Introduction
The milestone results reported by Altschul et
al (1) >50 years ago
demonstrated that nicotinic acid (NA) has the capacity to decrease
plasma lipids. As a result, this water soluble vitamin B family
member has been widely used clinically for the treatment and
prevention of atherosclerosis and other lipid-metabolic disorders
(2,3). At present, NA is one of the most
effective agents that offers protection against cardiovascular risk
factors by increasing high density lipoprotein (HDL) levels, while
simultaneously decreasing very low density lipoprotein (VLDL) and
low density lipoprotein (LDL) levels (4). The major side effect of NA is
cutaneous vasodilatation, also known as ‘flush’, which limits its
clinical utility and applications (5). NA functions by downregulating
intracellular cyclic adenosine monophosphate (cAMP), the major
intracellular mediator of prolipolytic stimuli, and subsequently
decreases cellular levels of free fatty acids (5). Notably, prostaglandin has been
demonstrated to have a vital role in flushing (6,7).
Anti-lipid and flush effects are mediated by its G protein-coupled
receptor GPR109A (8,9). Despite extensive studies in the field
of lipid metabolism, the effects of NA on other aspects of cellular
physiology remain elusive. Previously, several groups have
demonstrated that NA elevates intracellular [Ca2+] in
neutrophil (10), macrophage
(8) and CHOK1 cell lines (9) in a GPR109A-dependent manner.
Elevation of intracellular [Ca2+] may transduce a number
of different signaling pathways in different cell types. In the
present study, variations in intracellular Ca2+ levels
were observed under incubation with different concentrations of NA,
and long-term (1 h) effects on the NIH3T3 cell line and its
cytoskeleton were analyzed.
Materials and methods
Cell culture
CHO-K1 cells (cat.no CCL-61; American Type Culture
Collection; Manassas, VA, USA) were grown in F12 medium (11765047;
Life Technologies, Grand Island, NY, USA) supplemented with 10%
fetal calf serum (FCS; 16170086; Life Technologies). The 293T cells
(CRL-3216; American Type Culture Collection) were grown in
Dulbecco’s modified Eagle medium (DMEM; 12430047; Life
Technologies) with 10% fetal calf serum (FCS). The NIH3T3 cells
(CRL-1658; American Type Culture Collection) were cultured in DMEM
with 10% FCS.
Time lapse measurement of intracellular
[Ca2+]
The cells (2×104/well) were allowed to
adhere to a sterile 96-well cell culture plate (Greiner Bio-One)
and incubated with Fluo3 acetoxymethyl (AM) Ca2+
indicator (Molecular Probes, Invitrogen Life Technologies,
Carlsbad, CA, USA) for 1 h at 37°C. The Ca2+ levels were
assessed by measuring the fluorescent intensity using a Zeiss LSM
510 META confocal microscope and Zeiss Lsm Image Examiner software
(FV10-ASW 2.1 Viewer; Carl Zeiss, Jena, Germany) was applied for
quantitative analysis.
Fluorescent immunohistochemistry
The cells were fixed for 10 min with 3.7%
paraformaldehyde (Sigma, St. Louis, MO, USA) and permeabilized with
0.2% Triton X-100 (Sigma). The F-actin stress fibers were labeled
with Texas Red-X phalloidin (Molecular Probes, Invitrogen Life
Technologies). The microtubule filaments were stained with
monoclonal mouse anti-β-tubulin antibody (1:200; E1C-601; EnoGene,
New York, NY, USA) and the secondary antibody was
goat-anti-mouse-fluorescein isothiocyanate (1:200; Sigma).
Western blot analysis
The cells were incubated with MG132 (10 μM; Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and/or NA and
collected at the appropriate time. The cells were boiled at 100°C
in Lämmli buffer for 5 min. The following antibodies were used:
Mouse anti-β-tubulin monoclonal antibody (1:10,000; EnoGene
E1C-601; EnoGene); mouse anti-β-actin monoclonal antibody
(1:10,000; ab6276; Abcam, Massachusetts, MA, USA); rabbit anti-H3
polyclonal antibody (1:10,000; H0164; Sigma); horseradish
peroxidase (HRP)-goat anti mouse antibody (1:10,000; A3673; Sigma);
HRP-goat anti-rabbit antibody (1:10,000; sc-2030; Santa Cruz
Biotechnology, Inc.).
Xenopus embryo manipulation and
microinjection
In vitro embryo fertilization and culture
were conducted as described previously (11). For each embryo, 70 ng of NA was
injected at the 2 cell stage. The microinjection procedure was
performed as described previously (12). The use of Xenopus embyos in the
study was approved by the Ethics Comittee of the Children’s
Hospital of Chongqing Medical University (Chongqing, China).
