Introduction
Hypertension is an important risk factor in the
pathogenesis of hypertensive renal injury, which is one of the
leading causes of chronic kidney disease worldwide (1–4).
Analysis of epidemiological data from dialysis registries in Japan
and the USA (5–7) has shown that the incidence of
end-stage renal disease due to hypertensive nephrosclerosis is
increasing in a number of countries. It is therefore crucial to
identify novel preventive and therapeutic compounds for use in
hypertensive renal disease (8,9).
3-n-Butylphthalide (NBP) is a compound extracted
from Chinese celery and is used as an antihypertensive herbal
traditional Chinese medicine in the treatment of stroke patients
(10,11). Several mechanisms may underlie its
therapeutic effects. NBP can promote the nitric oxide (NO)
production of endothelial cells, resulting in vasodilative effects
(12). In addition, NBP can
increase the number of cerebral microvessels via upregulation of
the expression of vascular endothelial growth factor and hypoxia
inducible factor-1α (13). A
randomized, double-blind placebo-controlled trial involving 573
patients in China has shown that NBP is safe and effective for
patients with acute noncardioembolic ischemic stroke, particularly
for those cases of moderate severity (14). One study demonstrated that NBP
decreased blood pressure (BP) and increased heart rate (HR) in
hypertensive rats, but had no effect on normotensive rats (10). Furthermore, NBP protects
endothelial cells in the microvessels from oxidative and
nitrosative stress, mitochondrial damage and subsequent cell death,
following oxygen and glucose deprivation in vitro. These are
also important risk factors for chronic renal dysfunction induced
by hypertension (12,15,16).
It was hypothesized that NBP may have a protective effect against
the development of hypertensive nephropathy. The current study
established a chronic renal injury model, induced by long-term
hypertension, to examine the effect of NBP on this process.
Spontaneously hypertensive rats (SHRs) have been
widely used as a primary hypertension animal model, in which the
hypertensive nephropathy is characterized by multiple renal
structural and functional alterations. It has been found that the
mechanism underlying renal injury in SHRs comprises a complex
pathological network, involving renin, angiotensin II (Ang II),
monocytes, macrophages, inflammatory cytokines and oxidative stress
(17,18). Generally, in SHRs older than six
months, there is automatic progression into severe renal injury
characterized by marked proteinuria, elevation of serum creatinine
(Scr) and blood urea nitrogen (BUN), reduced creatinine clearance
ratio (CCr), glomerulosclerosis, interstitial fibrosis and renal
vascular arteriosclerosis. These characteristics render SHRs a good
animal model of human hypertensive nephropathy (19).
Previous studies have shown that the use of
renin-angiotensin system (RAS) inhibitors, such as Ang-converting
enzyme inhibitors and Ang receptor blockers, can effectively
suppress the progression of established renal disease (20,21).
The present study used losartan as a positive control to compare
the renal protective effect of NBP on SHRs. It aimed to investigate
whether NBP alleviates chronic kidney injury under hypertensive
conditions.
Materials and methods
Ethical considerations
This study was approved by the Institutional Animal
Care Committee of Tianjin Medical University General Hospital
(Tianjin, China), and was conducted in accordance with the US
National Institute of Health Guide for the Care and Use of
Laboratory Animals.
Animal treatment protocol
Male spontaneously hypertensive rats (aged 16 weeks
and weighing 300–340 g) and normal male Wistar rats (aged 16 weeks
and weighing 300–340 g), were obtained from the Institute of
Laboratory Animal Science at the Chinese Academy of Medical
Sciences (Beijing, China). They were kept in a
specific-pathogen-free facility under constant temperature (22±2°C)
conditions, with 12 h (7am to 7pm) illumination, and provided with
food and water ad libitum.
The hypertensive rats were randomized into four
groups (n=10 per group). These were: Saline control; 10 mg/kg, per
oral (p.o.) losartan treatment; 15 mg/kg, p.o. NBP treatment; and
30 mg/kg p.o. NBP treatment. NBP was purchased from CSPC-NBP
Pharmaceutical Co., Ltd. (Shijiazhuang, China) and losartan was
purchased from Merck Co., Inc. (Darmstadt, Germany). Losartan and
NBP were diluted in sodium carboxymethycellulose-Na. The treatment
lasted for up to 20 weeks, during which time blood pressure was
measured every four weeks by tail-cuff plethysmography (BP-98A;
Softron, Tokyo, Japan) with prior training of investigators to
minimize variability in the blood pressure measurement.
