1. Introduction
The Circadian Locomotor Output Cycles Kaput
(CLOCK) and other genes, including brain and muscle
ARNT-like protein 1 (bmal1), period circadian clock
(per)1/2/3, cryptochrome (cry)1/2,
nuclear receptor subfamily 1, group D, member 1 (NR1D1, also
known as rev-erbα), and differentiated embryonic chondrocyte
gene (dec)1/2, predominantly participate in circadian
rhythm feedback and regulation of clock-controlled genes in a
feed-forward loop (1), accounting
for 99% of the regulation. In humans, the CLOCK gene is
crucial for maintaining a 24-hour circadian rhythm in behavior,
physiology and the endocrine system (2,3). The
CLOCK gene is mainly expressed in specific groups of the
suprachiasmatic nucleus (SCN), the ventromedial hypothalamus,
olfactory bulb, amygdala, lateral habenula, hippocampus and
hypothalamus (4,5). The per2 gene is expressed in
the central and peripheral nervous systems and operates as a
pacemaker of circadian rhythm in the brain of humans and other
mammals, regulating spontaneous activity, metabolism and behavior.
Rhythmic per2 expression is regulated by the SCN, but also,
via negative feedback regulation from the bed nucleus of the stria
terminalis (BNST) and the central nucleus of the amygdala (CeA) in
the limbic system, as well as from corticosterone (6–8).
Per2 is crucial for regulating visceral cyclic rhythmic
activities through the HPA axis of the limbic system (6–8).
Current research has addressed the mechanism of action of PER2 on
target organs, the HPA axis and the limbic system (6–8).
However, it remains poorly understood whether per2 is
involved in regulatory mechanisms of the limbic system on
lower-level brain structures. Moreover, the relationship between
functional areas of the limbic system is still unclear. In this
review, we summarize per2 biological features, what is known
on its participation in limbic system regulation of the HPA axis,
and on its effects on target organs.
2. Biological features of per2
The human per2 gene maps to chromosome 2, at
2q37.3 (gene ID: 8864), and is 51,529 base pairs (bp) long. The
per2 mRNA is 6,342 bp and contains 23 exons (http://www.ncbi.nlm.nih.gov/gene/8864);
its transcription and translation give the PER2 protein, which
contains two PER-ARNT-SIM (PAS) domains (PAS-A and PAS-B) to
mediate homo-mPER interactions and interactions with transcription
factors (9). The per2 gene
is a member of the Period gene family. PER2-immunoreactive (ir)
nuclei reaches the peak in expression at ZT13 (9 p.m.) and down to
the lowest level of expression at ZT1 (9 a.m.) (10).
PER2 is strongly associated with the biological
functions of the CLOCK/BMAL1 complex. BMAL1 and CLOCK are members
of the basic helix-loop-helix/PAS family of transcription factors
(11). The regulation of circadian
periodicity involves an induction of transcription and translation
of per1, per2 and per3 genes by the
CLOCK/BMAL1 complex; these genes inhibit CLOCK/BMAL1-induced
transcription, serving as a determinant of negative regulation
(Fig. 1). However, only
per2 can regulate rhythmic periodicity and its amplitude in
the central and peripheral nervous systems (12,13),
although its regulatory mechanism in the central nervous system is
complex (14,15). Per2-ir neurons receive
neuronal projections from the SCN and the sympathetic nervous
system and are regulated by the neuroendocrine system via
corticosterone, melatonin and adrenaline (14,15).
Per2 is mainly expressed in the oval nucleus of the BNST and
central nucleus of the amygdala (CeA) in the limbic system. These
nuclear groups regulate motion and emotion and exhibit rhythmic
alterations (16,17). Studies of the molecular mechanisms
underlying PER2 functions have shown that cyclic adenosine
monophosphate, protein kinase A and C, and mitogen-activated
protein kinase are involved in the intracellular signaling pathway
regulated by PER2 in the central and peripheral nervous systems.
Moreover, per2 cyclical variation is directly regulated by
the SCN and its lower-level brain structures (Fig. 2) (18–20).