Results
NA regulates intracellular
Ca2+ mobilization
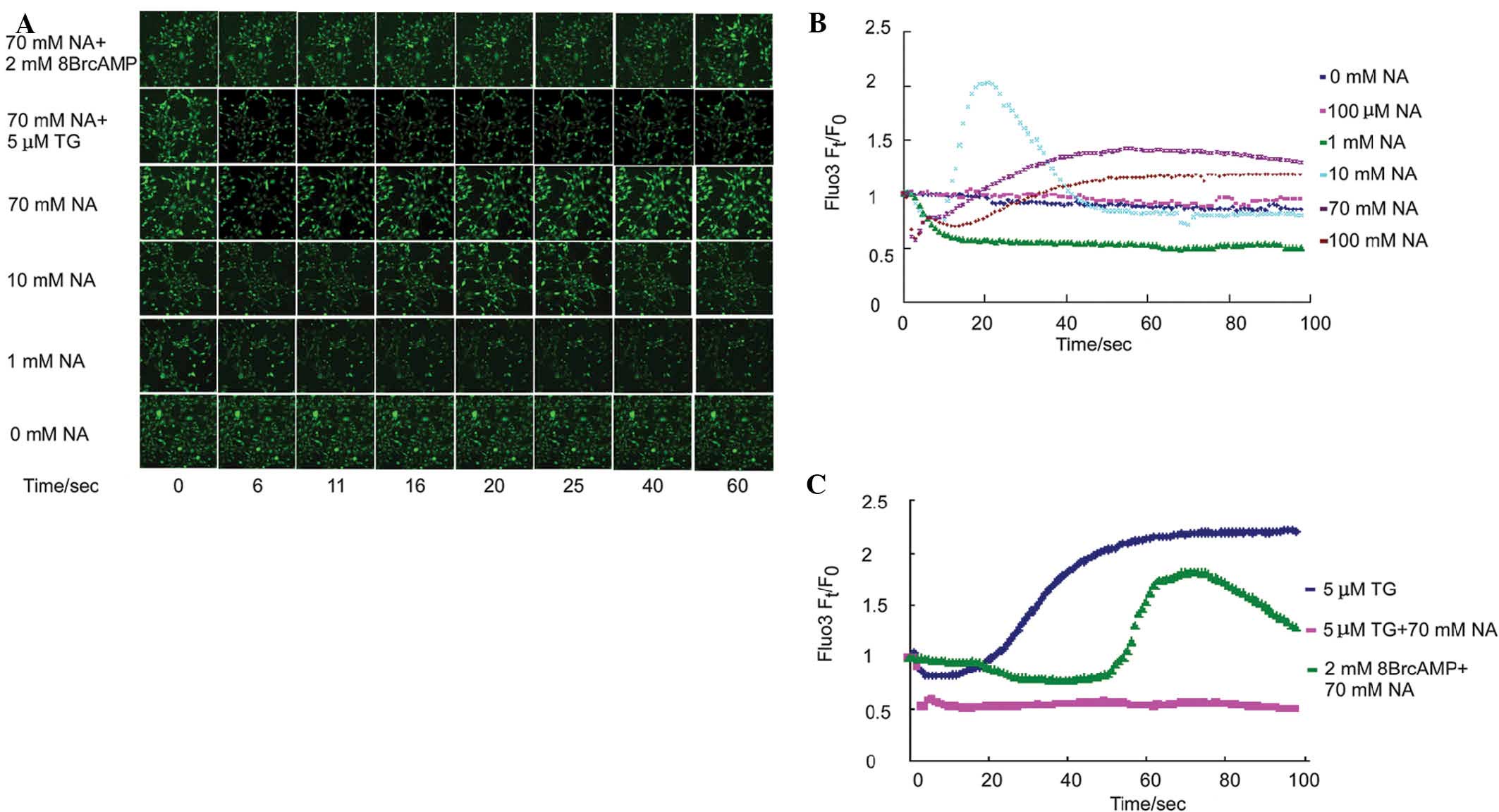
To examine the time lapse effect of NA on
intracellular free Ca2+ mobilization, Fluo3-labeled
NIH3T3 cells were incubated with different concentrations of NA,
and the fluorescence intensity was simultaneously assessed over 100
sec. The fluorescence intensity reflected the intracellular free
Ca2+ concentration. Previous studies have demonstrated
that 100 μM NA induced transient intracellular [Ca2+]
elevation in CHO-K1 cells (9),
macrophages (8) and matured
neutrophils (10) within one to
several minutes. In the NIH3T3 cell line, 100 μM NA did not alter
the intracellular Ca2+ mobilization (Fig. 1B, pink curve). The NIH3T3 cells
were further exposed to a wider span of NA concentration gradients
and the Ca2+ mobilization was assessed. At a 1 mM NA,
the intracellular Ca2+ levels decreased by 50% within 10
sec and no elevation was detected during the entire process
(Fig. 1A and B, green curve). In
the 10 mM NA exposure group, intracellular free Ca2+
reduced precipitously similarly to the observations at 1 mM NA;
however, a transient sharp elevation-reduction n-turn like curve of
Ca2+ mobilization was observed (Fig. 1A and B, light blue curve).
