Introduction
Dendritic cells (DCs) are specific
antigen-presenting cells critical for the induction of adaptive
immunity and tolerance by interacting with T cells (1). DC differentiation from monocytes is a
key step in infections and numerous other conditions. DC turnover
is similarly important for maintaining the steady state of the
immune system. Circulating monocytes usually undergo spontaneous
apoptosis within days (2);
however, the life span of monocytes is extended to weeks following
differentiation into DCs, induced by treatment with interleukin
(IL)-4/granulocyte macrophage colony-stimulating factor
(GM-CSF).
Previous studies have observed that T-helper (Th)1
cytokines, including IL-2 and IL-12, inhibit myeloid cell
apoptosis, whereas Th2 cytokines, such as IL-4 and IL-10, enhance
apoptosis in these cells (3,4).
IL-10-induced myeloid cell apoptosis is mediated through the
caspase-dependent signaling pathway, which is blocked by caspase-3
inhibitors and pan-caspase inhibitors (2). Galectin-1 (Gal-1) exhibits the
ability to induce IL-10 expression in T cells (5,6) and
in DCs (7,8), but does not induce apoptosis in
monocytes (9,10).
Granulocyte colony-stimulating factor (G-CSF, also
termed CSF3) was identified in an attempt to define the normal
regulators present in cell supernatants that induced terminal
differentiation of the WEHI-3B D+ murine myeloid
leukemia cell line (11).
Recently, Romero-Weaver et al reported the ability of G-CSF
to promote the proliferation of bone marrow stem cells and inhibit
granulocyte apoptosis (12). G-CSF
also improved the recovery from spinal cord injury in mice
(13) and improved memory and
neuro-behavior in an amyloid-β-induced experimental model of
Alzheimer’s disease (14).
However, the direct effects of G-CSF on differentiating monocytes
have not been discussed. In present study, the role of G-CSF in
galectin-1-treated monocytes was examined, particularly its role in
preventing cell apoptosis.
Materials and methods
Materials
Gal-1 and G-CSF were purchased from ProsPec-Tany
TechnoGene, Ltd. (Ness-Ziona, Israel). Human recombinant IL-10 was
purchased from R&D Systems (Minneapolis, MN, USA). Human
recombinant GM-CSF and IL-4 were purchased from Millipore Corp.
(Billerica, MA, USA).
Isolation and culture of human
monocytes
Human CD14+ monocytes were isolated from
the peripheral blood mononuclear cells (PBMCs) of healthy donors
without any known cancers or immunological disease. Briefly, PBMCs
were collected from interphase subsequent to Ficoll paque plus
separation (GE Healthcare Bio-Sciences, Little Chalfont, UK) and
washed twice in phosphate-buffered saline (PBS). CD14+
monocytes were isolated using the MACS® system (MACS
MicroBeads; Miltenyi Biotec Ltd, Bergisch Gladbach, Germany)
following the manufacturer’s instructions and cultured in RPMI-1640
containing 10% fetal bovine serum (Invitrogen Life Sciences,
Carlsbad, CA, USA) for five days in the presence of 20 ng/ml
IL-4/GM-CSF with or without 1 μg/ml Gal-1, 10 ng/ml G-CSF and IL-10
as indicated. Monocyte viability was determined by trypan blue
exclusion staining.
The Institutional Review Board of Kaohsiung Medical
University Hospital (Kaohsiung, Taiwan) approved the study. All
patients provided informed consent in accordance with the
Declaration of Helsinki.
Flow cytometry and detection of Annexin V
staining and CD14 expression
Two-color flow cytometry was performed by
FACSarray™ (BD Biosciences, Franklin Lakes, NJ, USA)
using the Annexin V-fluorescein isothiocyanate (FITC) Apoptosis
Detection kit I (BD Biosciences) according to the manufacturer’s
instructions. Briefly, the treated cells were centrifuged at 200 ×
g for 5 min and washed twice with cold PBS. The cells were
resuspended in 100 μl 1× binding buffer, and 5 μl Annexin V-FITC
and 5 μl propidium iodide (PI) were added. The cells were gently
vortexed and incubated for 15 min at room temperature in the dark.
Subsequently, the cells were centrifuged at 200 × g for 5 min,
washed twice with 1× binding buffer and resuspended in 100 μl 1×
binding buffer. The samples were analyzed using a
FACSarray™ flow cytometer.