 | Figure 1The Circadian Locomotor Output Cycles
Kaput (CLOCK) gene feedback pathway. The CLOCK/BMAL1 complex
activates transcription of per-, cry-, rora
and rev-erbα genes. PER and CRY proteins inhibit (−) the
transcription of the CLOCK/BMAL1 complex. REV-ERBα inhibits and
RORA promotes (+) bmal1 transcription. Figure reproduced
from references (8,12). BMAL1, brain and muscle ARNT-like
protein 1; PER, period circadian clock; CRY, cryptochrome;
REV-ERBα, nuclear receptor subfamily 1, group D, member 1; and
RORa, retinoic acid-related orphan nuclear receptor a. |
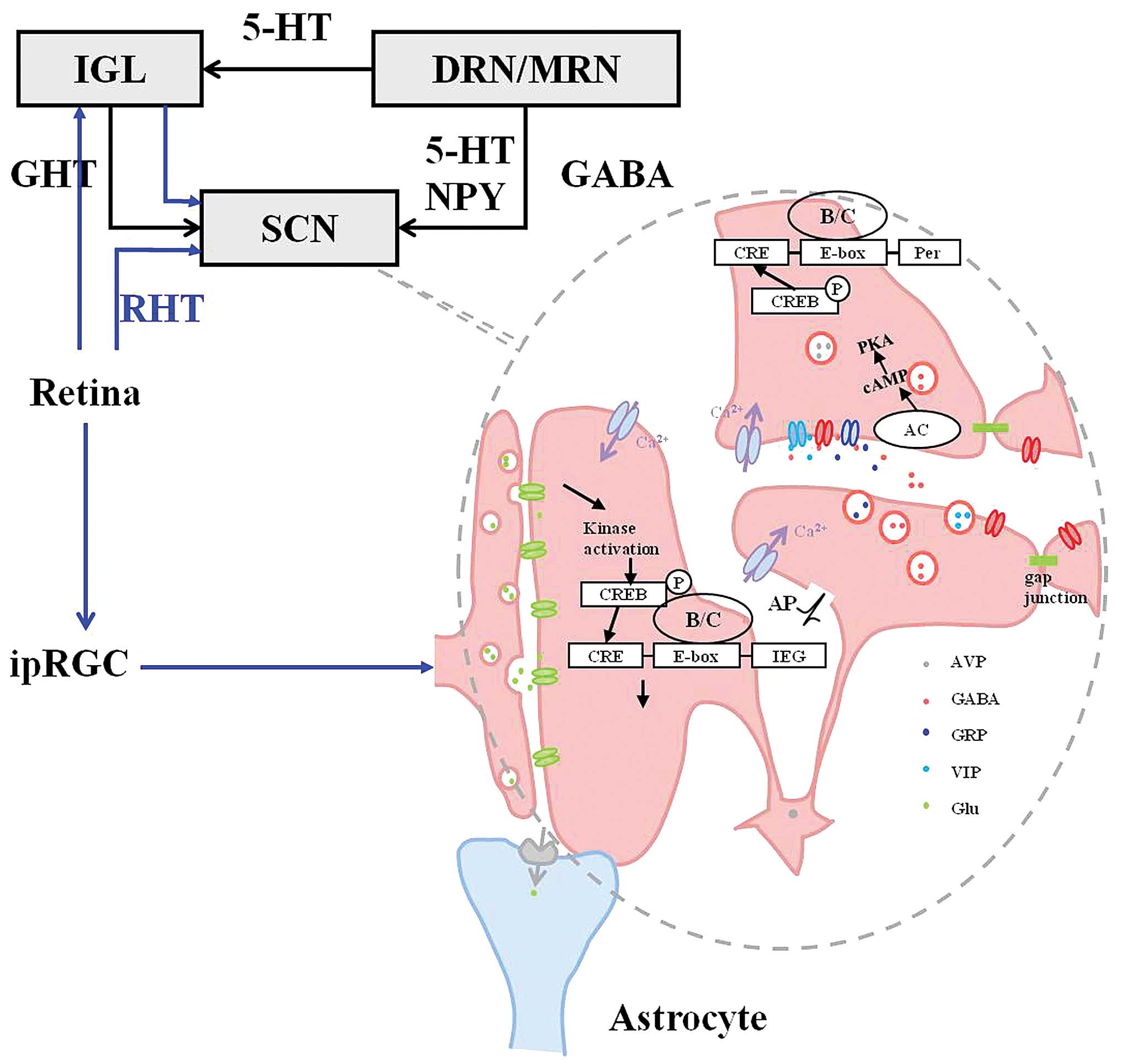 | Figure 2Intracellular mechanism of Circadian
Locomotor Output Cycles Kaput (CLOCK) gene regulation in the
suprachiasmatic nucleus (SCN). Blue arrows, optical signal afferent
pathway to the SCN (i); and black arrows, non-optical signal
afferent pathway (ii). i) Photic input to the SCN. Intrinsically
photosensitive retinal ganglion cells (ipRGCs) project into the SCN
via the retinohypothalamic tract (RHT). Presynaptic membranes
release glutamate (Glu), leading to kinase activation and a series
of CLOCK gene expression alterations, promoting expression
of immediate early genes (IEG). Glu is introduced from the synaptic
cleft into the cells by Glu transporters locating on the astrocyte
membrane; and ii) photic input. IGL, intergeniculate leaflet; DRN,
dorsal raphe nucleus; MRN, median raphe nucleus; GHT,
geniculohypothalamic tract; 5-HT, serotonin; NPY, neuropeptide Y;
GABA, γ-aminobutyric acid; cAMP, cyclic adenosine monophosphate;
CRE, cAMP-response element; CREB, CRE-binding protein; B/C, brain
and muscle ARNT-like protein 1 (BMAL1)/CLOCK; AP, action potential;
AVP, arginine vasopressin; GRP, gastrin-releasing peptide; VIP,
vasointestinal peptide; AC, adenylate cyclase; and PKA, protein
kinase A. Figure reproduced from reference (19). |
3. Effects of PER2 and
corticotropin-releasing factor (CRF) on visceral circadian rhythm
motion in the limbic system
Biological function of PER2 in the limbic
system
Rhythmic expression of the PER2 protein results from
rhythmic transcription-translation of the per2-CLOCK genes.
The PER2 protein is ideal for observing rhythmic changes of the
per2 gene (21). The BNST,
CeA, basolateral amygdala and hippocampal dentate gyrus (DG) are
four regions expressing the PER2 protein in the rat limbic system
(4,5). These regions are important
neuroendocrine areas, involved in motion, emotion, behavior and
mental adjustment during stress, which indicates that per2
in the limbic system may be involved in stress- and drug
abuse-related behavior and neuroendocrine responses, regulation of
ingestion, learning and memory, maternal behavior and reproduction,
and may overally target multiple organs (22–24).
