Introduction
Tumor cell lysate (TCL) refers to the tumor lysate
mixture that results following the artificial lysis of tumor cells.
This mixture has multiple tumor antigens and thus possesses the
ability to induce the activation of antitumor cells. However, TCL
has a dual effect on immune cells. TCL not only activates these
immune cells, but may also induce immunosuppression. For patients
with myeloma that receive treatment with an autologous dendritic
cell (DC) vaccine, DCs should be incubated with the TCL derived
from myeloma in vitro. During this incubation, TCL may
induce a subset of DCs with stimulatory activity, while having the
ability to induce immunotolerance in another subset of DCs
(1). However, a transfusion of
this subset of DCs may attenuate the specific immune response of
effector T cells to tumor-associated antigens, resulting in
antigen-specific immunotolerance of T cells and subsequently a low
tumor response rate and short survival time (1). In a previous study, a TCL derived
from Lewis lung cancer cells, in combination with mycobacterial
heat shock protein 65 (MHSP65), was used to prepare MHSP65-TCL,
which was found to activate the immune cells of mice during the
early phase of immunization and was characterized by an
upregulation of CD69 expression. However, during the late phase,
the immune cell response to the vaccine decreased, accompanied by a
reduction in the expression levels of CD69 (2).
The dual effects of TCL are ascribed to the
different characteristics of the lysate components. As a complex
mixture, TCL contains not only antigens, but also other substances
that inhibit the immune cells or even induce cell apoptosis.
Hyaluronan (HA) is a component of the extracellular matrix and can
be secreted by nine types of cancer cells, including human lung
cancer 95D cells and liver cancer HepG2 cells. The HA secreted by
these cells may induce the production of immunosuppressive
monocyte/macrophages (MΦ) and DCs in vitro (3). Although these cells are in an active
state, they may induce the apoptosis of T lymphocytes, while they,
themselves, are also susceptible to apoptosis (4). In addition to the inhibitory effect
on immune cells, certain proteins secreted by cancer cells may
directly induce cell apoptosis. Fas ligand (Fas-L) and transforming
growth factor-β (TGF-β) are proteins that are closely associated
with the apoptosis of immune cells and are potentially localized in
the TCL. Fas-L can bind to Fas on immune cells to induce the
activation of caspase in immune cells and to further induce cell
apoptosis (5). However, TGF-β may
act on the TGF-β receptor to activate the extracellular
signal-regulated kinase/mitogen-activated protein kinase signaling
pathway resulting in the apoptosis of immune cells (6). In addition, immunohistochemistry and
western blotting demonstrated that the two proteins were expressed
in the cancer cells. Western blot analysis demonstrated that Fas-L
is expressed in 16 human lung cancer cell lines. In addition,
immunohistochemistry results have demonstrated the expression of
Fas-L in 23 out of 28 types of resected lung cancer (7). Furthermore, breast cancer cells also
express Fas-L and lymphocyte apoptosis has been observed in
adjacent normal tissues surrounding breast cancer tissues (8). Immunohistochemistry results have also
revealed that the expression of TGF-β was at a high level in 45
lung cancer samples (9). Patients
with high TGF-β expression levels in lung cancer cells were found
to have a significantly shorter survival time following surgery
(10). Immunohistochemistry and
western blot assays enable the detection of intracellular proteins
and thus, it was hypothesized that the TCL prepared from cancer
cells may contain Fas-L and TGF-β.
On the basis of the aforementioned findings, the
present study was undertaken to determine the concentration of HA,
pro-apoptotic Fas-L and TGF-β in the TCL from Lewis cells, and to
further investigate whether TCL induces the production of
immunosuppressive cells and the apoptosis of immune cells through
these proteins.
Materials and methods
Mice and cell lines
Female C57BL/6 mice were purchased from Nanjing
Qinglong Mountain Laboratory Animal Co., Ltd. (Nanjing, China) and
maintained in microisolator cages under pathogen-free conditions.
All mice were studied at 6–8 weeks of age. Experimental
manipulation of the mice was undertaken in accordance with the
National Institute of Health Guide for the Care and Use of
Laboratory Animals (Bethesda, MA, USA). A mouse Lewis lung cancer
cell line was purchased from the American Type Culture Collection
(Manassas, VA, USA) and maintained in high-glucose Dulbecco’s
modified Eagle’s medium (Wuhan Boshide Biotechnology Co., Wuhan,
China) supplemented with 10% fetal calf serum (FCS; Invitrogen Life
Technologies, Carlsbad, CA, USA), 100 U/ml penicillin and 100 μg/ml
streptomycin (Sigma-Aldrich, St. Louis, MO, USA). This study was
approved by the Ethics Committee of Wannan Medical College (Wuhu,
China).
Preparation of TCL
To prepare the TCL, cultured Lewis cells were lysed
using a freezing-thawing cycle in a 0.85% NaCl solution. This was
repeated five times in rapid succession, between −70°C and 37°C and
then refrozen and stored in a −70°C refrigerator until use. Each of
the TCLs were detected under a microscope (Olympus Corporation,
Tokyo, Japan) using trypan blue staining (Sigma-Aldrich, St. Louis,
MO, USA) following the final thawing.
Isolation of monocytes and culture of
DCs
Peritoneal MΦs were isolated using plastic adhesion
and further subset purification was performed with magnetic beads
(Miltenyi Biotech, Bergisch, Gladbach, Germany) and specific
biotin-conjugated antibodies (BD Biosciences, Franklin Lakes, NJ,
USA), yielding >98% cell purity. Subsequently, MΦ
(1×106 cells/ml) were cultured in DMEM medium (Wuhan
Boshide Biotechnology Co., Wuhan, China) with 10% FBS and added to
either 0.85% NaCl or a TCL prepared from 1×106 Lewis
cells for 72 h. The culture supernatant was collected every 24 h.