Measurement of secreted factors
The cultured supernatants from monocytes were
collected following centrifugation. The samples were analyzed for
IL-10 and G-CSF by multiple cytokine analyses using the cytometric
bead array (CBA; BD Biosciences). The CBA technique is based on two
bead populations with distinct fluorescence intensities that are
coated with capture antibodies specific for each cytokine. The
fluorescent dye had a maximal emission wavelength of ~650 nm
(FL-3), which was detectable by flow cytometry. The cytokine
capture beads were mixed with the phycoerythrin-conjugated
detection antibodies and then incubated with recombinant standards
or test samples to form sandwich complexes. Following the
acquisition of sample data on the FACSarray™ flow
cytometer, the sample results were analyzed using FCAP Array™
software version 3.0 (BD Biosciences). A standard calibration curve
was established for each cytokine; the maximum and minimum limits
of detection for each cytokine were 1.0 and 5,000 pg/ml,
respectively.
Statistical analysis
Data are expressed as the mean ± standard deviation.
Statistical comparisons of the results were performed by analysis
of variance and two-sided Student’s t-test using Excel 2010
(Microsoft Corp., Redmond, WA, USA). P<0.05 was considered to
indicate a statistically significant difference between the means
of the two groups.
Results
IL-10 induces apoptosis in monocytes
Monocytes isolated from PBMCs of healthy donors
usually died after several days due to a constitutively active cell
death program (15). This
spontaneous cell death was reduced by 20% following stimulation
with IL-4 and GM-CSF for five days (Fig. 1A). The viability of the stimulated
monocytes, determined by trypan blue exclusion assay, was reduced