Rhythmic changes in PER2 expression show reverse profiles in
different brain regions. Within the BNST and CeA, PER2 expression
is maximal during the transition between day and night and almost
synchronized with PER2 expression in the SCN. However, in the
basolateral amygdala and hippocampal DG, PER2 expression changes
are opposite to those observed in the SCN (Fig. 3). Previous studies have shown
region-specific periods of PER2 in different brain sites of the
limbic system (25), which may be
associated with the negative feedback of PER2 to the CLOCK-BMAL1
dimer, which suppresess its own expression (26).
 | Figure 3Period circadian clock 2 protein
expression, detected by immunocytochemical staining in the
suprachiasmatic nucleus (SCN) and the limbic system at zeitgeber
times (ZT) 1 and 13, where ZT1, day time, start of lighting; and
ZT13, night time, end of lighting. BNST-OV, oval nucleus of the bed
nucleus of the stria terminalis; CEA, central nucleus of the
amygdala; BLA, basolateral amygdala (400×400 μm frame); and DG,
dentate gyrus (200×400 μm frame). Figure reproduced from reference
(26). |
Similar patterns of PER2 rhythmic
expression in the limbic system and SCN
PER2 alterations in the SCN are strongly associated
with PER2 expression in the limbic system. Bilateral SCN lesions,
or long-term 24-hour room illumination, can eliminate PER2 rhythmic
expression in the SCN, resulting in a behavioral rhythm disorder
(16,17). Rhythmicity of PER2 expression is
also completely eliminated in the BNST, CeA, basolateral amygdala,
DG and their lower-level brain structures (16,17).
Unilateral SCN lesions do not affect circadian rhythm-related
behavior, but attenuate the significance of PER2 rhythmic
expression in the ipsilateral limbic system (16), suggesting similar patterns of PER2
rhythmic expression and the presence of an important neuronal link
between the SCN and specific limbic system areas (27).
CRF regulates PER2 in the limbic system
and the hypothalamus
CRF is abundantly expressed in the hypothalamic
paraventricular nucleus. CRF is a promoter of and is activated by,
the HPA axis during stress. In the paraventricular nucleus, CRF is
regulated by GABAergic neurons that are mediated by CRF and other
neurotransmitter projections from higher level brain structures,
including the prefrontal cortex, hippocampus, nucleus accumbens
septi, CeA and BNST (Fig. 5)
(28). There is a strong
interaction between dopamine and CRF, and this interaction
regulates PER2 expression and its rhythmic changes (29). The CeA and BNST receive dopamine
signals (30) and are enriched in
CRF-ir neurons (31–33). Unilateral 6-hydroxydopamine injury
of dopaminergic neurons in the medial forebrain bundle causes
reduced PER2 expression in the ipsilateral BNST and CeA, and also
decreased expression of CRF neurons (34,35).
Following unilateral CRF gene silencing in the BNST and CeA,
ipsilateral PER2 expression almost disappears. However, there is no
co-expression of CRF and PER2 (10), indicating that the regulatory
effect of intracerebral CRF on PER2 possibly depends on
intracellular signal transduction pathways.
 | Figure 5Role of glucocorticoids (GC) and
other neurotransmitters on corticotropin-releasing factor (CRF) and
the hypothalamus-pituitary-adrenal (HPA) axis. Upon external stress
stimulation, the amygdala is activated first, followed by CRF
neurons overexpressed in the amygdala and leading to increased CRF
release in the hypothalamic paraventricular nucleus.
Simultaneously, norepinephrine (NE) overexpression is detected in
the locus coeruleus (LC) and the bed nucleus of the stria
terminalis. Serotonin (5-HT) release increases in the dorsal raphe
nucleus, resulting in elevated excitability of the HPA axis,
increased GC release in surrounding tissues, and a visceral
rhythmic motion disorder. The combination of GC and GC receptors in
the hippocampus inhibits CRF overexpression in the amygdala and the
hypothalamus, resulting in inhibition of the HPA axis
hyperactivity. CeA, central nucleus of the amygdala; DG, dentate
gyrus; CA, cornu Ammon; CRFR, cotricotropin-releasing factor
receptor; 5HT-R, hydroxytryptamine receptor; β1R, β1-adrenergic
receptors; GR, glucocorticoid receptor; MR, mineralocorticoid
receptor. |
Glucocorticoids regulate rhythmic PER2
expression in the BNST and CeA
PER2 expression in the BNST and CeA is synchronized
with rhythmic PER2 alterations in the SCN affected by
glucocorticoids. Circadian corticosterone release is regulated by
the SCN, leading to rhythmic per2 mRNA expression in
hepatocytes and fibroblasts (36,37).
Glucocorticoid receptors are expressed in the BNST, CeA,
basolateral amygdala and hippocampal DG. Endogenous and exogenous
glucocorticoids profoundly affects rhythmic genes in these regions.
Moreover, glucocorticoid effects on the limbic system are regulated
by CRF, a promoter of the HPA axis, and HPA axis activation affects
numerous surrounding target organs (29,38,39).
Following bilateral adrenal gland removal, rhythmic PER2 expression
in the BNST and CeA disappears, while PER2 expression in the
basolateral amygdala and DG is unaffected (16,17).
Administering corticosterone in drinking water restores rhythmic
PER2 expression in the BNST and CeA, while persistent subcutaneous
pumping is ineffective (40),
suggesting that glucocorticoid signaling is likely an important
factor for regulating rhythmic PER2 expression in these brain
regions.
In summary, the per2 gene presents
region-specific rhythmicity in the central nervous system. Target
organs exhibit circadian rhythms through optical signal
integration, CRF and related transmitters in the stress-response
system, and regulation of the HPA axis excitability.
Glucocorticoids and glucocorticoid receptors play a role in the
negative feedback-type regulation of the period circadian clock 2
mRNA and protein and on the HPA axis in the limbic system, but the
mechanism that the limbic system participates in requires further
investigation.