To prepare murine DCs, bone marrow cells were harvested from the
tibiae and femurs of the C57/BL6 mice and depleted of red blood
cells using a red blood cell lysis buffer (Sigma-Aldrich). Bone
marrow cells were cultured in an RPMI-1640 medium containing 10%
FBS, 100 U/ml penicillin, 100 μg/ml streptomycin and 50 μM
2-mercaptoethanol (Invitrogen Life Technologies), supplemented with
20 ng/ml murine granulocyte-macrophage colony-stimulating factor
(GM-CSF) and IL-4 (Miltenyi Biotech) in the presence of NaCl or a
TCL prepared from Lewis cells (1×106). On days 3 and 6,
the culture medium was replaced with a fresh medium supplemented
with GM-CSF. From day 5, the culture supernatant was collected
every 24 h.
Flow cytometric analysis
The mouse spleen cells (1×106/ml) that
were co-cultured with either TCL prepared from Lewis lung cancer
cells (1×106) or 0.85% NaCl for 48 h were collected,
washed and resuspended in phosphate buffered-saline (PBS)
supplemented with 1% heat-inactivated fetal bovine serum.
Thereafter, the mouse spleen cells were stained with Annexin V and
propidium iodide (BD Pharmingen, San Diego, CA, USA) to detect
apoptosis or stained with fluorescein isothiocyanate-labeled
anti-CD4 and phycoerythrin-labeled anti-CD25 monoclonal antibodies
(mAB; BD Pharmingen) to analyze the activation of regulatory T
(Treg) cells. The cells were then put on ice in the dark for 30
min, washed with a fluorescence-activated cell sorting buffer (1X
PBS) and analyzed by flow cytometry (FACS Calibur;
Becton-Dickinson, San Jose, CA, USA).
Western blot analysis
The TCL was prepared from Lewis cells
(1×106) and was then separated using SDS-PAGE. The
protein was transferred on gel onto a nitrocellulose membrane. The
membrane was incubated with an anti-mFas-L mAb (Wuhan Boshide
Biotechnology Co.) and an anti-rabbit polyclonal IgG-horseradish
peroxidase successively (Wuhan Boshide Biotechnology Co.). To
detect the membrane protein, 0.01 g 3,3′-diaminobenzidine (Sigma)
was dissolved in 10 ml of 1X PBS with 0.25 g NiSO4 and
15% H2O2 (1.5 μl).
DNA ladder
The spleen cells (1×106) were treated
with TCL that was prepared from either Lewis lung cancer cells
(1×106) or 0.85% NaCl for 48 h and were then collected
and washed twice with PBS. The splenocyte DNA was extracted using
the Genomic DNA Mini Preparation kit with Spin Column (Beyotime
Institute of Biotechnology, Haimen, China). Subsequently, ~15 μg
DNA was loaded onto a 1.5% agarose gel and analyzed using
electrophoresis. The gel was stained with ethidium bromide and
visualized under ultraviolet light.
ELISA
Concentrations of tumor necrosis factor-α (TNF-α),
interleukin (IL)-10, HA and TGF-β were determined using ELISA kits
(Wuhan Boshide Biotechnology Co.).
Statistical analysis
In the present study, the data on cytokine
concentrations and surface marker expression are presented as the
mean ± standard deviation. Statistical significance was determined
using Student’s t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
HA in TCL from Lewis cells induces the
production of immunosuppressive MΦ and DCs in mice
