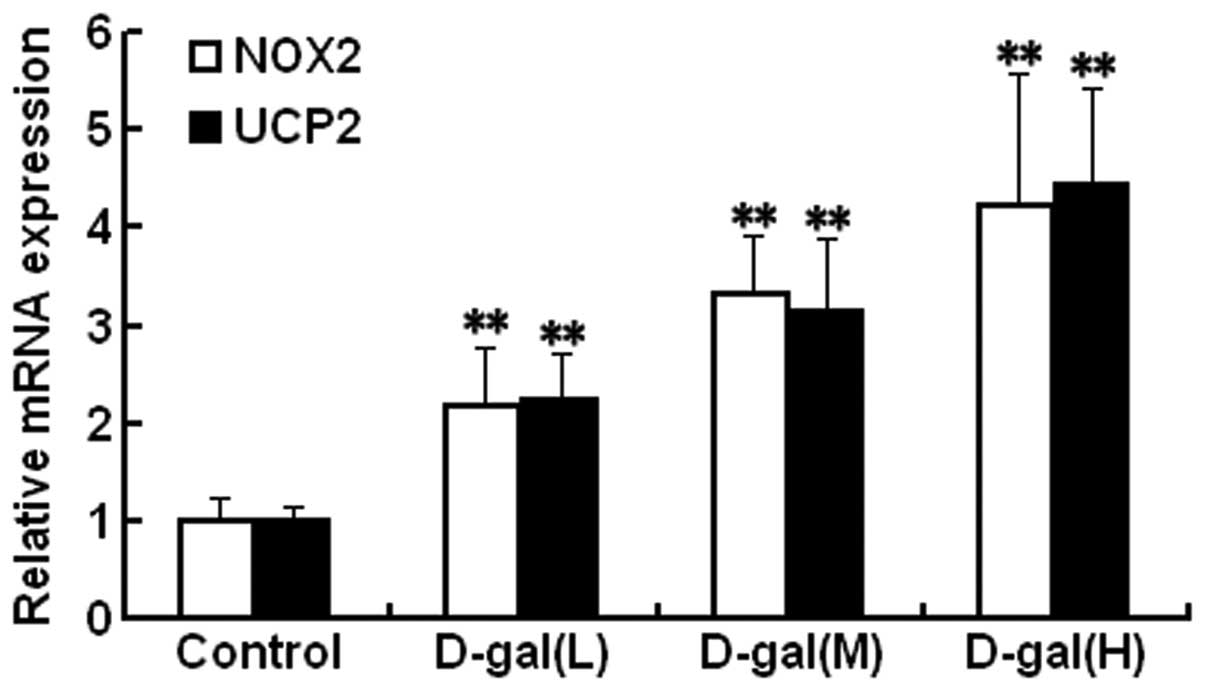
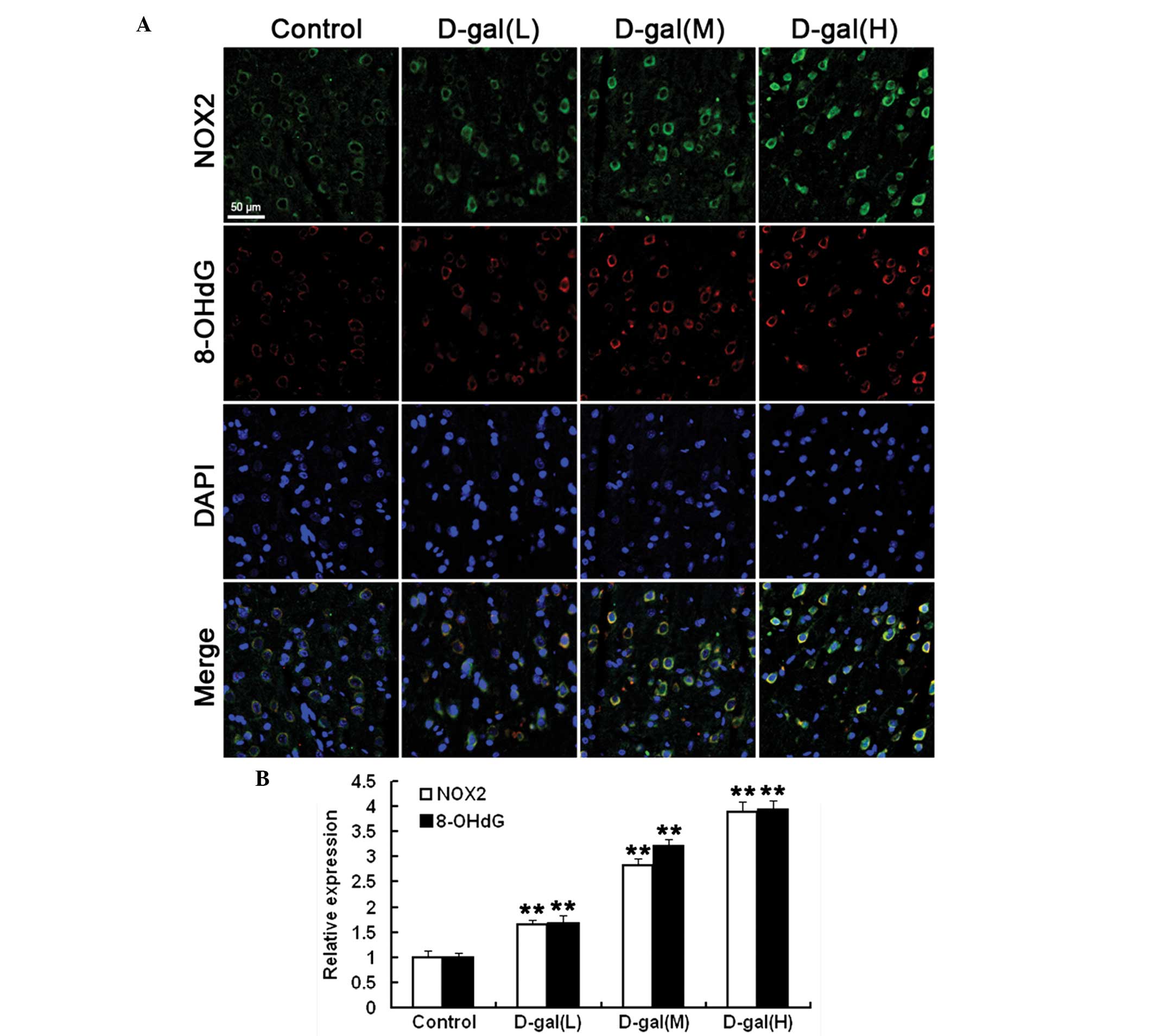
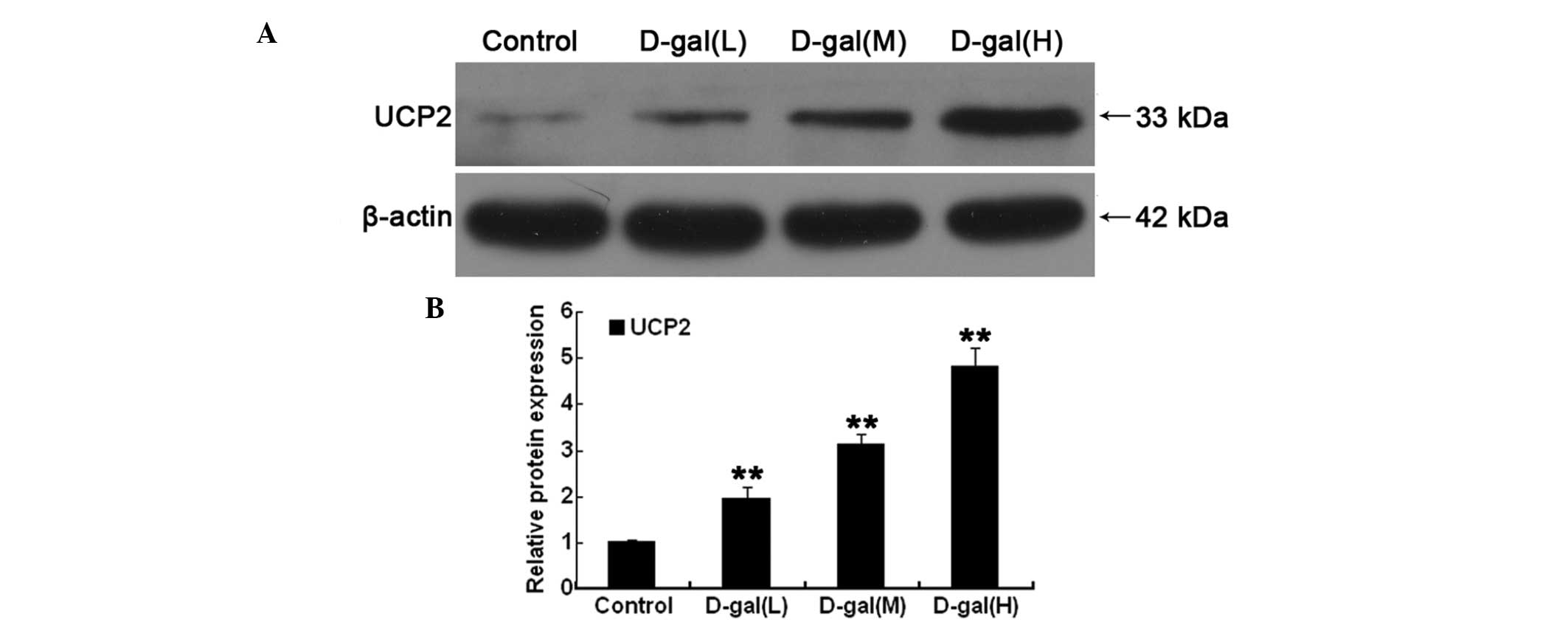
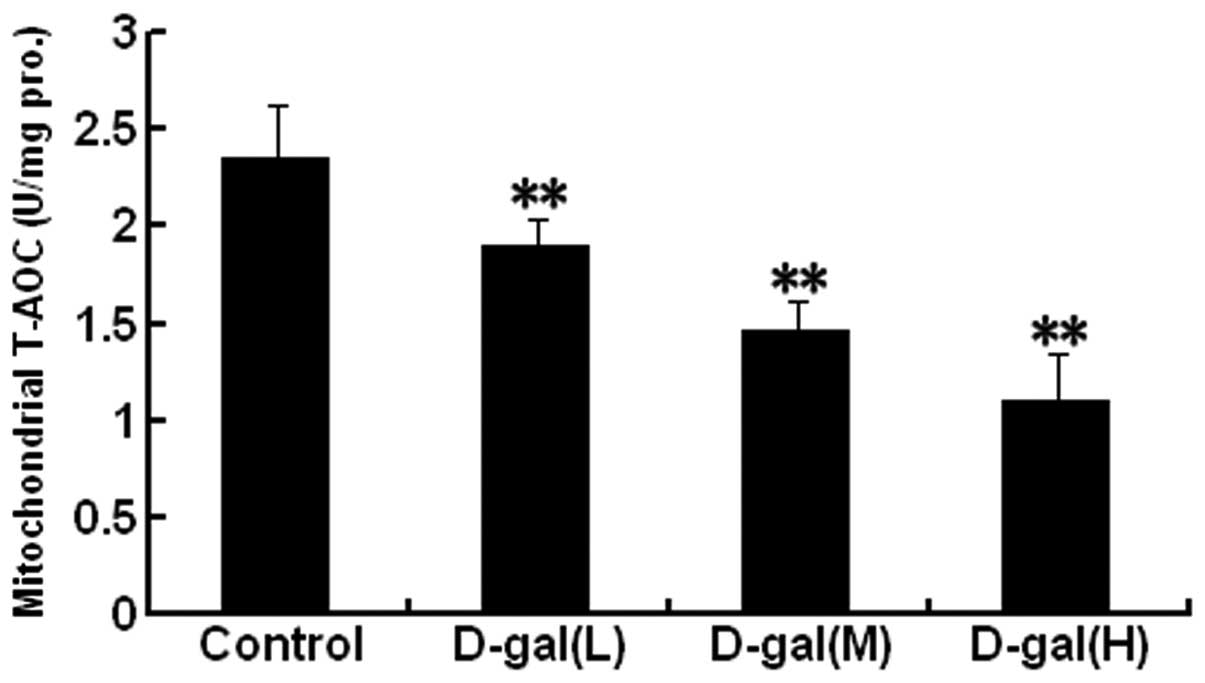
|
1
|
Frisina RD and Walton JP: Age-related
structural and functional changes in the cochlear nucleus. Hear
Res. 216–217:216–223. 2006.PubMed/NCBI
|
|
2
|
Howarth A and Shone GR: Ageing and the
auditory system. Postgrad Med J. 82:166–171. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wu L, Sun Y, Hu YJ, et al: Increased
p66Shc in the inner ear of D-galactose-induced aging mice with
accumulation of mitochondrial DNA 3873-bp deletion: p66Shc and
mtDNA damage in the inner ear during aging. PLoS One. 7:e504832012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhong Y, Hu Y, Peng W, et al: Age-related
decline of the cytochrome c oxidase subunit expression in