Consistent with the 10 mM group, both 70 mM (Fig. 1B, purple curve) and 100 mM
(Fig. 1B, brown curve) NA
decreased intracellular free [Ca2+] within the first
several seconds, and secondarily, triggered an elevation in
intracellular free [Ca2+]. Of note, secondary increase
in [Ca2+] was slower with increasing NA concentration.
In addition, NA-induced secondary [Ca2+] was inhibited
by thapsigargin (TG; Fig. 1A and
C, pink curve), an endoplasmic reticulum (ER)
Ca2+-ATPase pump inhibitor, which induces
Ca2+ release from the ER. Furthermore, the NA-induced
decrease in primary intracellular [Ca2+] was delayed by
the addition of 2 mM of the cAMP analog 8Br-cAMP (Fig. 1A and C, green curve). These data
suggested that the reduction of cAMP levels by NA may be
responsible for the primary transient ER decrease and
Ca2+ release by the endoplasmic reticulum (ER)
contributed to the later observed Ca2+ elevation.
NA disassembles the cytoskeleton and
deposits opaque materials at the perinuclear region
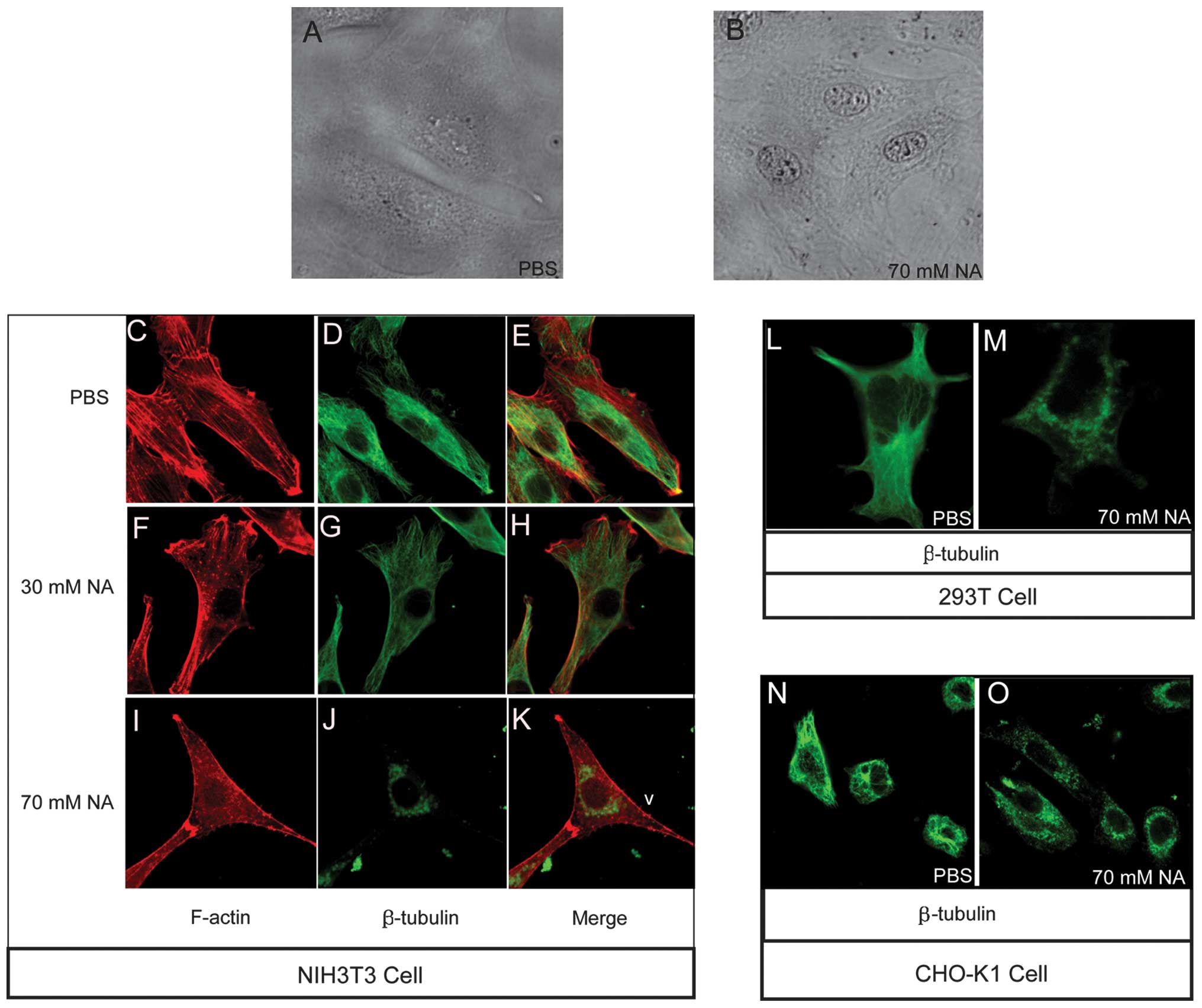
Besides intracellular Ca2+ wave
variation, the results revealed that an accumulation of
unidentified opaque material at the perinuclear region, forming a
ring-type structure, as well as at the nucleolus, was markedly
evident in the NIH3T3 cells following incubation with 70 mM NA
(Fig. 2B). However, in the
phosphate-buffered saline (PBS)-treated control group, the NIH3T3
cells exhibited a spread morphology and no perinuclear ring or dim
nucleolus phenotypes (Fig. 2A).