HA is a matrix required for the growth of cancer
cells. HA is secreted by cancer cells and may induce the production
of immunosuppressive cells, resulting in the inability of immune
cells to kill cancer cells (11–13).
Certain cells can synthesize HA, including liver cancer HepG2
cells, cervical cancer HeLa cells and lung cancer 95D cells
(3). To investigate whether HA is
present in the TCL of mouse Lewis lung cancer cells, Lewis cells
were subjected to repeated freezing and thawing to prepare the TCL.
ELISA was then performed to detect HA in the TCL. The results
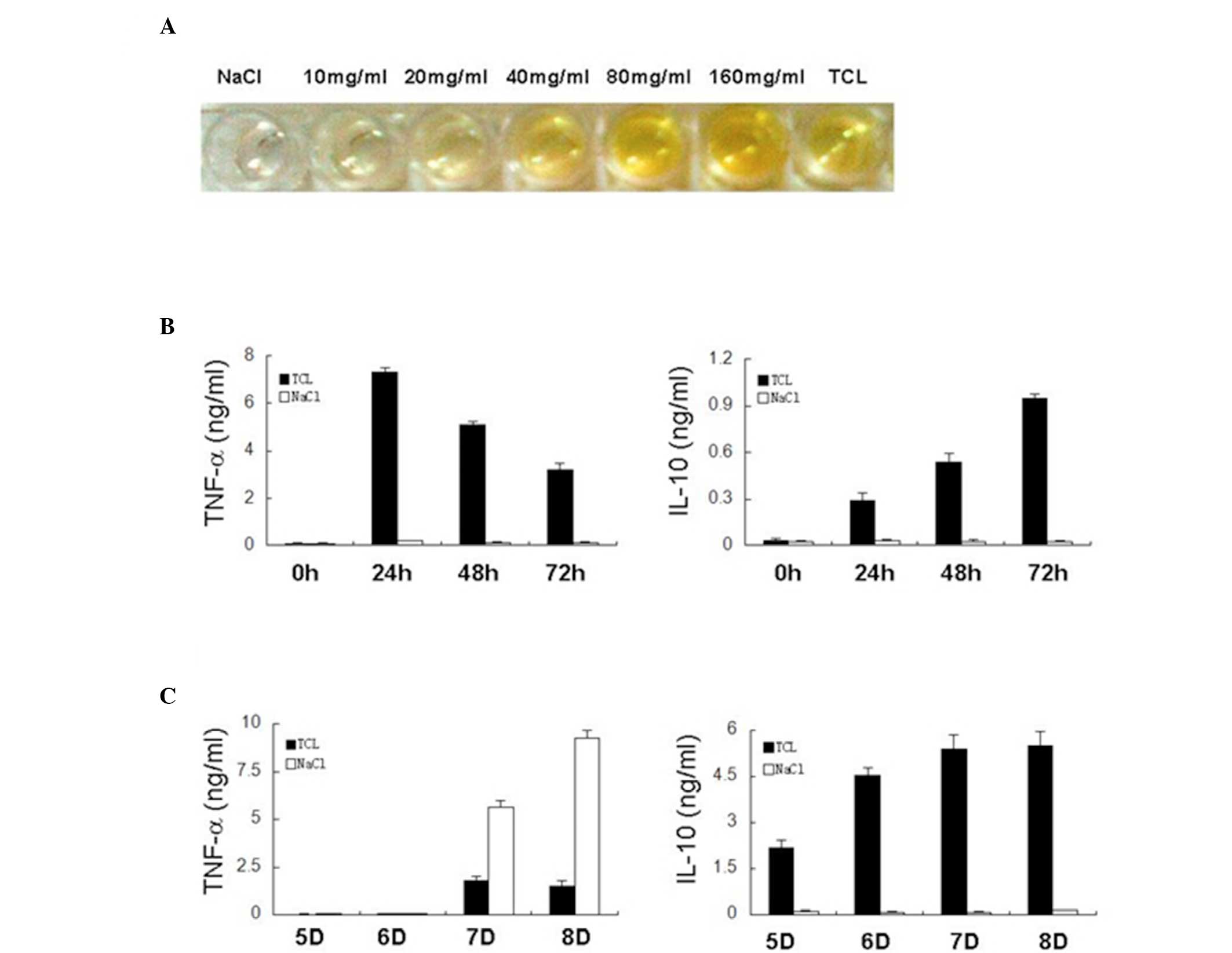
demonstrated the presence of mouse-derived HA in the TCL. Following
comparison of HA in the TCL with standard HA, the concentration of
HA in the TCL was 42 mg/ml in the Lewis lung cancer cells
(1×106; Fig. 1A). In
our previous study, the mice were immunized at four time points.
Thus, the concentration of accumulated HA may be >42 mg/ml in
vivo (2). HA may induce the
production of immunosuppressive MΦs and DCs. Following the
detection of HA in the TCL, whether TCL could induce the production
of these immunosuppressive cells was further investigated. TCL from
the Lewis cells was used to treat mouse MΦs and DCs independently
and then the levels of TNF-α and IL-10 secreted by the MΦs and DCs
were detected. As shown in Fig.
1B, after 24 h treatment with TCL, the concentrations of TNF-α
and IL-10 secreted by the MΦs were markedly increased. The
concentration of TNF-α from the MΦs began to decrease at 48 h.
However, the concentration of IL-10 continuously increased and
remained at a high level at 72 h. Overall, following incubation
with the TCL, the mouse MΦs had an increased secretion of TNF-α
during the early phase that continuously decreased thereafter.
However, IL-10 secreted by the mouse MΦs continuously increased.
Following NaCl treatment, the quantity of TNF-α and IL-10 from the
MΦs remained stable at 24, 48 and 72 h. At four time points, the
TNF-α concentration was 0.067, 0.174, 0.116 and 0.117 pg/ml,
respectively (P<0.01 vs. TCL group). The IL-10 concentration was
0.022, 0.029, 0.025 and 0.023 pg/ml, respectively (P<0.01 vs.
TCL group).
As with the mouse MΦs, the expression levels of
IL-10 gradually increased in the immature mouse DCs after 6 days
incubation with TCL in vitro (Fig. 1C). On day 5, the DCs remained
immature and IL-10 secretion began to increase. When these cells
had become completely mature on day 6, the IL-10 secretion
continuously increased on days 7 and 8. By contrast, the secretion
of IL-10 in the mouse DCs remained unaltered following NaCl
treatment regardless of the maturation of the DCs. However, when
NaCl-treated DCs had become mature, lipopolysaccharide (LPS) could
stimulate the excessive secretion of TNF-α by mature DCs. Following
a 24 (day 7) and 48 h (day 8) treatment with LPS, the TNF-α
concentration was 5.655 and 9.255 ng/ml, respectively. In addition,
the TCL-treated DCs were insensitive to LPS. After a 24 (day 7) and
48 h (day 8) treatment with LPS, the TNF-α concentration was 1.790
and 1.515 pg/ml, respectively (P<0.01 vs NaCl control).