Biochemical analyses of blood and
urine
At the end of the designated treatments, blood was
sampled through the eyes under anesthesia with diethyl ether (30%
concentration; Beihua Ltd, Beijing, China). Continuous collection
of urine samples for 24 h was performed in each animal after
placement in metabolic cages (Zhenghua Ltd, Hefei, China) the day
prior to blood sample collection. BUN, Scr and urinary albumin were
measured by the standard biochemical kits (BHKT Clinical Reagent
Co., Ltd., Beijing, China). Creatinine clearance (CCr) was
calculated according to the following formula: CCr = Urinary
creatinine (mg/ml) × urine volume (ml/kg)/creatinine in plasma
(mg/ml) (22).
Histological examination
Section preparation
The kidneys were fixed in 10% phosphate-buffered
formalin solution (PBS), and embedded in paraffin. Sections of 2 μm
thickness were cut and stained with hematoxylin and eosin (H&E)
and Periodic acid-Schiff (PAS). In order to assess the degree of
kidney injury, the following semi-quantitative scores were obtained
using light microscopy (Eclipse Ni-E, Nikon, Tokyo, Japan).
Glomerular sclerosis score (H&E
and PAS stain)
Glomerular sclerosis was determined as previously
described (22): Grade 0, no
sclerosis; grade 1, <25% of the glomerulus; grade 2, 26–50% of
the glomerulus; grade 3, 51–75% of the glomerulus; and grade 4,
76–100% of the glomerulus. Thirty glomeruli were observed from each
specimen under a microscope with ×200 magnification. The score of a
biopsy was calculated with the following equation: [(number grade 1
glomeruli) + (2 × number grade 2 glomeruli) + (3 × number grade 3
glomeruli) + (4 × number grade 4 glomeruli)] × 100/total number of
glomeruli examined (23).
Tubulointerstitial damage
A scoring system was applied, ranging from 0 to 4,
in which tubular atrophy, dilation, casts, interstitial
inflammation and fibrosis were assessed in 10 kidney fields at a
magnification of ×200. The scoring was as follows: 0, normal; 1,
lesions in <25% of the area; 2, lesions in 25–50% of the area;
3, lesions in >50% of the area; and 4, lesions involving the
entire area (24,25).
Immunohistochemistry
Paraffin-embedded sections were subjected to
immunohistochemical assays as previously reported (26). Briefly, the sections were
deparaffinized with xylene, rehydrated through a graded series of
ethanol to water, and then incubated in blocking solution (PBS plus
1% bovine serum) at room temperature for 1 h. The sections were
then incubated overnight at 4°C with one of the following primary
antibodies: Monoclonal antibody against rat tumor necrosis factor
(TNF)-α (1:50 dilution; R&D Systems, Minneapolis, MN, USA),
interleukin (IL)-6 (1:100 dilution; Santa Cruz Biotechnology, Santa
Cruz, CA, USA) or goat polyclonal anti-nuclear factor (NF)-κB p65
antibody that detects the endogenous level of total NF-κB p65
(1:150 dilution; Santa Cruz Biotechnology). All antibodies were
diluted with blocking solution. Negative controls consisted of
histological sections incubated with PBS rather than the primary
antibody. Immunostaining was conducted with an
avidinbiotin-peroxidase complex kit (Zhongshan Ltd., Beijing,
China) and counterstained with hematoxilin.
Enzyme-linked immunosorbent assay (ELISA)
and western blot analysis
Plasma transforming growth factor (TGF)-β1 levels
were measured by a Quantikine ELISA kit according to the
manufacturer’s instructions. Briefly, a monoclonal antibody
specific for rat TGF-β1 (MB100B; R&D systems, Minneapolis, MN
USA) was pre-coated onto a microplate. The standards and test
samples were then pipetted into the wells to allow binding of
TGF-β1 to the immobilized antibodies. After washing away any
unbound substances, an enzyme-linked polyclonal antibody specific
for TGF-β1 was added to the wells (antibody contained within
Quantikine ELISA kit MB100B; R&D systems). Following a wash
that removed any unbound antibody-enzyme reagent, a substrate
solution (contained within Quantikine ELISA kit MB100B; R&D
systems) was added to the wells and color was developed in
proportion to the quantity of TGF-β1 bound to the well. The optical
density of each well was measured at a wave length of 450 nm by a
microreader (S190, Molecular Devices, Sunnyvale, CA, USA).