4. The PER2-hypothalamus-pituitary-adrenal
(HPA) axis interaction regulates visceral activities
Per2 gene regulation on the HPA axis
Optical signals activate the CLOCK gene in
the SCN. The CRF/arginine-vasopressin immunoreactive neuronal
projection to the hypothalamic paraventricular nucleus causes
hypothalamic CRF release and leads to activation of the HPA axis,
inducing rhythmic release of glucocorticoids in the adrenal gland
(41–43) (Fig.
5). CRF mRNA expression in the paraventricular nucleus
synchronizes with per2 expression in the SCN (44). Rhythmic per2 expression
patterns are not similar in the different parts of the HPA axis,
and specifically in the hypothalamus (except the SCN), and the
pituitary and adrenal glands, with per2 expression patterns
being reversed between other regions of the hypothalamus and SCN.
Similar per2 expression patterns are found between the
pituitary gland, adrenal gland and SCN. Following SCN injury, the
function of the HPA axis is attenuated, and the release of
adrenocorticotropic hormone from the pituitary gland is reduced,
but rhythmicity is maintained. Moreover, the rhythmicity of
glucocorticoid release from the adrenal gland is unaffected by the
central nervous system. Therefore, rhythmic secretion of the
adrenocorticotropic hormone from the pituitary gland is possibly
associated with a negative feedback effect of glucocorticoids in
the peripheral nervous system (45).
Rhythmic glucocorticoid release, mediated by the
CLOCK gene within the central nervous system, plays an
important role in the regulation of circadian adaptability. The SCN
mediates autonomic nerve excitability, altering the sensitivity of
the adrenal cortex to the adrenocorticotropic hormone and
rhythmically released glucocorticoids from the adrenal gland
(41–43,46).
Previous experiments detected no significant difference in baseline
levels of serum glucocorticoids between per2 gene knock-out
and wild-type mice. However, the circadian rhythms were absent in
per2 knock-out mice, while the pituitary gland-released
adrenocorticotropic hormone lost its original circadian rhythm
(47,48). Another study demonstrated that in
per2Brdm1/Cry1−/− mice, glucocorticoid
biosynthesis is disrupted in both adrenal glands (46), suggesting the per2 gene
controls glucocorticoid synthesis in adrenal glands, which may also
be regulated by adrenocorticotropic hormone that is rhythmically
released from the pituitary gland. Thus, it is assumed that in the
central nervous system, the per2 gene can be regulated by
both optical signals and the rhythmic secretion of glucocorticoids
(products of the HPA axis), but its regulation may be either
dependent or independent of the HPA axis. Regulation of the
per2 gene within the adrenal gland by secretory products
from other parts of the HPA axis (for example, adrenocorticotropic
hormone released from the pituitary gland), also affects the
circadian rhythm of organs in the peripheral nervous system
(Fig. 5).
Effects of the HPA axis on the per2
gene
The HPA axis strongly affects per2 expression
within the majority of organs by regulating glucocorticoid release,
but minimally affects per2 expression in the central nervous
system. This is due to reduced glucocorticoid levels in the central
nervous system and decreased co-expression of PER2 and
glucocorticoid receptors in the BNST and CeA (49). Therefore, the circadian rhythm of
the CLOCK gene in the central nervous system is independent
of changes in the surrounding environment. However in the
peripheral nervous system, CLOCK gene expression exhibits
rhythmic phase shifts during adjustment to surrounding changes, and
is restored to normal rhythmic levels through regulation by
glucocorticoid negative feedback in the central nervous system.
Within organs, glucocorticoid-mediated intermittent phasic
resetting are associated with alterations in the CLOCK gene
rhythmicity, induced by adjustments to stress within each organ
(49–51).
5. Clinical significance of the
PER2-HPA axis interaction on regulation of visceral rhythmic
movement
Visceral rhythmic movements stimulated by the
environmental stressors and show rhythmic motion alterations. The
HPA axis integrates limbic system information and regulates target
organs (Figs. 4 and 5). The PER2 effects on visceral
activities include tissue metabolism and organ movement. The
PER2-HPA axis interaction is important for studies of metabolic
syndrome, functional intestinal tract disease, hepatic metabolism
and immune system-related diseases.
 | Figure 4Role of the
hypothalamus-pituitary-adrenal (HPA) axis on visceral organ
regulation modulated by the suprachiasmatic nucleus (SCN). The
Circadian Locomotor Output Cycles Kaput (CLOCK) gene and the
HPA axis affect each other. The CLOCK gene is affected by
light stimulation and regulates HPA axis excitability in the
central nervous system, leading to rhythmic glucocorticoid release.
In the peripheral nervous system, CLOCK is mainly expressed
in the adrenal gland and other HPA axis-related regions. The
pituitary is regulated by the CLOCK gene in the central
nervous system (via the sympathetic nervous system) and
rhythmically regulated by glucocorticoids secreted by the adrenal
gland. Glucocorticoids exert a feedback effect, likely involving
other genes related to the biological functions of CLOCK. Black
dashed lines, parasympathetic nerves; red solid lines, sympathetic
nerves; blue solid lines, glucocorticoids. IGL, intergeniculate
leaflet; DRN, dorsal raphe nucleus; MRN, median raphe nucleus; GHT,
geniculohypothalamic tract; 5-HT, serotonin; NPY, neuropeptide Y;
GABA, γ-aminobutyric acid; PVN, paraventricular nucleus; MPO,
medial preoptic region; DMH, dorsomedial nucleus of hypothalamus;
and DMV, dorsal motor nucleus of the vagus nerve. |
Metabolic syndrome
Previous studies examining PER2 effects on calorie
intake and metabolism have shown that the food intake rate is
delayed and the weight is increased in per2 gene knock-out
mice. The weight of per2 knock-out mice provided with the
same food intake daily as wild-type mice was similar to that of
high-fat diet-fed mice suffering from an orexin secretion disorder
(52,53). This disorder is associated with
PER2 effects on glucocorticoid synthesis, release and metabolism.