Fas-L and TGF-β in TCL from Lewis cells
induces the apoptosis of mouse lymphocytes
Cancer cells may induce the apoptosis of immune
cells by secreting pro-apoptotic factors (Fas-L and TGF-β), which
is one of mechanisms underlying the escape of cancer cells from
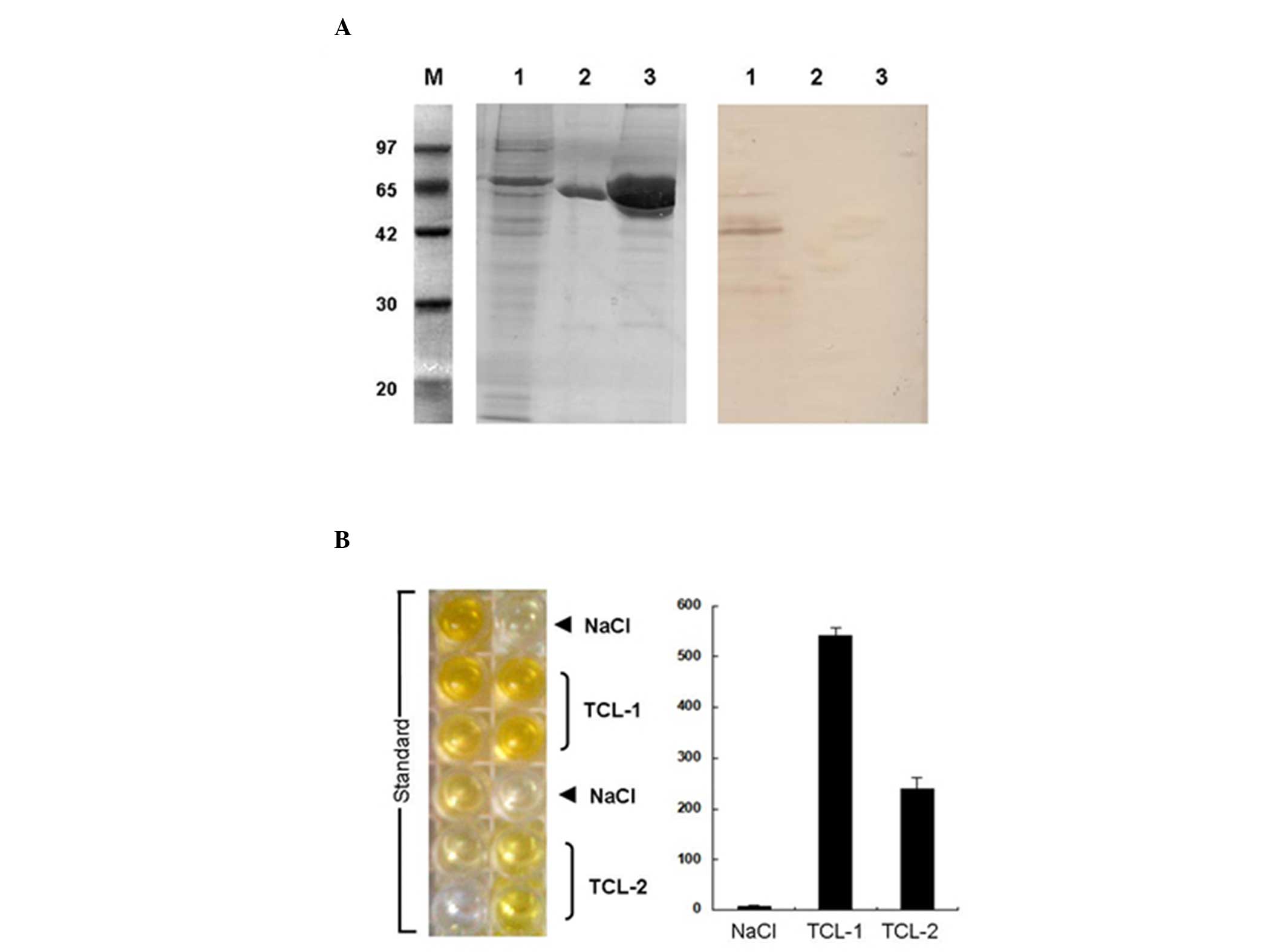
antitumor immunity (14–19). In the present study, western blot
analysis and ELISA were performed to detect Fas-L and TGF-β in the
TCL from Lewis lung cancer cells (Fig.
2A). Western blot analysis demonstrated that the Fas-L
concentration was 200 ng/ml in the TCL from the Lewis cells
(1×106) and that the Fas-L detected by the western blot
assay had a high specificity and could not react with non-specific
proteins (including MHSP65 and bovine serum albumin). ELISA
demonstrated that the TGF-β concentration was 239.64 pg/ml in the
TCL from the Lewis cells (1×106). However, no TGF-β was
identified in the TCL from the Lewis cells in the NaCl group
(Fig. 2B).
Subsequently, the mouse splenocytes were incubated
with the TCL for 48 h in vitro. The results demonstrated
that TCL was able to markedly stimulate the apoptosis of mouse
splenocytes (Fig. 3A). When
compared with the NaCl group, the apoptotic rate was as high as
34.82% (P<0.05). In addition, the apoptotic rate of the mouse
splenocytes decreased to 0.55% following incubation with LPS for 48
h. If the mouse splenocytes were simultaneously treated with TCL
and LPS, their apoptotic rate increased to 1.53% (P<0.05 vs. LPS
group). A DNA ladder assay also revealed that TCL was able to
promote the apoptosis of mouse splenocytes. In the TCL-treated
mouse splenocytes, electrophoresis of the genomic DNA revealed
evident DNA ladders. In the NaCl group and the LPS group,
electrophoresis of the genomic DNA failed to show any DNA ladders.
However, following simultaneous treatment with LPS and TCL,
electrophoresis of the genomic DNA from the mouse splenocytes again
demonstrated DNA ladders (Fig.
3B).