when IL-10 was added and the proportion of trypan blue- stained
cells increased following treatment with higher IL-10
concentrations (Fig. 1A).
Similarly, Annexin V-PI staining revealed that the proportion of
apoptotic cells was elevated with increasing IL-10 concentration
and increased culture duration (Fig.
1B and C). The apoptosis induced by recombinant human IL-10 was
significantly increased at concentrations >2.5 ng/ml.
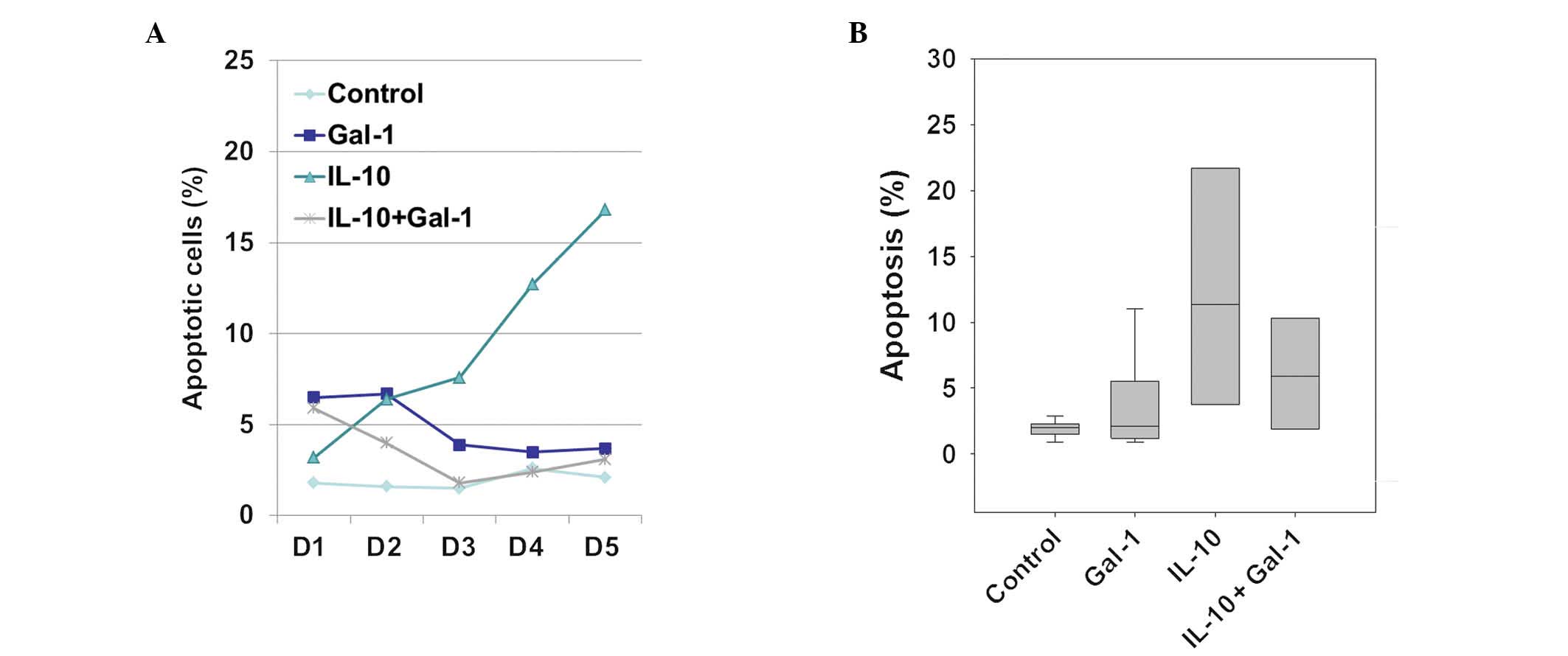
Gal-1 protects monocytes from
IL-10-induced apoptosis
The percentage of apoptotic cells was determined by
Annexin V-propidium iodide staining of the IL-4/GM-CSF-stimulating
monocyte culture media with and without 1 μg/ml Gal-1 and/or 10
ng/ml IL-10. Stimulated monocyte apoptosis in the IL-10-only group
continuously increased over five days. The Gal-1-only group
exhibited no increase in apoptosis after three days (Fig. 2A). Furthermore,
IL-10+Gal-1-stimulated monocyte apoptosis was not increased after
three days (Fig. 2A). The same
phenomenon was observed in monocytes isolated from five donors,
although the percentage of apoptotic cells varied (Fig. 2B).
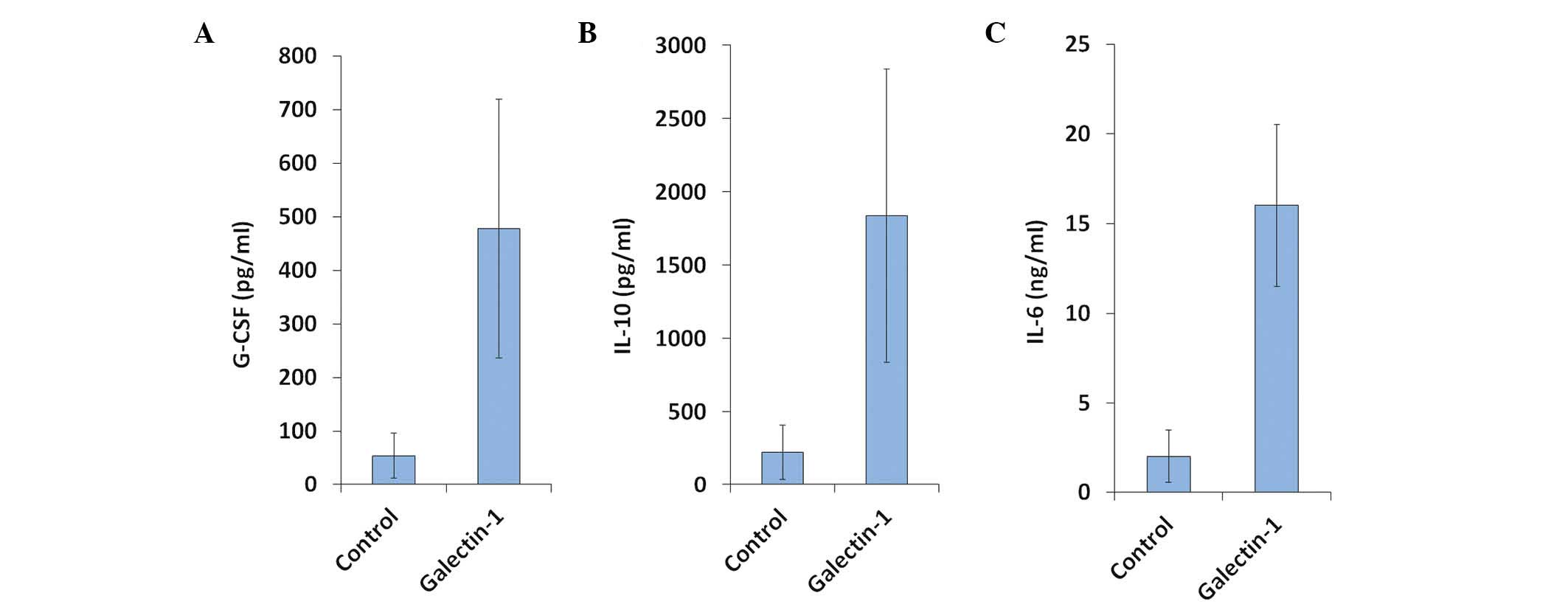
Gal-1 induces IL-10 and G-CSF in
stimulated monocytes
The supernatants of the Gal-1 only group were
collected after five days of incubation and analyzed by the CBA
system. The concentrations of >10 cytokines (i.e. IL-1, -4, -6,
-8, -10, -11, -12, -17 and -21, interferons (IFNs), the tumor
necrosis factors (TNFs), basic fibroblast growth factor, vascular
endothelial growth factor and G-CSF) were determined, with GM-CSF
serving as an internal control. Gal-1 enhanced the expression
levels of IL-6, IL-10 and G-CSF, but not those of the other
cytokines (Fig. 3A–C).