CRF participates in the negative feedback effect of glucocorticoids
on PER2. A number of observations support that PER2 is involved in
metabolic disease: i) hypercortisolism (or Cushing’s syndrome)
causes central obesity; ii) bilateral adrenal gland removal induces
a reduction in food intake and weight due to the permanent absence
of cortisol, which regulates per2 in the organs and provides
negative feedback to the hypothalamus, hippocampus and other brain
sites in the CNS; iii) cortisol plays an important role in
maintaining metabolic balance, including regulation of food intake
and insulin levels (54); iv)
following adrenalectomy, cortisol function is disrupted, while upon
hypophysectomy, the function of the HPA axis is disrupted. These
disorders can lead to cortisol release, circadian disappearance,
irregular food intake and even body weight gain (55); and v) Cushing’s syndrome patients
experience anxiety, one of the manifestations of acute or chronic
stress, which can result in increased cortisol release, rhythmic
destruction and reduced CRF levels in the hypothalamus and the
pituitary gland. Thus, patients are eager to have high-calorie and
-fat food, with the associated reduction in hypothalamic CRF
release, reducing stress-induced anxiety-like behavior (56). Short-term damage in the rhythmicity
of cortisol release induces increases in postprandial blood sugar,
serum insulin levels and mean arterial blood pressure, with
significant reductions in the level of leptin and sleep efficiency,
and even complete loss of control of the rhythmic movement of the
organs by cortisol (57).
Altogether, restoring the cortisol-PER2 interaction
may provide a novel research direction and method for the treatment
of Cushing’s syndrome in the clinic. However, a number of issues
need to be addressed in this direction. For example, it is
difficult to observe the rhythmic cortisol pattern in living
animals or humans. Thus, whether damage in the cortisol circadian
rhythms results in obesity, or obesity causes cortisol circadian
rhythms disturbance remains to be clarified.
Irritable bowel syndrome (IBS)
Time differences cause resets in per2 gene
expression in the intestinal tract, leading to intestinal
dysfunctions including abdominal pain, constipation and diarrhea,
which are the main causes and symptoms of IBS (58–62).
With regards to intestinal functional regulation in the peripheral
nervous system, the per2 gene is mainly expressed in the
myenteric plexus of the intestinal tract, where neurotransmitters
promoting intestinal movement are synthesized (8). The HPA axis plays an important
regulatory role in intestinal movement. Rhythmic CRF release in the
hypothalamic paraventricular nucleus regulates intestinal rhythmic
motion. The stress reaction, via CRF neuronal activation in the HPA
axis and the limbic system, regulates autonomic nervous system
excitability and affects intestinal movement (Figs. 4 and 5). Thus, adrenocorticotropic hormone
levels are increased in the HPA axis, rhythmicity decreased and the
colon cortisol level increased (63). It is important to study the
PER2-HPA axis interaction and its roles in IBS for the following
reasons: i) in recent years, the rapidly developing Asian economy
has been strongly associated with an increased incidence of IBS,
with fast-paced work, sleep, food and drink appearing as main
stress factors (64); ii) patients
with IBS show sleep disturbances (65,66),
which are a hallmark symptom of CLOCK gene abnormalities in
the central nervous system (60,67,68);
iii) a clinical randomized and placebo-controlled trial
demonstrated that melatonin, an important regulatory peptide of the
circadian rhythm, can reduce intestinal symptoms in IBS patients
(69); and v) there are
correlations between intestinal sensitivity increased induced
abdominal pain and circadian disorder in IBS patients (70). In addition, electroacupuncture
noticeably improves sleep quality in patients with sleep
disturbances (71), providing
therapeutic evidence for a sleep-related functional disorder in
other organs.
6. Perspectives
The PER2 protein and the HPA axis interact via
glucocorticoids to regulate visceral circadian activities.
Glucocorticoids in adjacent organs are regulated by rhythmic CRF
alterations in the HPA axis and its response to stress. In the HPA
axis, CRF is regulated by the limbic system, including the BNST,
CeA and hippocampus, and shows a direct or indirect connection to
rhythmic alterations in PER2 expression affecting the morphology
and behavior of emotion and food intake. In general, the HPA axis
is a mediator of the effects of PER2 on target organs. The limbic
system integrates stress-related (CRF, stress-related
neurotransmitters and neuropeptides) and optical signals,
regulating the HPA axis excitability. To date, numerous clinical
and fundamental research studies have examined the PER2-HPA axis
interaction in the context of metabolic syndrome and IBS. Although
the exact mechanism underlying the contributions of the PER2-HPA
axis interaction in these disorders remains unclear, associations
with the limbic system deserve further investigation. Future
studies of the per2 gene need to focus on the following
aspects: i) CRF rhythmic expression in the hypothalamus and
pituitary, rhythmic expression of ACTH in the pituitary and
cortisol rhythmic changes in the adrenal gland, are included in the
HPA axis circadian rhythm. Previous studies have focused on the
effects of SCN on the PER2-HPA axis interaction, including local
expression and biological function of PER2 in the SCN, the effect
of PER2 in the regulation of the circadian rhythm in target organs,
and the glucocorticoids negative feedback to the brain areas in the
limbic systems (41–44). A few studies have identified an
interaction between the limbic system and per2 expression in
the SCN (23,24). Conversely, the limbic system, in
particular the hippocampus and amygdala, plays an important role in
HPA axis priming and regulation. Therefore, the limbic system may
be an important connection between the HPA axis and the cerebral
cortex in the mediation of the PER2 effects (27); ii) intracellular signaling
mechanisms underlying the hypothalamic CRF-PER2 interaction. CRF
activates the HPA axis and is regulated by the hippocampus and
amygdala (25). Pharmacological
studies have confirmed that there is a correlation between the
biological function of PER2 and CRF, and although morphological
studies have not identified a direct correlation, it is likely to
be mediated by intracellular signaling, although the exact
molecular mechanism is unknown (31–33,
10, 35); and iii) studies examining the
contribution of the per2 gene in various metabolic,
neuroendocrine and neuroimmunological diseases (22–24).