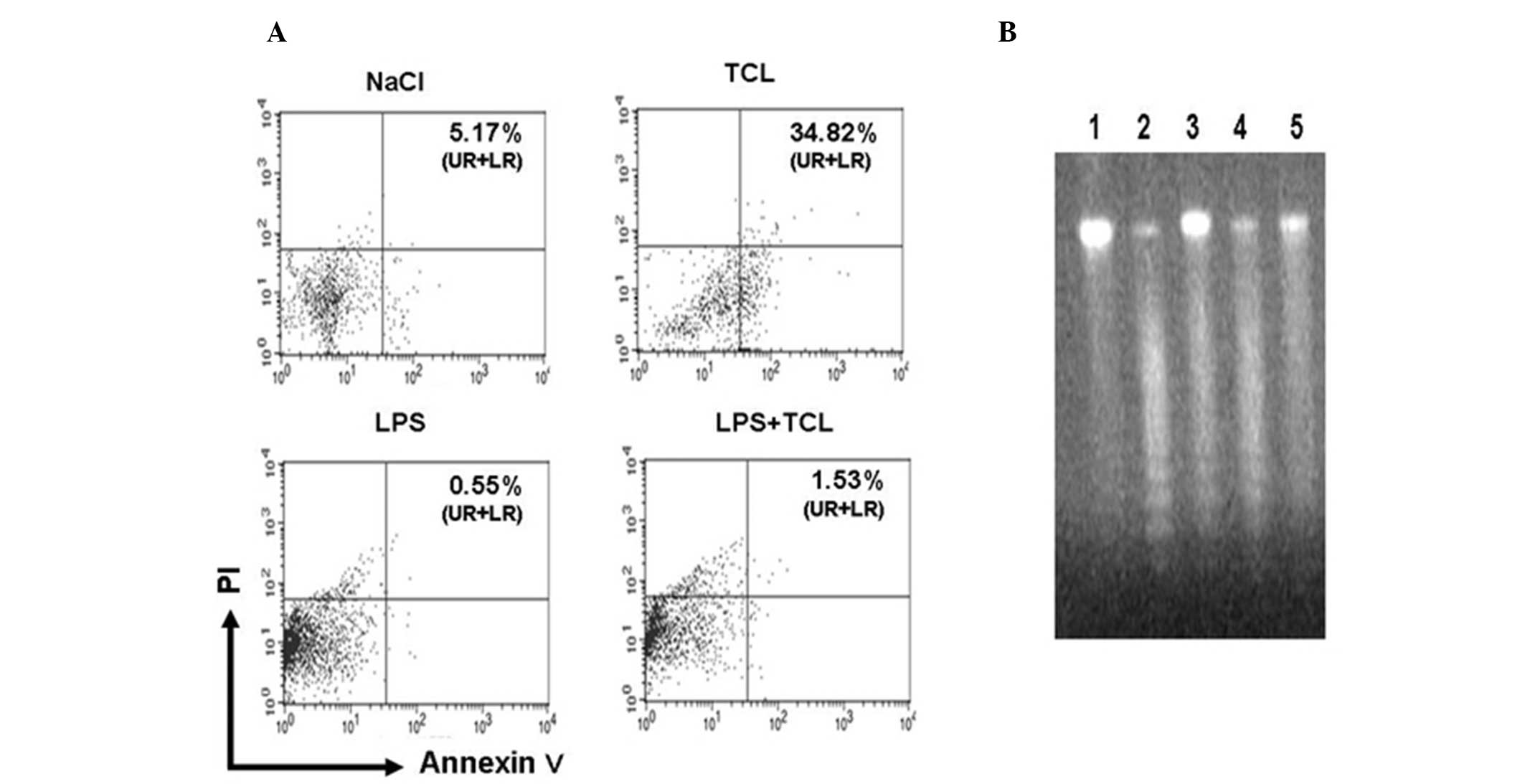 | Figure 3TCL induces the apoptosis of mouse
splenocytes. Mouse splenocytes were cultured for 48 h in a medium
containing 0.85% NaCl, TCL from Lewis lung cancer cells, MHSP65,
LPS alone or LPS + TCL. The level of apoptosis in slpenocytes was
determined by flow cytometry or DNA ladder. (A) The numbers in each
plot image represent the positive percentage of Annexin V (UR
percentage + LR percentage). Representative data from one of the
three experiments are shown. (B) Genomic DNA of mouse splenocytes
cultured with medium containing 0.85% NaCl (Lane 5), TCL from Lewis
lung cancer cells (Lane 2), MHSP65 (Lane 4), LPS alone (Lane 1) or
LPS + TCL (Lane 3) was analyzed by agar-gel. The ladder degraded
bands of genome DNA indicate apoptosis of splenocytes. MHSP65,
mycobacterial heat shock protein 65; LPS, lipopolysaccharide; TCL
tumor cell lysate; PI, propidium iodide; NaCl, sodium chloride; UR,
upper right; LR, lower right. |
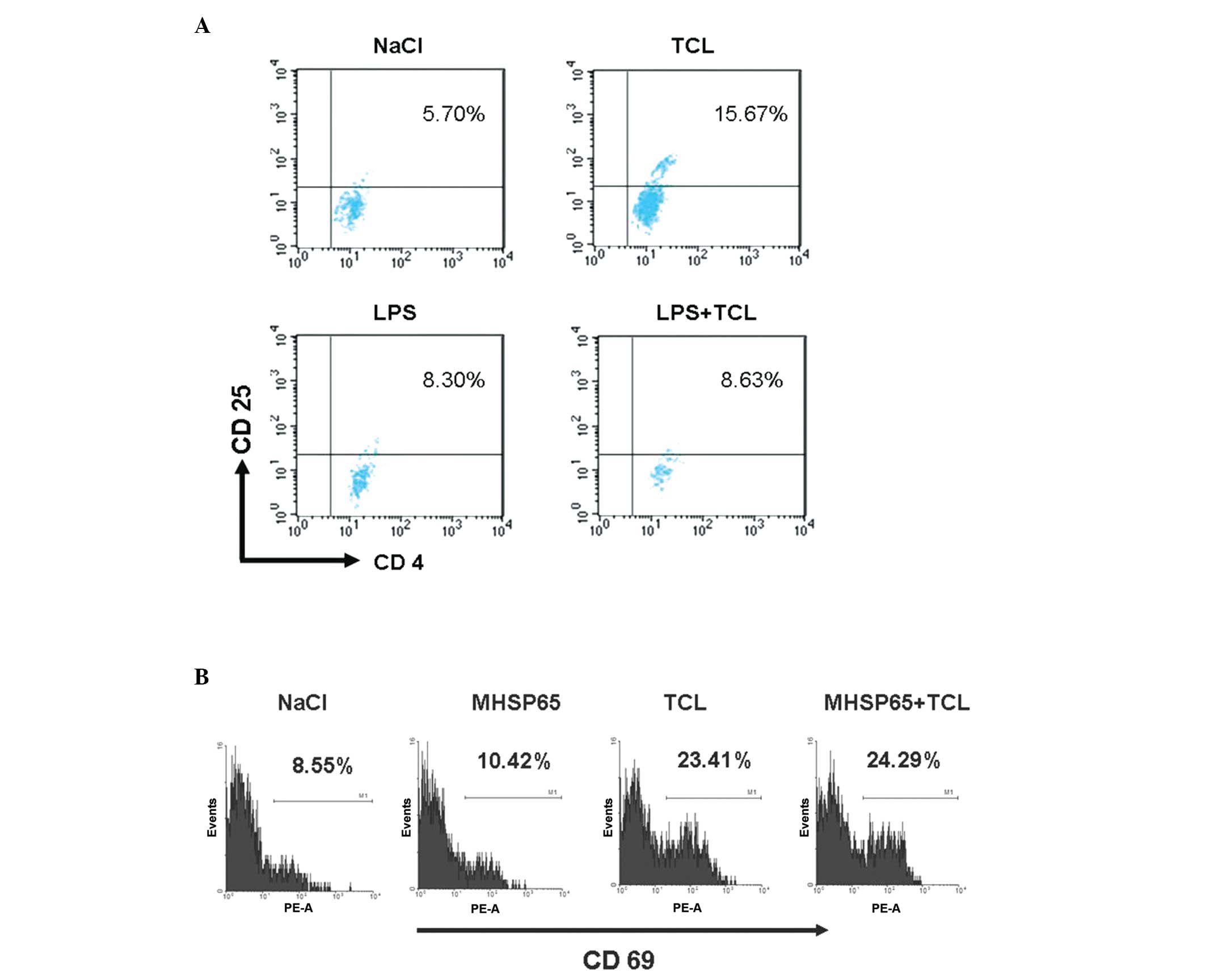
TCL from Lewis cells upregulates CD69
expression in mouse splenic lymphocytes and induces production of
Treg cells
The transformation of CD4+ T cells into
Treg cells is an inducer of tolerance to tumor vaccination
(20). In order to investigate
whether TCL-induced tolerance to tumor vaccines is associated with
Treg cells, mouse splenic lymphocytes were treated with TCL in
vitro for 48 h and then flow cytometry was performed to detect
the Treg cells (Fig. 4A). The
results demonstrated that following TCL treatment, the proportion
of Treg cells was 15.67% in the CD4+ T lymphocytes.
However, in the NaCl group, the proportion of Treg cells was 5.70%
(P<0.05). Notably, in the LPS-treated CD4+ T
lymphocytes, the proportion of Treg cells was 8.30% and in the
CD4+ T lymphocytes treated with LPS and TCL, the
proportion of Treg cells was 8.63%, suggesting that the number of
Treg cells was not significantly increased (P>0.05 vs. LPS
group). Treg cells are a group of immunosuppressive CD4+
T lymphocytes. The production of Treg cells is associated with CD69
expression and TGF-β secretion in immune cells (21). Consistently, after 48 h treatment
with TCL, the expression of CD69 was significantly increased in the
mouse splenic lymphocytes with CD69 expressed in 23.41% of the
splenic lymphocytes (Fig. 4B).
However, in the NaCl group, CD69 expression was observed only in
8.55% of the splenic lymphocytes (P<0.01).
Discussion
TCL has long been used as a stimulator of immune
cells and as an antitumor vaccine. However, there are substances in
TCL that can exert an immunosuppressive effect and induce
apoptosis, resulting in adverse effects on the action of TCL. Thus,
it is imperative to detect these substances and to investigate the
mechanisms underlying the effect of these substances on
TCL-mediated immunoactivation (22,23).
In our previous studies, TCL was prepared from Lewis lung cancer