The highly visible nucleolus in the NA-treated cells suggested the
activation of synthetic processes; however, the manner in which the
perinuclear opaque rings formed remains elusive. Cytoskeletal
organization has a number of important roles in intracellular
transport processes. The assembly-disassembly homeostasis of
F-actin and microtubules are regulated by numerous factors,
including variations in intracellular [Ca2+] (13–17).
The NA-induced phenotypes identified in the present study allowed
for the following hypothesis: NA changes intracellular
[Ca2+], thereby obstructing cytoskeletal integrated
organization, then affecting cytoskeleton-dependent intracellular
transport and finally causing the accumulation of material at the
perinuclear area, forming an opaque ring like structure. To confirm
this hypothesis, F-actin and microtubule structures were observed
with Texas Red-X phalloidin and anti-β-tubulin antibodies,
respectively, under different concentrations of NA. In the
PBS-treated control group, the F-actin (Fig. 2C) and microtubules (Fig. 2D) were normally patterened, as
shown in the merged image in Fig.
2E. The F-actin (Fig. 2F)
filaments began to disassemble, forming punctuated spots and the
microtubules (Fig. 2G) exhibited
weaker staining following treatment with 30 mM/1 h NA. Following
incubation with 70 mM/1 h NA, the F-actin (Fig. 2I) and microtubule (Fig. 2J) cytoskeletons (merged in Fig. 2K) were completely disassembled, and
the liberated microtubule residues accumulated at the distal end of
the filopodia and perinuclear region (Fig. 2J). Further analysis confirmed the
occurrence of microtubule disassembly with 70 mM NA in the 293T
(Fig. 2L and M) and CHO-K1 cell
lines (Fig. 2N and O). These data