The per2 regulatory effect on HPA axis circadian rhythms and
its roles on the autonomic nervous system, emotion and behavior, in
functional disorders of autonomic nerve-induced diseases (including
functional gastrointestinal disorders and heart disease), various
stresses (including psychological, social and post-trauma acute and
chronic stress), cognitive function and behavioral alterations. A
breakthrough of the central mechanism of per2 gene interacting with
the HPA axis is in urgent need to deepen the understanding of per2
circadian rhythm physiopathologically and contribute to the
treatment of per2 rhythm disturbance related diseases.
Acknowledgements
This study was supported by the grant no. 81202735
(2012) from the National Natural Science Foundation of China, and
the Excellent Backbone Teacher Program of Qinglan Engineering of
the Jiangsu Province in China.
References
|
1
|
Li MD, Ruan HB, Hughes ME, et al: O-GlcNAc
signaling entrains the circadian clock by inhibiting BMAL1/CLOCK
ubiquitination. Cell Metab. 17:303–310. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Korencic A, Bordyugov G, Kosir R, Rozman
D, Golicnik M and Herzel H: The interplay of cis-regulatory
elements rules circadian rhythms in mouse liver. PLoS One.
7:e468352012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Prendergast BJ, Cisse YM, Cable EJ and
Zucker I: Dissociation of ultradian and circadian phenotypes in
female and male Siberian hamsters. J Biol Rhythms. 27:287–298.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Granados-Fuentes D, Ben-Josef G, Perry G,
Wilson DA, Sullivan-Wilson A and Herzog ED: Daily rhythms in
olfactory discrimination depend on clock genes but not the
suprachiasmatic nucleus. J Biol Rhythms. 26:552–560. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Valnegri P, Khelfaoui M, Dorseuil O, et
al: A circadian clock in hippocampus is regulated by interaction
between oligophrenin-1 and Rev-erbα. Nat Neurosci. 14:1293–1301.
2011.PubMed/NCBI
|
|
6
|
Amir S and Stewart J: Motivational
modulation of rhythms of the expression of the clock protein PER2
in the limbic forebrain. Biol Psychiatry. 65:829–834. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Amir S and Stewart J: Behavioral and
hormonal regulation of expression of the clock protein, PER2, in
the central extended amygdala. Prog Neuropsychopharmacol Biol
Psychiatry. 33:1321–1328. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yamaguchi M, Kotani K, Sakane N, et al:
The CLOCK 3111T/C SNP is associated with morning gastric motility
in healthy young women. Physiol Behav. 107:87–91. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kucera N, Schmalen L, Hennig S, et al:
Unwinding the differences of the mammalian PERIOD clock proteins
from crystal structure to cellular function. Proc Natl acad Sci
USA. 109:3311–3316. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hood S, Cassidy P, Cossette MP, et al:
Endogenous dopamine regulates the rhythm of expression of the clock
protein PER2 in the rat dorsal striatum via daily activation of D2
dopamine receptors. J Neurosci. 30:14046–14058. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang Y, Yao Q and Chen KP: Progress of
studies on family members and functions of animal bHLH
transcription factors. Yi Chuan. 32:307–330. 2010.(In Chinese).
|
|
12
|
Swanson G, Forsyth CB, Tang Y, et al: Role
of intestinal circadian genes in alcohol-induced gut leakiness.
Alcohol Clin Exp Res. 35:1305–1314. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Konturek PC, Brzozowski T and Konturek SJ:
Gut clock: implication of circadian rhythms in the gastrointestinal
tract. J Physiol Pharmacol. 62:139–150. 2011.PubMed/NCBI
|
|
14
|
Manfredini R and Portaluppi F: Night shift
and impaired endothelial function: circadian out-of-synch may play
a role. Int J Cardiol. 154:94–95. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tonsfeldt KJ and Chappell PE: Clocks on
top: the role of the circadian clock in the hypothalamic and
pituitary regulation of endocrine physiology. Mol Cell Endocrinol.
349:3–12. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Harbour VL, Robinson B and Amir S:
Variations in daily expression of the circadian clock protein,
PER2, in the rat limbic forebrain during stable entrainment to a
long light cycle. J Mol Neurosci. 45:154–161. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mueller AD, Mear RJ and Mistlberger RE:
Inhibition of hippocampal neurogenesis by sleep deprivation is
independent of circadian disruption and melatonin suppression.
Neuroscience. 193:170–181. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pezuk P, Mohawk JA, Wang LA and Menaker M:
Glucocorticoids as entraining signals for peripheral circadian
oscillators. Endocrinology. 153:4775–4783. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Albrecht U: Timing to perfection: the
biology of central and peripheral circadian clocks. Neuron.