cells and the results demonstrated that the use of TCL could induce
antitumor immunity that was not maintained during the late phase
(2). It was hypothesized that this
may be attributed to certain components in the TCL that could
inhibit immune cells. In the present study, the results
demonstrated the presence of HA in the TCL from Lewis lung cancer
cells and it has been confirmed that HA is closely associated with
the production of immunosuppressive immune cells (MΦ and DC). The
concentration of HA was 42 mg/ml in the TCL from the Lewis lung
cancer cells (1×106). In prior animal studies, the
animals were immunized at several time points and thus, the in
vivo HA concentration may be higher. At this concentration, HA
was able to induce the production of immunosuppressive cells
(24). The TCL-treated MΦs were
consistently found to possess an elevated capability to secrete
TNF-α and the secretion of TNF-α decreased over time. However, the
secretion of IL-10 gradually and continuously increased. In the
NaCl-treated MΦs, the secretion of TNF-α and IL-10 demonstrated an
opposite tendency to that of the TCL-treated MΦs. Similar findings
in the secretion of TNF-α and IL-10 by mouse DCs were also
demonstrated following TCL or NaCl treatment. These findings
suggest that following TCL treatment, the MΦs and DCs transform
into a special group of immunosuppressive MΦs. The
immunosuppressive MΦs and DCs are characterized by a reduction in
the secretion of TNF-α and IL-12 and an increase in the secretion
of IL-10, an inhibitory cytokine (25). These immunosuppressive MΦs and DCs
may be derived from immature mouse MΦs and DCs following incubation
with the HA in the TCL. If immunosuppressive MΦs and DCs are
present, they may secrete a large quantity of IL-10 to induce the
apoptosis of T lymphocytes. In addition, immunosuppressive MΦs and
DCs are also susceptible to a secondary apoptosis following
transient activation, which may result in a reduction in the
proportion of antigen-presenting cells in the immune cells and may
affect TCL-activated antitumor immune cells (4,26).
In addition to HA, two pro-apoptotic factors (Fas-L
and TGF-β) were also detected in the TCL. In the TCL from Lewis
lung cancer cells (1×106), the concentration of Fas-L
and TGF-β was 200 ng/ml and 239.64 pg/ml, respectively. The two
proteins may assist cancer cells in escaping antitumor immunity.
Fas-L may bind to Fas on the immune cells to trigger a cysteine
protease cascade to induce the apoptosis of antitumor immune cells
and thus, these immune cells fail to exert an antitumor effect
(27). TGF-β is a multifunctional
cytokine that can be secreted by cancer cells and at the same time,
act on themselves by exerting an antitumor effect. If there is
TGF-β in the TCL, the TGF-β may act on the immune cells to inhibit
their function or even induce the apoptosis of these immune cells.