the auditory cortex of the mimetic aging rat model associated with
the common deletion. Hear Res. 294:40–48. 2012.PubMed/NCBI
|
|
5
|
Du Z, Yang Y, Hu Y, et al: A long-term
high-fat diet increases oxidative stress, mitochondrial damage and
apoptosis in the inner ear of D-galactose-induced aging rats. Hear
Res. 287:15–24. 2012. View Article : Google Scholar
|
|
6
|
Zhong Y, Hu YJ, Chen B, et al:
Mitochondrial transcription factor A overexpression and base
excision repair deficiency in the inner ear of rats with
D-galactose-induced aging. FEBS J. 278:2500–2510. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen B, Zhong Y, Peng W, et al: Increased
mitochondrial DNA damage and decreased base excision repair in the
auditory cortex of D-galactose-induced aging rats. Mol Biol Rep.
38:3635–3642. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhong Y, Hu YJ, Yang Y, et al:
Contribution of common deletion to total deletion burden in
mitochondrial DNA from inner ear of d-galactose-induced aging rats.
Mutat Res. 712:11–19. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen B, Zhong Y, Peng W, Sun Y and Kong
WJ: Age-related changes in the central auditory system: comparison
of D-galactose-induced aging rats and naturally aging rats. Brain
Res. 1344:43–53. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kong WJ, Wang Y, Wang Q, Hu YJ, Han YC and
Liu J: The relation between D-galactose injection and mitochondrial
DNA 4834 bp deletion mutation. Exp Gerontol. 41:628–634. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kong WJ, Hu YJ, Wang Q, et al: The effect
of the mtDNA4834 deletion on hearing. Biochem Biophys Res Commun.
344:425–430. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bedard K and Krause KH: The NOX family of
ROS-generating NADPH oxidases: physiology and pathophysiology.
Physiol Rev. 87:245–313. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bánfi B, Malgrange B, Knisz J, Steger K,
Dubois-Dauphin M and Krause KH: NOX3, a superoxide-generating NADPH
oxidase of the inner ear. J Biol Chem. 279:46065–46072.
2004.PubMed/NCBI
|
|
14
|
Quinn MT, Ammons MC and Deleo FR: The
expanding role of NADPH oxidases in health and disease: no longer
just agents of death and destruction. Clin Sci (Lond). 111:1–20.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Serrano F, Kolluri NS, Wientjes FB, Card
JP and Klann E: NADPH oxidase immunoreactivity in the mouse brain.
Brain Res. 988:193–198. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Raha S and Robinson BH: Mitochondria,
oxygen free radicals, disease and ageing. Trends Biochem Sci.