indicated that NA dissociates the F-actin and microtubule
cytoskeleton, which may affect intracellular transport in a
dose-dependent manner.
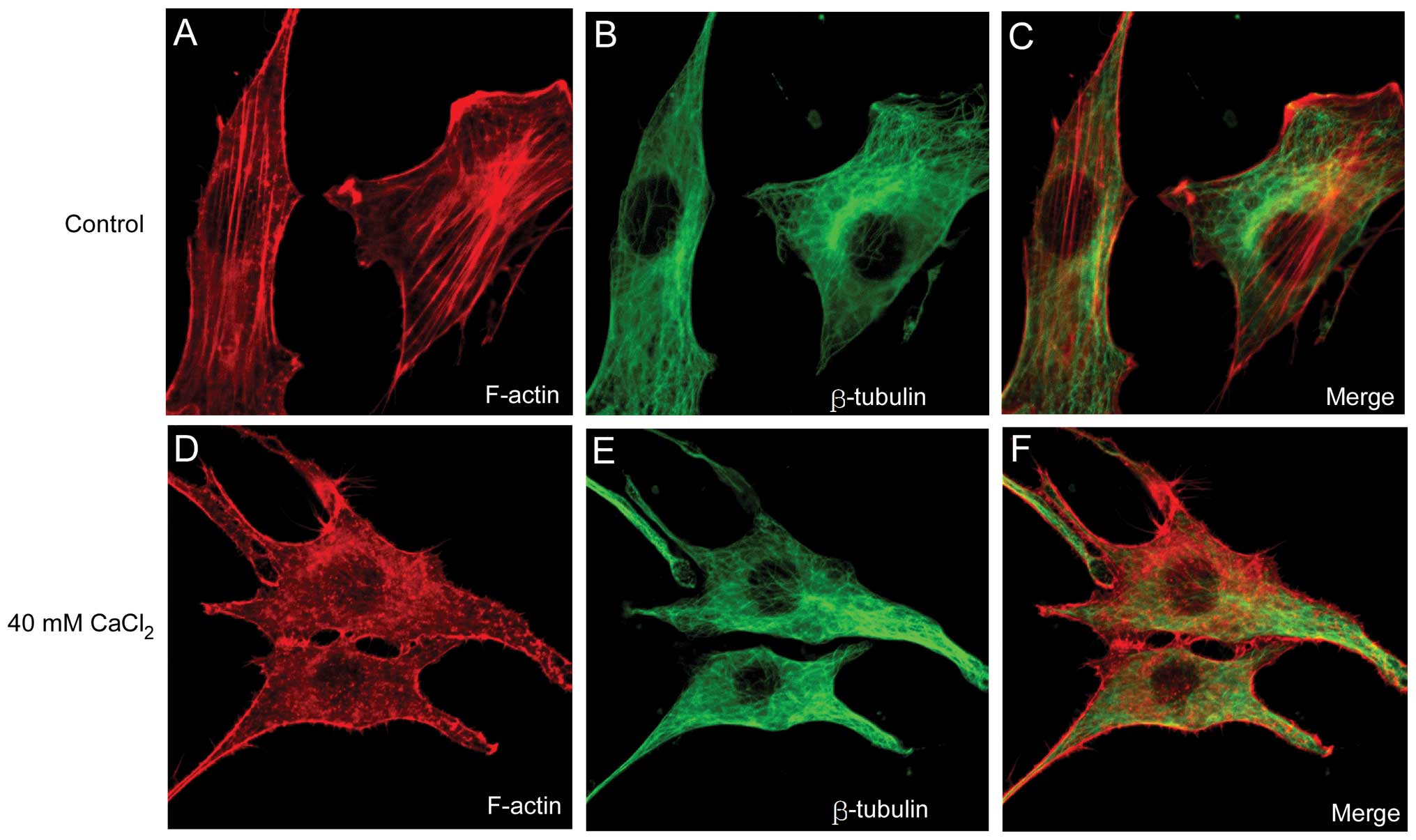
Abnormal increases in the Ca2+
concentration contribute to the disassembly of F-actin
To further elucidate the association between changes
in [Ca2+] and the disassembly of the cytoskeleton, a
CaCl2 solution was added directly into the NIH3T3 cell
culture media and the cytoskeleton structure was examined following
1 h of incubation. Consistent with the effect of NA demonstrated
above, 40 mM CaCl2 disrupted F-actin filaments (Fig. 3A) into punctuate G-actin spots
(Fig. 3D). However, artificial
increases in [Ca2+] did not affect the microtubular
structure (Fig. 3E) compared with
that of the control group (Fig.
3B). The results implied that the Ca2+ wave induced
by NA may be involved in the disruption of F-actin filaments, but
not in the disassembly of the microtubular polymer structure.
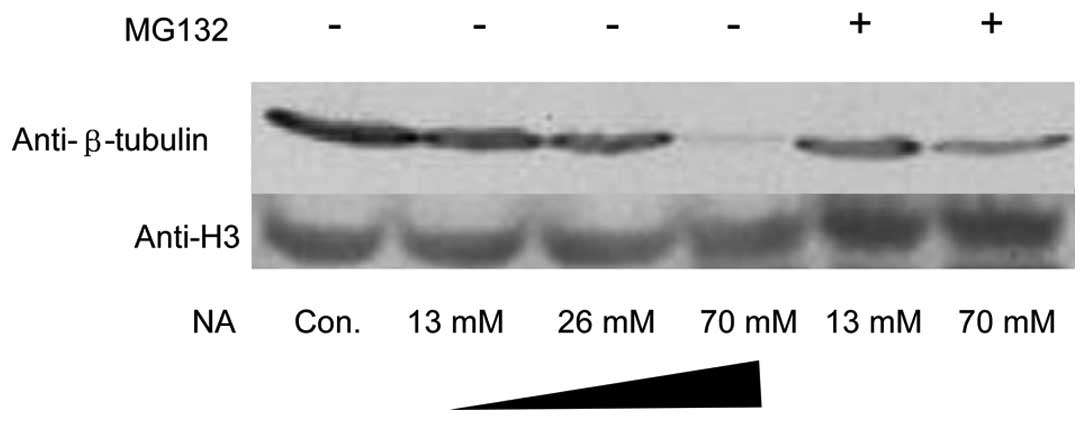
Depolymerized microtubule subunits
undergo ubiquitin-proteasome degradation
Microtubules consist of α-tubulin and β-tubulin
hetero-subunits. The microtubules were labeled with anti-β tubulin
antibody. Under exposure to 70 mM NA for 1 h, not only did the
microtubule-stained pattern change, but also its immunofluorescent
intensity decreased significantly (Fig. 2J, M and O). To confirm these
results, a total amount of β tubulin was analyzed using western
blot analysis. As expected, NA markedly downregulated β-tubulin at
the protein level. In addition, MG132, an inhibitor of the
ubiquitin-proteasome pathway, was able to reverse β-tubulin
reduction (Fig. 4). However, there
were no significant changes in F-actin monomer protein G-actin
(data not shown).