Thus, TGF-β assists cancer cells in evading the antitumor immunity
and promotes cancer formation (28). In the present study, TCL was
directly used to treat mouse splenocytes over 48 h and the
apoptosis of these cells was observed (apoptotic rate: 34.82%).
This may be attributed to the direct contact between the
splenocytes and Fas-L/TGF-β in the TCL. The ability of TCL to
induce cell apoptosis is potent and, as a potent immune activator,
LPS fails to completely inhibit this effect. Thus, although there
are cancer antigens in the TCL, which could activate immune cells
as LPS does, other factors, including Fas-L and TGF-β, may also
induce the apoptosis of immune cells (apoptotic rate of mouse
splenocytes: 34.82%). This undoubtedly affects TCL-mediated
antitumor immunity.
The apoptosis of immune cells is associated with not
only the pro-apoptotic factors, but also with the upregulation of
the expression of CD69 on these cells (29). If the expression of CD69 on T
lymphocytes and natural killer NK cells increases, the CD69 cells
may activate secondary messengers and protein kinases, including
Ca2+ and protein kinase C, which further activate
signaling pathways resulting in increased secretion of TGF-β by the
T lymphocytes and NK cells. TGF-β acts on immune cells to induce
their apoptosis (30). In our
previous study and in the present study, the results demonstrated
that CD69 expression in the mouse splenic lymphocytes significantly
increased following treatment with TCL and the proportion of CD69
positive cells was 23.41%. Of note, of the TCL-treated mouse
splenic lymphocytes, CD4+/CD25+ Treg cells
were detectable. Treg cells are a group of CD4+ T
lymphocytes with immunosuppressive capability and can inhibit the
activation of certain immune cells, including CD4+
cells, CD8+ T cells, NK cells and monocytes (31). In addition, the inhibitory activity
of Treg cells is associated with the excessive expression of CD69
on them (32). In our previous
study, CD69 was used as a marker for the activation of immune cells
(21). Cancer antigens in the TCL
could effectively activate immune cells that were characterized by
an increase in the expression of CD69 on these cells (33). However, although CD69 may be used
as a marker for immune cell activation, the expression of CD69 is
not involved in the activation of immune cells and the occurrence
of antitumor immunity (34).
CD69−/− mice were found to have a more potent antitumor
capability (35). If an anti-CD69
antibody was used to inhibit CD69 expression, an antitumor effect
was observed in cancer-bearing mice, suggesting the antitumor
effect of the CD69 antibody (30).
Thus, as for the detection of immune activators in the TCL, other
cellular surface molecules, including HLA-DR and CD38, may be used
(36). In addition, inhibition of
the expression of CD69 assists in increasing the immunocompetence
of the TCL.
Taken together, the results of the present study
demonstrated that HA, Fas-L and TGF-β were present in the TCL from
Lewis lung cancer cells, and may induce the production of
immunosuppressive MΦs and DCs, or directly induce the apoptosis of
immune cells. These molecules may significantly compromise the
immunocompetence of TCL or cause a non-response to TCL. In
addition, TCL may activate CD69 expression, leading to the
production of Treg cells, which may further increase immune cell
inhibition and apoptosis, and thus further reduce the antitumor
activity of the TCL. On the basis of the abovementioned findings,
removal of HA, Fas-L and TGF-β from the TCL preparation and
inhibition of CD69 expression may improve the antitumor activity of
TCL.
Acknowledgements
This study was supported by the Anhui Provincial
Natural Science Foundation (no. 1208085QC49), the Anhui Provincial
Natural Science Research Subject of Provincial University (no.
KJ2012B200) and the Doctor Research Foundation of Wan Nan Medical
College (no. 201215).
References
|
1
|
Yang DH, Park JS, Jin CJ, et al: The
dysfunction and abnormal signaling pathway of dendritic cells
loaded by tumor antigen can be overcome by neutralizing VEGF in
multiple myeloma. Leuk Res. 33:665–670. 2009. View Article : Google Scholar
|
|
2
|
Dong B, Sun L, Wu X, et al: Vaccination
with TCL plus MHSP65 induces anti-lung cancer immunity in mice.
Cancer Immunol Immunother. 59:899–908. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kuang DM, Zhao Q, Xu J, Yun JP, Wu C and
Zheng L: Tumor-educated tolerogenic dendritic cells induce CD3
down-regulation and apoptosis of T cells through oxygen-dependent
pathways. J Immunol. 181:3089–3098. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kuang DM, Wu Y, Chen N, Cheng J, Zhuang SM
and Zheng L: Tumor-derived hyaluronan induces formation of
immunosuppressive macrophages through transient early activation of
monocytes. Blood. 110:587–595. 2007. View Article : Google Scholar
|
|
5
|
Li H, Zhu H, Xu CJ and Yuan J: Cleavage of
BID by caspase 8 mediates the mitochondrial damage in the Fas
pathway of apoptosis. Cell. 94:491–501. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chuster N and Krieglstein K: Mechanisms of
TGF-beta-mediated apoptosis. Cell Tissue Res. 307:1–14. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Niehans GA, Brunner T, et al: Human lung
carcinomas express Fas ligand. Cancer Res. 57:1007–1012.
1997.PubMed/NCBI
|
|
8
|
Gutierrez LS, Eliza M, Niven-Fairchild T,
Naftolin F and Mor G: The Fas/Fas-ligand system: a mechanism for
immune evasion in human breast carcinomas. Breast Cancer Res Treat.
54:245–253. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Saji H, Nakamura H, Awut H, et al:
Significance of expression of TGF-β in pulmonary metastasis in
non-small cell lung cancer tissues. Ann Thorac Cardiovasc Surg.