G-CSF inhibits IL-10-induced apoptosis in
monocytes
When IL-10 (10 ng/ml) was added to the
IL-4/GM-CSF-stimulated monocyte culture media with and without
Gal-1 (1 μg/ml) and G-CSF (10 ng/ml), analysis of stimulated
monocyte apoptosis revealed that recombinant human G-CSF or Gal-1
significantly inhibited IL-10-induced apoptosis (P<0.05 as
compared with IL-10-only treated cells; Fig. 4A and B).
Discussion
The fate of monocytes is regulated by different
signaling pathways, including those of NF-κB, Fas-Fas ligand (FasL)
and the B-cell lymphoma 2 (Bcl-2) family. A previous study reported
that spontaneous monocyte apoptosis was inhibited by treatment with
inflammatory mediators, including TNF, lipopolysaccharide (LPS),
CD40 ligand (CD154), growth factors and cytokines, including GM-CSF
and IFN-γ (16). Alone, IL-4 does
not inhibit spontaneous apoptosis, and may inhibit the
anti-apoptotic effects of IL-1 and LPS (3,17).
However, co-treatment with GM-CSF and IL-4, according to the
monocyte-derived DC protocol, inhibits the spontaneous apoptosis of
monocytes (17). This implies that
the signaling pathway involved in the anti-apoptotic effect
mediated by GM-CSF may be different from the signaling pathway
induced by IL-1 and LPS.
Receptors of pro-inflammatory mediators, including
TNF receptor, IL-1R, Toll-like receptor 4 and CD14, activate the
NF-κB signaling pathway and upregulate anti-apoptotic genes
(18). Conversely, the GM-CSF
receptor activates the Janus kinase (JAK)/signal transducer and
activator of transcription (STAT)5 signaling pathway and
upregulates Bcl-2 in neural progenitor cells and mouse
hematopoietic precursors (19,20).
Studies regarding IL-4 and IL-6 in monocytes support
the hypothesis that IL-4 inhibits IL-6 production by reducing
nuclear NF-κB levels (21,22). However, the interaction between the
IL-4 signaling pathway and STAT5 in monocytes has not been
reported. Notably, in the present study, apoptosis enhanced by
another Th2 cytokine, IL-10, was not inhibited by the presence of
GM-CSF, suggesting a difference between IL-10-induced apoptosis and
apoptosis enhanced by IL-4. Hashimoto et al (23) obtained similar results and further
demonstrated that IL-10 inhibited the phosphorylation of STAT5
induced by GM-CSF. In another study, Schmidt et al (24) found that CD95 ligand-neutralizing
antibody significantly inhibited IL-10-induced apoptosis. In
conclusion, IL-10 may induce apoptosis by inhibiting STAT5 and by
activating the Fas/FasL signaling pathway.
Galectins are a family of 15 β-galactoside-binding
proteins. Gal-1 is a 14.5 kDa protein and was the first galectin
family member to be described. Dimeric Gal-1 binds to glycoproteins
and activates signaling pathways, including those of CD4, CD7, CD43
and CD45 (25–28). Numerous studies have demonstrated
that Gal-1 induces apoptosis in T cells (25,28–32)
and macrophages (33), which may
be involved in the regulation of immune responses. The signaling
pathway involved in Gal-1-mediated T-cell death requires
clarification, as data remain inconclusive due to variations in
Gal-1 interacting proteins and concentrations (34).