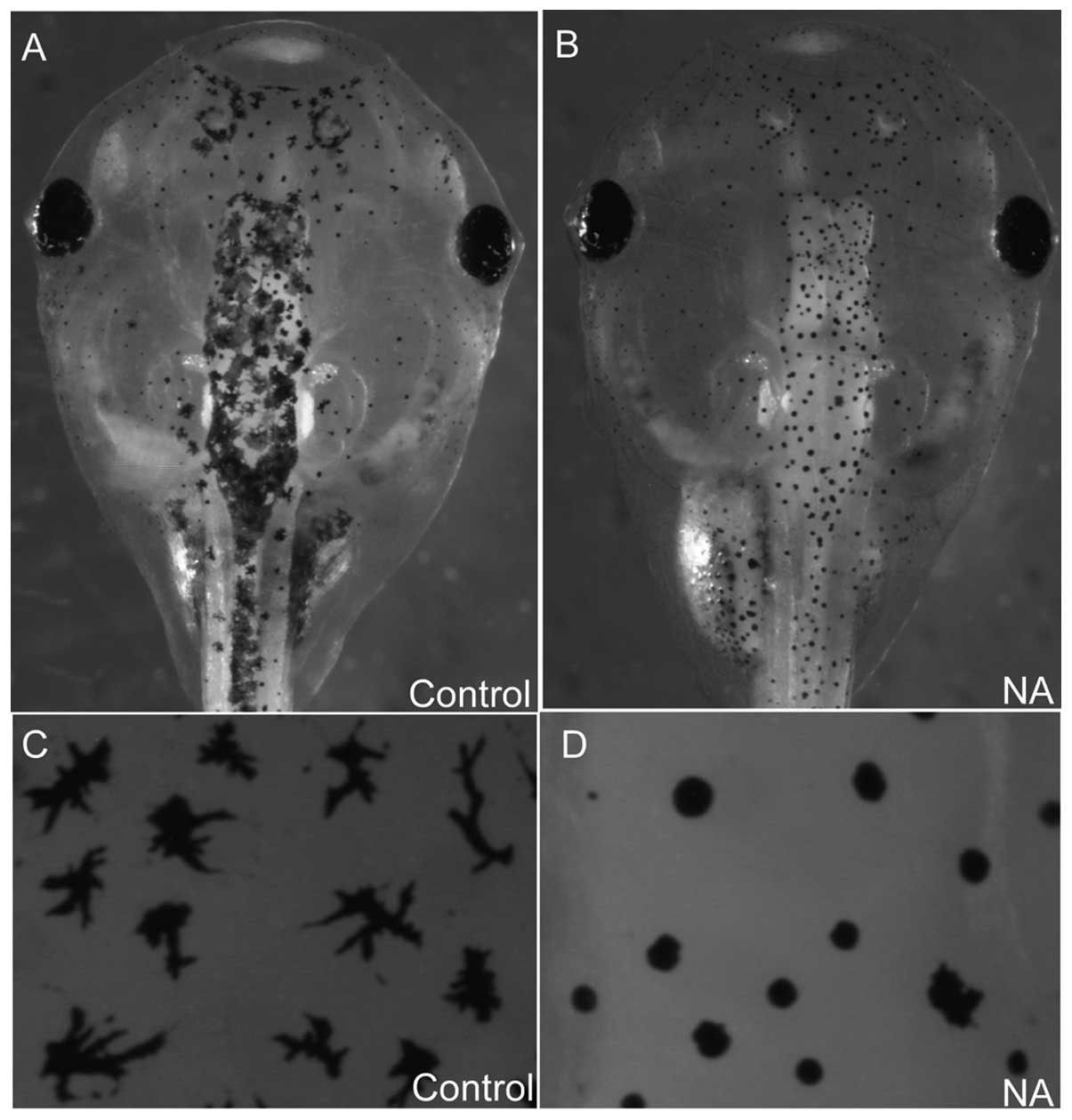
NA blocks melanosome intracellular
transport in xenopus embryos
In the cultured cells NA disrupted cytoskeletal
integrity and may have inhibited intracellular trafficking. To
investigate the effect of NA on intracellular transport processes
in vivo, 70 ng NA was microinjected into xenopus embryos and
its effect on melanosome transport in melanocytes was observed.
Melanosomes either disperse or aggregate along microtubules and F
actin-filaments (18). As they are
easy to observe, melanocytes represent a reliable system for
investigating intracellular transport. In normal embryos, the
melanosomes disperse uniformly in a dendritic type manner (Fig. 5A and C). By contrast, in
NA-microinjected embryos, melanosome transport was blocked and
exhibited an aggregated disc type morphology (Fig. 5B and D), suggesting that NA blocked
intracellular transport processes in vivo.
Discussion
Previous studies have demonstrated that 100 μM NA
evokes intracellular [Ca2+] within several minutes
(8–10); however, the detailed mechanisms
underlying this effect remains elusive. In the present study,
intracellular [Ca2+] was assessed in a time lapse manner
upon exposure to NA. NIH3T3 cells required higher quantities of NA
to evoke any effects on intracellular Ca2+. As expected,
the first response of NIH3T3 cells to NA was not an elevation but a
reduction in intracellular [Ca2+]. The [Ca2+]
increase following [Ca2+] reduction may be disrupted by
ruining Ca2+ storage in the ER by TG, an endoplasmic
reticulum Ca2+-ATPase pump inhibitor. Therefore, it was
suggested that the overall effect of [Ca2+] increase may
be divided into two steps. Firstly, NA reduces intracellular
[Ca2+], possibly via triggering the efflux of
Ca2+ ions out of the cell membrane channels. Secondly,
the Ca2+ release from the ER contributes at least in
part to the [Ca2+] elevation. Since small amounts of NA
(1 mM) do not elevate but only reduce [Ca2+], the
release of Ca2+ from the ER requires higher
concentrations of NA to trigger this process.