9:295–300. 2003.
|
|
10
|
Imai K, Minamiya Y, Goto A, et al:
Bronchioloalveolar invasion in non-small cell lung cancer is
associated with expression of transforming growth factor-β1. World
J Surg Oncol. 11:1132013.PubMed/NCBI
|
|
11
|
Kim HR, Wheeler MA, Wilson CM, et al:
Hyaluronan facilitates invasion of colon carcinoma cells in vitro
via interaction with CD44. Cancer Res. 64:4569–4576. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Anttila MA, Tammi RH, Tammi MI, Syrjanen
KJ, Saarikoski SV and Kosma VM: High levels of stromal hyaluronan
predict poor disease outcome in epithelial ovarian cancer. Cancer
Res. 60:150–155. 2000.PubMed/NCBI
|
|
13
|
Auvinen P, Tammi R, Parkkinen J, et al:
Hyaluronan in peritumoral stroma and malignant cells associates
with breast cancer spreading and predicts survival. Am J Pathol.
156:529–536. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Subramanian G, Schwarz RE and Higgins L:
Targeting endogenous transforming growth factor beta receptor
signaling in SMAD4-deficient pancreatic carcinoma cells inhibits
their invasive phenotypel. Cancer Res. 64:5200–5211. 2004.
View Article : Google Scholar
|
|
15
|
Grimm M, Gasser M, Bueter M, et al:
Evaluation of immunological escape mechanisms in a mouse model of
colorectal liver metastases. BMC Cancer. 10:822010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jansen T, Tyler B, Mankowski JL, et al:
FasL gene knock-down therapy enhances the antiglioma immune
response. Neuro Oncol. 12:482–489. 2010.PubMed/NCBI
|
|
17
|
Hahne M, Rimoldi D, Schroter M, et al:
Melanoma cell expressing of Fas (Apo1-1/CD95) ligand: implications
for tumor immune escape. Science. 274:1363–1366. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Haidong D, Scott ES, Diva RS, et al:
Tumor-associated B7-H1 promotes T-cell apoptosis: a potential
mechanism of immune evasion. Nature Med. 8:793–800. 2002.PubMed/NCBI
|
|
19
|
Quesnel B: Tumor dormancy and
immunoescape. APMIS. 116:685–694. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Attia P, Powell DJ Jr, Maker AV, Kreitman
RJ, Pastan I and Rosenberg SA: Selective elimination of human
regulatory T lymphocytes in vitro with the recombinant immunotoxin
LMB-2. J Immunother. 29:208–214. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Radstake TR, van Bon L, Broen J, et al:
Increased frequency and compromised function of T regulatory cells
in systemic sclerosis (SSc) is related to a diminished CD69 and
TGFb expression. PLoS One. 4:e59812009. View Article : Google Scholar
|
|
22
|
Chiang CL, Benencia F and Coukos G: Whole
tumor antigen vaccines. Semin Immunol. 22:132–143. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kawahara M and Takaku H: Intradermal
immunization with combined baculovirus and tumor cell lysate
induces effective antitumor immunity in mice. Int J Oncol.
43:2023–2030. 2013.PubMed/NCBI
|
|
24
|
Mytar B, Woloszyn M, Szatanek R, et al:
Tumor cell-induced deactivation of human monocytes. J Leukoc Biol.
74:1094–1101. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mantovani A, Sozzani S, Locati M, Allavena
P and Sica A: Macrophage polarization: tumor-associated macrophages
as a paradigm for polarized M2 mononuclear phagocytes. Trends
Immunol. 23:549–555. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kryczek I, Wei S, Zou L, et al: Cutting
edge: induction of B7–H4 on APCs through IL-10: novel suppressive
mode for regulatory T cells. J Immunol. 177:40–44. 2006.
|
|
27
|
Bennett MW, O’connell J, O’sullivan GC, et
al: Expression of Fas ligand by human gastric adenocarcinomas: a
potential mechanism of immune escape in stomach cancer. Gut.
44:156–162. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Derynck R, Akhurst RJ and Balmain A:
TGF-beta signaling in tumor suppression and cancer progression. Nat
Genet. 29:117–129. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pajusto M, Ihalainen N, Pelkonen J, et al:
Human in vivo activated CD45R0(+) CD4(+) T
cells are susceptible to spontaneous apoptosis that can be
inhibited by the chemokine CXCL12 and IL-2, -6. -7 and -15. Eur J
Immunol. 34:2771–2780. 2004.PubMed/NCBI
|
|
30
|
Esplugues E, Vega-Ramos J, Cartoixa D, et
al: Induction of tumor NK-cell immunity by anti-CD69 antibody
therapy. Blood. 105:4399–4406. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ralainirina N, Poli A, Michel T, Poos L,
Andrès E, Hentges F and Zimmer J: Control of NK cell functions by
CD4+CD25+ regulatory T cells. J Leukoc Biol. 81:144–153. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Radosavljević GD, Jovanović IP, Kanjevac
TV and Arsenijević NN: The role of regulatory T cells in the
modulation of anti-tumor immune response. Srp Arh Celok Lek.
141:262–267. 2013.(In Serbian).
|
|
33
|
David S, Manuel G and Francisco SM: CD69
is an immunoregulatory molecule induced following activation.
Trends Immunol. 26:136–140. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Freedman RS, Kudelka AP, Kavanagh JJ, et
al: Clinical and biological effects of intraperitoneal injections
of recombinant interferongamma and recombinant interleukin 2 with
or without tumor-infiltrating lymphocytes in patients with ovarian
or peritoneal carcinoma. Clin Cancer Res. 6:2268–2278. 2000.
|
|
35
|
Esplugues E, Sancho D, Vega-Ramos J, et
al: Enhanced antitumor immunity in mice deficient in CD69. J Exp
Med. 197:1093–1106. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Schwenk R, Banania G, Epstein J, et al: Ex
vivo tetramer staining and cell surface phenotyping for early
activation markers CD38 and HLA-DR to enumerate and characterize
malaria antigen-specific CD8+ T-cells induced in human
volunteers immunized with a Plasmodium falciparum
adenovirus-vectored malaria vaccine expressing AMA1. Malar J.
12:3762013. View Article : Google Scholar
|