The type 2
diabetes drug liraglutide reduces chronic inflammation induced by
irradiation in the mouse brain. Eur J Pharmacol. 700:42–50. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hendarto H, Inoguchi T, Maeda Y, et al:
GLP-1 analog liraglutide protects against oxidative stress and
albuminuria in streptozotocin-induced diabetic rats via protein
kinase A-mediated inhibition of renal NAD(P)H oxidases. Metabolism.
61:1422–1434. 2012. View Article : Google Scholar
|
|
10
|
Sturis J, Gotfredse CF, Rømer J, et al:
GLP-1 derivative liraglutide in rats with β-cell deficiencies:
influence of metabolic state on β-cell mass dynamics. British J
Pharmacol. 140:123–132. 2003.
|
|
11
|
Shiju TM, Rajesh NG and Viswanathan P:
Renoprotective effect of aged garlic extract in
streptozotocin-induced diabetic rats. Indian J Pharmacol. 45:18–23.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hill HR and Martins TB: The flow
cytometric analysis of cytokines using multi-analyte fluorescence
microarray technology. Methods. 38:312–316. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rigato M and Fadini GP: Comparative
effectiveness of liraglutide in the treatment of type 2 diabetes.
Diabetes Metab Syndr Obes. 7:107–120. 2014.PubMed/NCBI
|
|
14
|
Vretenar J, Jindal K and Senior PA: Effect
of liraglutide on metabolic and renal outcomes over 12 months in
community diabetic nephropathy clinics. Can J Diabetes O.
37S4:S252013. View Article : Google Scholar
|
|
15
|
García C, Nuñez-Anita RE, Thebault S, et
al: Requirement of phosphorylatable endothelial nitric oxide
synthase at Ser-1177 for vasoinhibin-mediated inhibition of
endothelial cell migration and proliferation in vitro. Endocrine.
45:263–270. 2013.PubMed/NCBI
|
|
16
|
Parthsarathy V and Hölscher C: The type 2
diabetes drug liraglutide reduces chronic inflammation induced by
irradiation in the mouse brain. Eur J Pharmacol. 700:42–50. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xie X, Lan T, Chang X, et al: Connexin43
mediates NF-κB signalling activation induced by high glucose in
GMCs: involvement of c-Src. Cell Commun Signal.
11:382013.PubMed/NCBI
|
|
18
|
Okazaki T, Sakon S, Sasazuki T, et al:
Phosphorylation of serine 276 is essential for p65 NF-kappaB
subunit-dependent cellular responses. Biochem Biophys Res Commun.
300:807–812. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang M, Liu R, Jia X, Mu S and Xie R:
N-acetyl-seryl-aspartyl-lysyl-proline attenuates renal inflammation
and tubulointerstitial fibrosis in rats. Int J Mol Med. 26:795–801.
2010.PubMed/NCBI
|
|
20
|
Jain SK, Velusamy T, Croad JL, Rains JL
and Bull R: L-cysteine supplementation lowers blood glucose,
glycated hemoglobin, CRP, MCP-1, and oxidative stress and inhibits
NF-kappaB activation in the livers of Zucker diabetic rats. Free
Radical Bio Med. 46:1633–1638. 2009. View Article : Google Scholar
|
|
21
|
Wada J and Makino H: Inflammation and the
pathogenesis of diabetic nephropathy. Clin Sci (Lond). 124:139–152.
2013. View Article : Google Scholar : PubMed/NCBI
|