A study revealed that Gal-1 regulates the T-cell
immune response through upregulating IL-10 expression; Gal-1 did
not induce apoptosis in myeloid lineage and Th cells, but did
increase the regulatory T-cell population (35). In another model, recombinant Gal-1
enhanced IL-10 expression levels up to seven-fold, but the
apoptosis induced by high dosages of IL-10 was not observed,
implying that other signaling pathways activated by Gal-1 inhibit
the pro-apoptotic effects of IL-10 (36). In the present study, Gal-1 enhanced
IL-6 and G-CSF expression levels up to twelve- and nine-fold,
respectively, but not the expression levels of pro-inflammatory
cytokines (i.e. TNF, IFN and IL-12; data not shown). Mangan and
Wahl (37) reported that IL-6
exerted no effect on non-stimulating apoptosis; this was also
observed in later studies (5,6). The
present study demonstrated that IL-6 did not inhibit IL-10-induced
apoptosis in IL-4/GM-CSF-stimulated monocytes. However, another
hematopoietic growth factor induced by Gal-1, G-CSF, was found to
reduce IL-10-induced apoptosis.
G-CSF is the predominant regulator of neutrophil
production under basal conditions of hematopoiesis. G-CSF maintains
neutrophil survival (38,39) and regulates the survival and
mobilization of cardiomyocytes and neurons (40–42).
The G-CSF receptor belongs to the cytokine receptor type I
superfamily, which engages the canonical JAK/STAT,
Ras/Raf/mitogen-activated protein kinase and protein kinase B
signaling pathways, all of which are crucial for the anti-apoptotic
function of G-CSF (43,44).
The present study demonstrated that G-CSF not only
exerted an anti-apoptotic effect on monocytes, but also inhibited
IL-10-induced apoptosis without affecting the tolerogenic function
of IL-10 (data not shown). Examining the network of cytokines that
regulate the fate of monocytes, this implies that Gal-1 reinforces
its immune modulating effects by simultaneously upregulating IL-10
and G-CSF. Therefore, G-CSF may be further applied in immune
therapy, particularly in the IL-10-presenting microenvironment.
Acknowledgements
This study was supported by grants from the National
Science Council of Taiwan (no. NSC 101-2628-B-037-001-MY3) and the
Kaohsiung Medical University Hospital (no. KMUH102-2M09). The
authors would like to thank the Centre for Resources, Research and
Development of Kaohsiung Medical University for support with the
instrumentation.
References
|
1
|
Janikashvili N, Bonnotte B, Katsanis E and
Larmonier N: The dendritic cell-regulatory T lymphocyte crosstalk
contributes to tumor-induced tolerance. Clin Dev Immunol.
2011:4303942011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fahy RJ, Doseff AI and Wewers MD:
Spontaneous human monocyte apoptosis utilizes a caspase-3-dependent
pathway that is blocked by endotoxin and is independent of
caspase-1. J Immunol. 163:1755–1762. 1999.
|
|
3
|
Estaquier J and Ameisen JC: A role for
T-helper type-1 and type-2 cytokines in the regulation of human
monocyte apoptosis. Blood. 90:1618–1625. 1997.PubMed/NCBI
|
|
4
|
Ludewig B, Graf D, Gelderblom HR, Becker
Y, Kroczek RA and Pauli G: Spontaneous apoptosis of dendritic cells
is efficiently inhibited by TRAP (CD40-ligand) and TNF-alpha, but
strongly enhanced by interleukin-10. Eur J Immunol. 25:1943–1950.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
van der Leij J, van den Berg A, Harms G,
et al: Strongly enhanced IL-10 production using stable galectin-1
homodimers. Mol Immunol. 44:506–513. 2007.
|
|
6
|
van der Leij J, van den Berg A, Blokzijl
T, et al: Dimeric galectin-1 induces IL-10 production in
T-lymphocytes: an important tool in the regulation of the immune
response. J Pathol. 204:511–518. 2004.PubMed/NCBI
|
|
7
|
Kuo PL, Hung JY, Huang SK, et al: Lung
cancer-derived galectin-1 mediates dendritic cell anergy through
inhibitor of DNA binding 3/IL-10 signaling pathway. J Immunol.