74:246–260. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Glass JD, Brager AJ, Stowie AC and Prosser
RA: Cocaine modulates pathways for photic and nonphotic entrainment
of the mammalian SCN circadian clock. Am J Physiol Regul Integr
Comp Physiol. 302:R740–R750. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ripperger JA and Albrecht U: The circadian
clock component PERIOD2: from molecular to cerebral functions. Prog
Brain Res. 199:233–245. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hagenauer MH and Lee TM: The
neuroendocrine control of the circadian system: Adolescent
chronotype. Front Neuroendocrinol. 33:211–229. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jiang WG, Li SX, Zhou SJ, Sun Y, Shi J and
Lu L: Chronic unpredictable stress induces a reversible change of
PER2 rhythm in the suprachiasmatic nucleus. Brain Res. 1399:25–32.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ogawa Y, Koike N, Kurosawa G, Soga T,
Tomita M and Tei H: Positive autoregulation delays the expression
phase of mammalian clock gene Per2. PLoS One. 6:e186632011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Myung J, Hong S, Hatanaka F, Nakajima Y,
De Schutter E and Takumi T: Period coding of Bmal1 oscillators in
the suprachiasmatic nucleus. J Neurosci. 32:8900–8918. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Duong HA, Robles MS, Knutti D and Weitz
CJ: A molecular mechanism for circadian clock negative feedback.
Science. 332:1436–1439. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Perrin JS, Segall LA, Harbour VL, Woodside
B and Amir S: The expression of the clock protein PER2 in the
limbic forebrain is modulated by the estrous cycle. Proc Natl Acad
Sci USA. 103:5591–5596. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Maras PM and Baram TZ: Sculpting the
hippocampus from within: stress, spines, and CRH. Trends Neurosci.
35:315–324. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Carrasco J, Marquez C, Nadal R, Tobena A,
Fernandez-Teruel A and Armario A: Characterization of central and
peripheral components of the hypothalamus-pituitary-adrenal axis in
the inbred Roman rat strains. Psychoneuroendocrinology. 33:437–445.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hill MN, Patel S, Campolongo P, Tasker JG,
Wotjak CT and Bains JS: Functional interactions between stress and
the endocannabinoid system: from synaptic signaling to behavioral
output. J Neurosci. 30:14980–14986. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Costine BA, Oberlander JG, Davis MC, et
al: Chronic anabolic androgenic steroid exposure alters
corticotropin releasing factor expression and anxiety-like
behaviors in the female mouse. Psychoneuroendocrinology.
35:1473–1485. 2010. View Article : Google Scholar
|
|
32
|
Silberman Y, Matthews RT and Winder DG: A
corticotropin releasing factor pathway for ethanol regulation of
the ventral tegmental area in the bed nucleus of the stria
terminalis. J Neurosci. 33:950–960. 2013. View Article : Google Scholar
|
|
33
|
Cruz MT, Herman MA, Kallupi M and Roberto
M: Nociceptin/orphanin FQ blockade of corticotropin-releasing
factor-induced gamma-aminobutyric acid release in central amygdala
is enhanced after chronic ethanol exposure. Biol Psychiatry.
71:666–676. 2012. View Article : Google Scholar
|
|
34
|
Malloy JN, Paulose JK, Li Y and Cassone
VM: Circadian rhythms of gastrointestinal function are regulated by
both central and peripheral oscillators. Am J Physiol Gastrointest
Liver Physiol. 303:G461–G473. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gaszner B, Van Wijk DC, Korosi A, Jozsa R,
Roubos EW and Kozicz T: Diurnal expression of period 2 and
urocortin 1 in neurones of the non-preganglionic Edinger-Westphal
nucleus in the rat. Stress. 12:115–124. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Huang TS, Ruoff P and Fjelldal PG: Effect
of continuous light on daily levels of plasma melatonin and
cortisol and expression of clock genes in pineal gland, brain, and
liver in atlantic salmon postsmolts. Chronobiol Int. 27:1715–1734.
2010. View Article : Google Scholar
|
|
37
|
Kino T: Circadian rhythms of
glucocorticoid hormone actions in target tissues: potential
clinical implications. Sci Signal. 5(pt4)2012. View Article : Google Scholar
|
|
38
|
Schulkin J: Evolutionary conservation of
glucocorticoids and corticotropin releasing hormone: behavioral and
physiological adaptations. Brain Res. 1392:27–46. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Son GH, Chung S and Kim K: The adrenal
peripheral clock: glucocorticoid and the circadian timing system.
Front Neuroendocrinol. 32:451–465. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nader N, Chrousos GP and Kino T:
Interactions of the circadian CLOCK system and the HPA axis. Trends
Endocrinol Metab. 21:277–286. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Charmandari E, Chrousos GP, Lambrou GI, et
al: Peripheral CLOCK regulates target-tissue glucocorticoid
receptor transcriptional activity in a circadian fashion in man.
PLoS One. 6:e256122011. View Article : Google Scholar
|
|
42
|
Lilley TR, Wotus C, Taylor D, Lee JM and
de la Iglesia HO: Circadian regulation of cortisol release in
behaviorally split golden hamsters. Endocrinology. 153:732–738.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
So AY, Bernal TU, Pillsbury ML, Yamamoto
KR and Feldman BJ: Glucocorticoid regulation of the circadian clock
modulates glucose homeostasis. Proc Natl Acad Sci USA.
106:17582–17587. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Girotti M, Weinberg MS and Spencer RL:
Diurnal expression of functional and clock-related genes throughout
the rat HPA axis: system-wide shifts in response to a restricted
feeding schedule. Am J Physiol Endocrinol Metab. 296:E888–E897.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Helms CM, McClintick MN and Grant KA:
Social rank, chronic ethanol self-administration, and diurnal
pituitary-adrenal activity in cynomolgus monkeys.