The present study sought to elucidate the mechanisms
underlying NA-induced changes in intracellular [Ca2+]
waves. Based on other studies and the data of the present study, a
hypothesis was proposed that the metabolism of NA adenine
dinucleotide phosphate (NAADP) is important during NA modulation of
cellular Ca2+. A putative synthesis pathway for NAADP
exists, NAADP is a well established Ca2+ mobilizing
agent that releases Ca2+ from intracellular stores
(19). In the presence of NA and
nicotinamide adenine dinucleotide phosphate (NADP), ADP-ribosyl
cyclase catalyzes the synthesis of NAADP by a base exchange
reaction and cAMP is a stimulator during this process (20–22).
Notably, NA reduces the intracellular cAMP concentration (5). At a high concentration, NA may
inhibit cAMP and thereby limit the synthesis of NAADP. The decrease
of NAADP may be responsible for the first [Ca2+] drop
upon exposure to NA. Excessive NA may rapidly overcome the
cAMP-limited step and promote the synthesis of NAADP. Therefore, a
marked [Ca2+] elevation was observed. Furthermore, a
markedly high concentration of NA (100 mM) may completely eradicate
cAMP and re-establish cAMP as a rate-limiting step. Therefore, the
[Ca2+] elevation curve following 100 mM NA treatment is
not as steep as that following 10 mM NA treatment. In the present
study, 2 mM 8-Br-cAMP (a cAMP analog) delayed and alleviated the
first [Ca2+] drop in response to NA, suggesting that
cAMP has a key role in the changes in the [Ca2+] wave
induced by NA.
It is well established that the intracellular
Ca2+ wave may modulate the cytoskeletal structure
(23–28). Although F-actins and microtubules
underwent disassembly upon incubation with NA, the external
addition of high concentrations of CaCl2 only disrupted
the F-actin filaments. It was hypothesized that besides the
[Ca2+] elevation, other pathways must also be involved
in the disassembly of microtubules. The disruption of F-actin and
microtubule cytoskeleton may definitely negatively effect the
intracellular traffic process. In cultured cells, an opaque
material accumulated around the nucleus when incubated with 70 mM
NA. It appears that the minus end (nuclear region) to plus end
(cell membrane region)-directed transport process was inhibited and
therefore, cargo was deposited in the perinuclear region. Further
evidence in the xenopus melanocyte system confirmed that NA induced
an intracytic transport deficiency.
In conclusion, the present study showed that NA
regulated the intracellular calcium concentration depending on its
initial concentration and exposure time. High concentrations of
nicotinic acid induced cytoskeletal disassembly and promoted
β-tubulin degradation in a proteasome-dependent manner. The
cytoskeletal disassembly may finally contribute to the disruption
of the intracellular transport process. Further investigations aim
to minimize the functional concentration of NA and characterize the
function of NA in different biological systems, particularly in
cancer cells and animal models. As the cytoskeleton is essential
during cell migration and EMT, interrupting the dynamic arrangement
of the cytoskeletion may break the fundamental cancerous processes
of metastasis. NA provides potential for clinical use in the
future.
Acknowledgements
The authors are grateful to Dr Bingyu Mao for
providing the experimental reagents. This study was supported by
the National Natural Science Foundation of China (grant nos.
81102519 to Y.S. and 81200878 to J.L.), the China Postdoctoral
Science Foundation funded project (no. 2012M511914 to Y.S.), and
the Chongqing Science and Technology Committee (no. cstc2012jjA0147
to Y.S.). The funders had no role in the study design, data
collection and analysis, decision to publish or preparation of the
manuscript.
References
|
1
|
Altschul R, Hoffer A and Stephen JD:
Influence of nicotinic acid on serum cholesterol in man. Arch
Biochem Biophys. 54:558–559. 1955. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Carlson LA: Nicotinic acid: the
broad-spectrum lipid drug. A 50th anniversary review. J Intern Med.
258:94–114. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Figge HL, Figge J, Souney PF, et al:
Nicotinic acid: a review of its clinical use in the treatment of
lipid disorders. J Pharm Pharmacol. 8:287–294. 1988.PubMed/NCBI
|
|
4
|
Offermanns S: The nicotinic acid receptor
GPR109A (HM74A or PUMA-G) as a new therapeutic target. Trends
Pharmacol Sci. 27:384–390. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gille A, Bodor ET, Ahmed K, et al:
Nicotinic acid: pharmacological effects and mechanisms of action.
Annu Rev Pharmacol Toxicol. 48:79–106. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Morrow JD, Parsons WG IIIrd and Roberts LJ
IInd: Release of markedly increased quantities of prostaglandin D2
in vivo in humans following the administration of nicotinic acid.