186:1521–1530. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ilarregui JM, Croci DO, Bianco GA, et al:
Tolerogenic signals delivered by dendritic cells to T cells through
a galectin-1-driven immunoregulatory circuit involving interleukin
27 and interleukin 10. Nat Immunol. 10:981–991. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stowell SR, Qian Y, Karmakar S, et al:
Differential roles of galectin-1 and galectin-3 in regulating
leukocyte viability and cytokine secretion. J Immunol.
180:3091–3102. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Barrionuevo P, Beigier-Bompadre M,
Ilarregui JM, et al: A novel function for galectin-1 at the
crossroad of innate and adaptive immunity: galectin-1 regulates
monocyte/macrophage physiology through a nonapoptotic ERK-dependent
pathway. J Immunol. 178:436–445. 2007. View Article : Google Scholar
|
|
11
|
Welte K, Platzer E, Lu L, et al:
Purification and biochemical characterization of human pluripotent
hematopoietic colony-stimulating factor. Proc Natl Acad Sci USA.
82:1526–1530. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Romero-Weaver AL, Wan XS, Diffenderfer ES,
Lin L and Kennedy AR: Kinetics of neutrophils in mice exposed to
radiation and/or granulocyte colony-stimulating factor treatment.
Radiat Res. 180:177–188. 2013. View
Article : Google Scholar
|
|
13
|
Guo Y, Zhang H, Yang J, et al: Granulocyte
colony-stimulating factor improves alternative activation of
microglia under microenvironment of spinal cord injury.
Neuroscience. 15:2382013.PubMed/NCBI
|
|
14
|
Prakash A, Medhi B and Chopra K:
Granulocyte colony stimulating factor (GCSF) improves memory and
neurobehavior in an amyloid-β induced experimental model of
Alzheimer’s disease. Pharmacol Biochem Behav. 110:46–57.
2013.PubMed/NCBI
|
|
15
|
Doseff AI: Apoptosis: the sculptor of
development. Stem Cells Dev. 13:473–483. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kiener PA, Davis PM, Starling GC, et al:
Differential induction of apoptosis by Fas-Fas ligand interactions
in human monocytes and macrophages. J Exp Med. 185:1511–1516. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mangan DF, Robertson B and Wahl SM: IL-4
enhances programmed cell death (apoptosis) in stimulated human
monocytes. J Immunol. 148:1812–1816. 1992.PubMed/NCBI
|
|
18
|
Gaur U and Aggarwal BB: Regulation of
proliferation, survival and apoptosis by members of the TNF
superfamily. Biochem Pharmacol. 66:1403–1408. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Choi JK, Kim KH, Park H, Park SR and Choi
BH: Granulocyte macrophage-colony stimulating factor shows
anti-apoptotic activity in neural progenitor cells via
JAK/STAT5-Bcl-2 pathway. Apoptosis. 16:127–134. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Feldman GM, Rosenthal LA, Liu X, et al:
STAT5A-deficient mice demonstrate a defect in
granulocyte-macrophage colony-stimulating factor-induced
proliferation and gene expression. Blood. 90:1768–1776. 1997.
|
|
21
|
Donnelly RP, Crofford LJ, Freeman SL, et
al: Tissue-specific regulation of IL-6 production by IL-4.
Differential effects of IL-4 on nuclear factor-kappa B activity in
monocytes and fibroblasts. J Immunol. 151:5603–5612.
1993.PubMed/NCBI
|
|
22
|
Takeshita S, Gage JR, Kishimoto T,
Vredevoe DL and Martínez-Maza O: Differential regulation of IL-6
gene transcription and expression by IL-4 and IL-10 in human
monocytic cell lines. J Immunol. 156:2591–2598. 1996.PubMed/NCBI
|
|
23
|
Hashimoto SI, Komuro I, Yamada M and
Akagawa KS: IL-10 inhibits granulocyte-macrophage
colony-stimulating factor-dependent human monocyte survival at the
early stage of the culture and inhibits the generation of
macrophages. J Immunol. 167:3619–3625. 2001. View Article : Google Scholar
|
|
24
|
Schmidt M, Lügering N, Pauels HG,
Schulze-Osthoff K, Domschke W and Kucharzik T: IL-10 induces
apoptosis in human monocytes involving the CD95 receptor/ligand
pathway. Eur J Immunol. 30:1769–1777. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nguyen JT, Evans DP, Galvan M, et al: CD45
modulates galectin-1-induced T cell death: regulation by expression
of core 2 O-glycans. J Immunol. 167:5697–5707. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pang M, He J, Johnson P and Baum LG:
CD45-mediated fodrin cleavage during galectin-1 T cell death
promotes phagocytic clearance of dying cells. J Immunol.