Psychopharmacology (Berl). 224:133–143. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chung S, Son GH and Kim K: Adrenal
peripheral oscillator in generating the circadian glucocorticoid
rhythm. Ann NY Acad Sci. 1220:71–81. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Qu X, Metz RP, Porter WW, Neuendorff N,
Earnest BJ and Earnest DJ: The clock genes period 1 and period 2
mediate diurnal rhythms in dioxin-induced Cyp1A1 expression in the
mouse mammary gland and liver. Toxicol Lett. 196:28–32. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yang S, Liu A, Weidenhammer A, et al: The
role of mPer2 clock gene in glucocorticoid and feeding rhythms.
Endocrinology. 150:2153–2160. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Imanishi M, Yamamoto K, Yamada H, Hirose
Y, Okamura H and Futaki S: Construction of a rhythm transfer system
that mimics the cellular clock. ACS Chem Biol. 7:1817–1821. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Nebzydoski SJ, Pozzo S, Nemec L, Rankin MK
and Gressley TF: The effect of dexamethasone on clock gene mRNA
levels in bovine neutrophils and lymphocytes. Vet Immunol
Immunopathol. 138:183–192. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Verwey M and Amir S: Variable restricted
feeding disrupts the daily oscillations of Period2 expression in
the limbic forebrain and dorsal striatum in rats. J Mol Neurosci.
46:258–264. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sherman H, Genzer Y, Cohen R, Chapnik N,
Madar Z and Froy O: Timed high-fat diet resets circadian metabolism
and prevents obesity. FASEB J. 26:3493–3502. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Milagro FI, Gomez-Abellan P, Campion J,
Martinez JA, Ordovas JM and Garaulet M: CLOCK, PER2 and BMAL1 DNA
methylation: association with obesity and metabolic syndrome
characteristics and monounsaturated fat intake. Chronobiol Int.
29:1180–1194. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Solomon MB, Sakai RR, Woods SC and Foster
MT: Differential effects of glucocorticoids on energy homeostasis
in Syrian hamsters. Am J Physiol Endocrinol Metab. 301:E307–E316.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Nyberg CH, Leonard WR, Tanner S, et al:
Diurnal cortisol rhythms and child growth: exploring the life
history consequences of HPA activation among the Tsimane’. Am J Hum
Biol. 24:730–738. 2012.PubMed/NCBI
|
|
56
|
Parylak SL, Cottone P, Sabino V, Rice KC
and Zorrilla EP: Effects of CB1 and CRF1 receptor antagonists on
binge-like eating in rats with limited access to a sweet fat diet:
lack of withdrawal-like responses. Physiol Behav. 107:231–242.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Scheer FA, Hilton MF, Mantzoros CS and
Shea SA: Adverse metabolic and cardiovascular consequences of
circadian misalignment. Proc Natl Acad Sci USA. 106:4453–4458.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Hoogerwerf WA: Role of clock genes in
gastrointestinal motility. Am J Physiol Gastrointest Liver Physiol.
299:G549–G555. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Iwashina I, Mochizuki K, Inamochi Y and
Goda T: Clock genes regulate the feeding schedule-dependent diurnal
rhythm changes in hexose transporter gene expressions through the
binding of BMAL1 to the promoter/enhancer and transcribed regions.
J Nutr Biochem. 22:334–343. 2011. View Article : Google Scholar
|
|
60
|
Wells MM, Roth L and Chande N: Sleep
disruption secondary to overnight call shifts is associated with
irritable bowel syndrome in residents: a cross-sectional study. Am
J Gastroenterol. 107:1151–1156. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Vyas MV, Garg AX, Iansavichus AV, et al:
Shift work and vascular events: systematic review and
meta-analysis. BMJ. 345:e48002012. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Nojkov B, Rubenstein JH, Chey WD and
Hoogerwerf WA: The impact of rotating shift work on the prevalence
of irritable bowel syndrome in nurses. Am J Gastroenterol.
105:842–847. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Heitkemper MM, Cain KC, Deechakawan W, et
al: Anticipation of public speaking and sleep and the
hypothalamic-pituitary-adrenal axis in women with irritable bowel
syndrome. Neurogastroenterol Motil. 24:626–631. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Khan S and Chang L: Diagnosis and
management of IBS. Nat Rev Gastroenterol Hepatol. 7:565–581. 2010.
View Article : Google Scholar
|
|
65
|
Bellini M, Gemignani A, Gambaccini D, et
al: Evaluation of latent links between irritable bowel syndrome and
sleep quality. World J Gastroenterol. 17:5089–5096. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Hongo M: Epidemiology of FGID symptoms in
Japanese general population with reference to life style. J
Gastroenterol Hepatol. 26(Suppl 3): 19–22. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Burioka N, Koyanagi S, Endo M, et al:
Clock gene dysfunction in patients with obstructive sleep apnoea
syndrome. Eur Respir J. 32:105–112. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Miyamoto H, Nakamaru-Ogiso E, Hamada K and
Hensch TK: Serotonergic integration of circadian clock and
ultradian sleep-wake cycles. J Neurosci. 32:14794–14803. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Mozaffari S, Rahimi R and Abdollahi M:
Implications of melatonin therapy in irritable bowel syndrome: a
systematic review. Curr Pharm Des. 16:3646–3655. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Enck P, Kaiser C, Felber M, et al:
Circadian variation of rectal sensitivity and gastrointestinal
peptides in healthy volunteers. Neurogastroenterol Motil. 21:52–58.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Freire AO, Sugai GC, Togeiro SM, Mello LE
and Tufik S: Immediate effect of acupuncture on the sleep pattern
of patients with obstructive sleep apnoea. Acupunct Med.
28:115–119. 2010. View Article : Google Scholar : PubMed/NCBI
|



