Prostaglandins. 38:263–274. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Andersson RGG, Aberg G, Brattsand R, et
al: Studies on the mechanism of flush induced by nicotinic acid.
Acta Pharmacol Toxicol (Copenh). 41:1–10. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Benyó Z, Gille A, Kero J, et al: GPR109A
(PUMA-G/HM74A) mediates nicotinic acid–induced flushing. J Clin
Invest. 115:3634–3640. 2005.PubMed/NCBI
|
|
9
|
Tunaru S, Kero J, Schaub A, et al: PUMA-G
and HM74 are receptors for nicotinic acid and mediate its
anti-lipolytic effect. Nat Med. 9:352–355. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kostylina G, Simon D, Fey MF, et al:
Neutrophil apoptosis mediated by nicotinic acid receptors
(GPR109A). Cell Death Differ. 15:134–142. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao S, Jiang H, Wang W, et al: Cloning
and developmental expression of the Xenopus Nkx6 genes. Dev Genes
Evol. 217:477–483. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shi Y, Zhao S, Li J, et al: Islet-1 is
required for ventral neuron survival in Xenopus. Biochem Biophys
Res Commun. 388:506–510. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Downey GP, Chan CK, Trudel S, et al: Actin
assembly in electropermeabilized neutrophils: role of intracellular
calcium. J Cell Biol. 110:1975–1982. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yoneda M, Nishizaki T, Tasaka K, et al:
Changes in actin network during calcium-induced exocytosis in
permeabilized GH3 cells: calcium directly regulates F-actin
disassembly. J Endocrinol. 166:677–687. 2000. View Article : Google Scholar
|
|
15
|
Forscher P: Calcium and
polyphosphoinositide control of cytoskeletal dynamics. Trends
Neurosci. 12:468–474. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rosado JA and Sage SO: The actin
cytoskeleton in store-mediated calcium entry. J Physiol.
526:221–229. 2000. View Article : Google Scholar
|
|
17
|
Wilson MT, Kisaalita WS and Keith CH:
Glutamate-induced changes in the pattern of hippocampal dendrite
outgrowth: a role for calcium-dependent pathways and the
microtubule cytoskeleton. J Neurobiol. 43:159–172. 2000. View Article : Google Scholar
|
|
18
|
Sheets L, Ransom DG, Mellgren EM, et al:
Zebrafish melanophilin facilitates melanosome dispersion by
regulating dynein. Curr Biol. 17:1721–1734. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yamasaki M, Churchill GC and Galione A:
Calcium signalling by nicotinic acid adenine dinucleotide phosphate
(NAADP). FEBS J. 272:4598–4606. 2005. View Article : Google Scholar
|
|
20
|
Aarhus R, Graeff RM, Dickey DM, et al:
ADP-ribosyl cyclase and CD38 catalyze the synthesis of a
calcium-mobilizing metabolite from NADP. J Biol Chem.
270:30327–30333. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wilson H and Galione A: Differential
regulation of nicotinic acid–adenine dinucleotide phosphate and
cADP-ribose production by cAMP and cGMP. Biochem J. 331:837–843.
1998.
|
|
22
|
Rah SY, Mushtaq M, Nam TS, et al:
Generation of cyclic ADP-ribose and nicotinic acid adenine
dinucleotide phosphate by CD38 for Ca2+ signaling in
interleukin-8-treated lymphokine-activated killer cells. J Biol
Chem. 285:21877–21887. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sobue K, Kanda K, Adachi J, et al:
Calmodulin-binding proteins that interact with actin filaments in a
Ca2+-dependent flip-flop manner: survey in brain and
secretory tissues. Proc Natl Acad Sci USA. 80:6868–6871. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shin DM, Zhao XS, Zeng W, et al: The
mammalian Sec6/8 complex interacts with Ca2+ signaling
complexes and regulates their activity. J Cell Biol. 150:1101–1112.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Constantin B, Meerschaert K,
Vandekerckhove J, et al: Disruption of the actin cytoskeleton of
mammalian cells by the capping complex actin-fragmin is inhibited
by actin phosphorylation and regulated by Ca2+ ions. J
Cell Sci. 111:1695–1706. 1998.
|
|
26
|
Brown SS, Yamamoto K and Spudich JA: A
40,000-dalton protein from Dictyostelium discoideum affects
assembly properties of actin in a Ca2+-dependent manner.
J Cell Biol. 93:205–210. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yamamoto H, Fukunaga K, Tanaka E, et al:
Ca2+- and calmodulin-dependent phosphorylation of
microtubule-associated protein 2 and tau factor, and inhibition of
microtubule assembly. J Neurochem. 41:1119–1125. 1983.
|
|
28
|
Gradin HM, Marklund U, Larsson N, et al:
Regulation of microtubule dynamics by
Ca2+/calmodulin-dependent kinase IV/Gr-dependent
phosphorylation of oncoprotein 18. Mol Cell Biol. 17:3459–3467.
1997.PubMed/NCBI
|