25:502–508. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brand MD and Esteves TC: Physiological
functions of the mitochondrial uncoupling proteins UCP2 and UCP3.
Cell Metab. 2:85–93. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kujoth GC, Hiona A, Pugh TD, et al:
Mitochondrial DNA mutations, oxidative stress, and apoptosis in
mammalian aging. Science. 309:481–484. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ma Y, Mehta SL, Lu B and Li PA: Deficiency
in the inner mitochondrial membrane peptidase 2-like (Immp21) gene
increases ischemic brain damage and impairs mitochondrial function.
Neurobiol Dis. 44:270–276. 2011. View Article : Google Scholar
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
|
|
21
|
Nicklas JA, Brooks EM, Hunter TC, Single R
and Branda RF: Development of a quantitative PCR (TaqMan) assay for
relative mitochondrial DNA copy number and the common mitochondrial
DNA deletion in the rat. Environ Mol Mutagen. 44:313–320. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang CH, Wu SB, Wu YT and Wei YH:
Oxidative stress response elicited by mitochondrial dysfunction:
implication in the pathophysiology of aging. Exp Biol Med
(Maywood). 238:450–460. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Krause KH: Aging: a revisited theory based
on free radicals generated by NOX family NADPH oxidases. Exp
Gerontol. 42:256–262. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mukherjea D, Jajoo S, Kaur T, Sheehan KE,
Ramkumar V and Rybak LP: Transtympanic administration of short
interfering (si)RNA for the NOX3 isoform of NADPH oxidase protects
against cisplatin-induced hearing loss in the rat. Antioxid Redox
Signal. 13:589–598. 2010. View Article : Google Scholar
|
|
25
|
Mukherjea D, Jajoo S, Sheehan K, et al:
NOX3 NADPH oxidase couples transient receptor potential vanilloid 1
to signal transducer and activator of transcription 1-mediated
inflammation and hearing loss. Antioxid Redox Signal. 14:999–1010.
2011. View Article : Google Scholar
|
|
26
|
Du Z, Hu Y, Yang Y, et al: NADPH
oxidase-dependent oxidative stress and mitochondrial damage in
hippocampus of D-galactose-induced aging rats. J Huazhong Univ Sci
Technolog Med Sci. 32:466–472. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kitahara T, Li-Korotky HS and Balaban CD:
Regulation of mitochondrial uncoupling proteins in mouse inner ear
ganglion cells in response to systemic kanamycin challenge.
Neuroscience. 135:639–653. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kitahara T, Horii A, Kizawa K, Maekawa C
and Kubo T: Changes in mitochondrial uncoupling protein expression
in the rat vestibular nerve after labyrinthectomy. Neurosci Res.
59:237–242. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Druzhyna NM, Wilson GL and LeDoux SP:
Mitochondrial DNA repair in aging and disease. Mech Ageing Dev.
129:383–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Meissner C, Bruse P, Mohamed SA, et al:
The 4977 bp deletion of mitochondrial DNA in human skeletal muscle,
heart and different areas of the brain: a useful biomarker or more?
Exp Gerontol. 43:645–652. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yowe DL and Ames BN: Quantitation of
age-related mitochondrial DNA deletions in rat tissues shows that
their pattern of accumulation differs from that of humans. Gene.
209:23–30. 1998. View Article : Google Scholar
|
|
32
|
Markaryan A, Nelson EG and Hinojosa R:
Quantification of the mitochondrial DNA common deletion in
presbycusis. Laryngoscope. 119:1184–1189. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bai U, Seidman MD, Hinojosa R and Quirk
WS: Mitochondrial DNA deletions associated with aging and possibly
presbycusis: a human archival temporal bone study. Am J Otol.
18:449–453. 1997.PubMed/NCBI
|
|
34
|
Ueda N, Oshima T, Ikeda K, Abe K, Aoki M
and Takasaka T: Mitochondrial DNA deletion is a predisposing cause
for sensorineural hearing loss. Laryngoscope. 108:580–584. 1998.
View Article : Google Scholar : PubMed/NCBI
|