182:7001–7008. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fulcher JA, Chang MH, Wang S, et al:
Galectin-1 co-clusters CD43/CD45 on dendritic cells and induces
cell activation and migration through Syk and protein kinase C
signaling. J Biol Chem. 284:26860–26870. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Perillo NL, Pace KE, Seilhamer JJ and Baum
LG: Apoptosis of T cells mediated by galectin-1. Nature.
378:736–739. 1995. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Stillman BN, Hsu DK, Pang M, et al:
Galectin-3 and galectin-1 bind distinct cell surface glycoprotein
receptors to induce T cell death. J Immunol. 176:778–789. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Garín MI, Chu CC, Golshayan D,
Cernuda-Morollón E, Wait R and Lechler RI: Galectin-1: a key
effector of regulation mediated by CD4+ CD25+
T cells. Blood. 109:2058–2065. 2007.PubMed/NCBI
|
|
31
|
Pace KE, Hahn HP, Pang M, Nguyen JT and
Baum LG: CD7 delivers a pro-apoptotic signal during
galectin-1-induced T cell death. J Immunol. 165:2331–2334. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Perillo NL, Uittenbogaart CH, Nguyen JT
and Baum LG: Galectin-1, an endogenous lectin produced by thymic
epithelial cells, induces apoptosis of human thymocytes. J Exp Med.
185:1851–1858. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Paclik D, Werner L, Guckelberger O,
Wiedenmann B and Sturm A: Galectins distinctively regulate central
monocyte and macrophage function. Cell Immunol. 271:97–103. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cedeno-Laurent F and Dimitroff CJ:
Galectin-1 research in T cell immunity: past, present and future.
Clin Immunol. 142:107–116. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
van der Leij J, van den Berg A, Harms G,
et al: Strongly enhanced IL-10 production using stable galectin-1
homodimers. Mol Immunol. 44:506–513. 2007.PubMed/NCBI
|
|
36
|
Stowell SR, Qian Y, Karmakar S, et al:
Differential roles of galectin-1 and galectin-3 in regulating
leukocyte viability and cytokine secretion. J Immunol.
180:3091–3102. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mangan DF and Wahl SM: Differential
regulation of human monocyte programmed cell death (apoptosis) by
chemotactic factors and pro-inflammatory cytokines. J Immunol.
147:3408–3412. 1991.PubMed/NCBI
|
|
38
|
Liu F, Wu HY, Wesselschmidt R, Kornaga T
and Link DC: Impaired production and increased apoptosis of
neutrophils in granulocyte colony-stimulating factor
receptor-deficient mice. Immunity. 5:491–501. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lieschke GJ, Grail D, Hodgson G, et al:
Mice lacking granulocyte colony-stimulating factor have chronic
neutropenia, granulocyte and macrophage progenitor cell deficiency,
and impaired neutrophil mobilization. Blood. 84:1737–1746.
1994.
|
|
40
|
Shim W, Mehta A, Lim SY, et al: G-CSF for
stem cell therapy in acute myocardial infarction: friend or foe?
Cardiovasc Res. 89:20–30. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Schneider A, Krüger C, Steigleder T, et
al: The hematopoietic factor G-CSF is a neuronal ligand that
counteracts programmed cell death and drives neurogenesis. J Clin
Invest. 115:2083–2098. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
42
|
Schneider A, Kuhn HG and Schäbitz WR: A
role for G-CSF (granulocyte-colony stimulating factor) in the
central nervous system. Cell Cycle. 4:1753–1757. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Harada M, Qin Y, Takano H, et al: G-CSF
prevents cardiac remodeling after myocardial infarction by
activating the Jak-Stat pathway in cardiomyocytes. Nat Med.
11:305–311. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
44
|
Fukada T, Hibi M, Yamanaka Y, et al: Two
signals are necessary for cell proliferation induced by a cytokine
receptor gp130: involvement of STAT3 in anti-apoptosis. Immunity.
5:449–460. 1996. View Article : Google Scholar : PubMed/NCBI
|