TGF-β1 expression in renal tissues was examined by
western blotting. Renal tissue lysate was prepared using a lysis
buffer (Applygen Technologies Inc, Beijing, China) containing 25
mmol/l Tris-HCl, 150 mmol/l NaCl, 5 mmol/l ethyleneglycol
bis(2-aminoethyl ether)tetraacetic acid, 5 mmol/l ethylenediamine
tetraacetic acid, 10 mmol/l sodium fluoride, 1 mmol/l phenylmethyl
sulfonylfluoride, 1% TritonX-100, 0.5% Nonidet P40, 10 mg/l
aprotinin and 10 mg/l leupeptin, and quantified by the Bradford
dye-binding procedure (Applygen Technologies Inc.). Equal
quantities of protein were separated by SDS-PAGE (5% stacking gel
and 10% separating gel for β-actin or 12% separating gel for
TGF-β1) and electroblotted onto nitrocellulose membranes (Merck
Millipore, Darmstadt, Germany). After blocking with 3% bovine serum
albumin, the membranes were incubated with rabbit anti-rat
monoclonal TGF-β1 (Cell Signaling Technology, Inc., Beverly, MA,
USA) and mouse anti-rat polyclonal β-actin (Santa Cruz
Biotcehnology, Inc.) antibodies overnight. Membranes were then
incubated with a monoclonal horseradish peroxidase-conjugated goat
anti-rabbit secondary antibody (ZSGB-BIO, Beijing, China) at a
1:2,000 dilution for 1 h at room temperature after being washed.
Reactive proteins were viewed by enhanced chemoluminescence
(Applygen Technologies Inc.). The signals were detected with
FujiFilm Las-3000 (Tokyo, Japan). The intensity of the detected
bands was analyzed using the Image J program (National Institutes
of Health, Bethesda, MD, USA).
Measurement of nicotinamide adenine
dinucleotide phosphate (NAD(P)H) oxidase activity in isolated
glomeruli
The renal cortex was separated from the medulla.
Cortical sections were placed into ice-cold RPMI-1640 medium
(Sigma-Aldrich, St. Louis, MO, USA) and cut into three 2-mm
sections. Glomeruli were isolated using the technique of
differential sieving with stainless steel grids of 80, 150 and 200
mesh size and then resuspended in RPMI-1640 medium (27). NAD(P)H oxidase activities in
glomeruli were measured using tiron-inhibitable lucigenin
chemiluminescence, as previously described (28).
Statistical analysis
All data are expressed as the mean ± standard
deviation. Statistical comparisons were performed using Student’s
t-test, except the histological analyses in interstitium-tubular
lesions, which were analyzed by Pearson’s χ2 test. SPSS
version 19.0 software (SPSS Inc., Chicago, IL, USA) was used to
perform the Student’s t-test. P<0.05 was considered to indicate
a statistically significant difference.
Results
Effect of NBP on blood pressure
Prior to drug treatment, the average systolic
pressure (SAP) of SHRs was 200 mmHg, 56 mmHg higher than that of
normotensive rats (data not shown) and the average diastolic
pressure (DAP) was 172 mmHg, 50 mmHg higher than that of
normotensive rats (data not shown).
Following drug treatment, blood pressure was
measured every four weeks. The results are shown in Fig. 1A and B. Compared with the saline
control group, SHRs in the NBP treatment groups showed lower SAP
and DAP, and this effect occurred in a dose-dependent manner
(P<0.05). However, the blood pressure-lowering effect of NBP was
less marked than that of losartan.
Benefits of NBP on renal function
Table 1 shows the
results of the effects of NBP and losartan on the levels of BUN,
CCr and urinary albumin excretion (UAE) in SHRs. Compared with
normotensive rats, these three parameters were initially above the
normal ranges (UAE, 20.57±4.92 mg/ml/day; BUN, 16.77±1.26 mg/dl;
and CCr, 1.61±0.37 ml/min/100 g bdy weight). As shown in Table 1, treatment with NBP at 15 and 30
mg/kg significantly decreased the levels of BUN and UAE and
increased the CCr compared with the saline control (P<0.05).
Losartan produced similar effects.
 | Table IBUN, CCr and UAE values of SHRs after
20 weeks of treatment with saline, losartan or NBP. |
Table I
BUN, CCr and UAE values of SHRs after
20 weeks of treatment with saline, losartan or NBP.
| Parameter | SHR-saline | Losartan (10
mg/kg) | NBP (15 mg/kg) | NBP (30 mg/kg) |
|---|
| Mice, n | 10 | 10 | 10 | 10 |
| UAE (mg/ml/day) | 25.76±7.25 | 18.11±5.66a | 20.08±10.04a | 17.52±4.46a |
| BUN (mg/dl) | 23.69±1.64 | 20.45±1.32a | 20.06±0.95a | 19.84±2.15a |
| CCr (ml/min/100 g
body weight) | 0.79±0.35 | 1.19±0.37a | 0.09±0.16 | 1.15±0.12a |
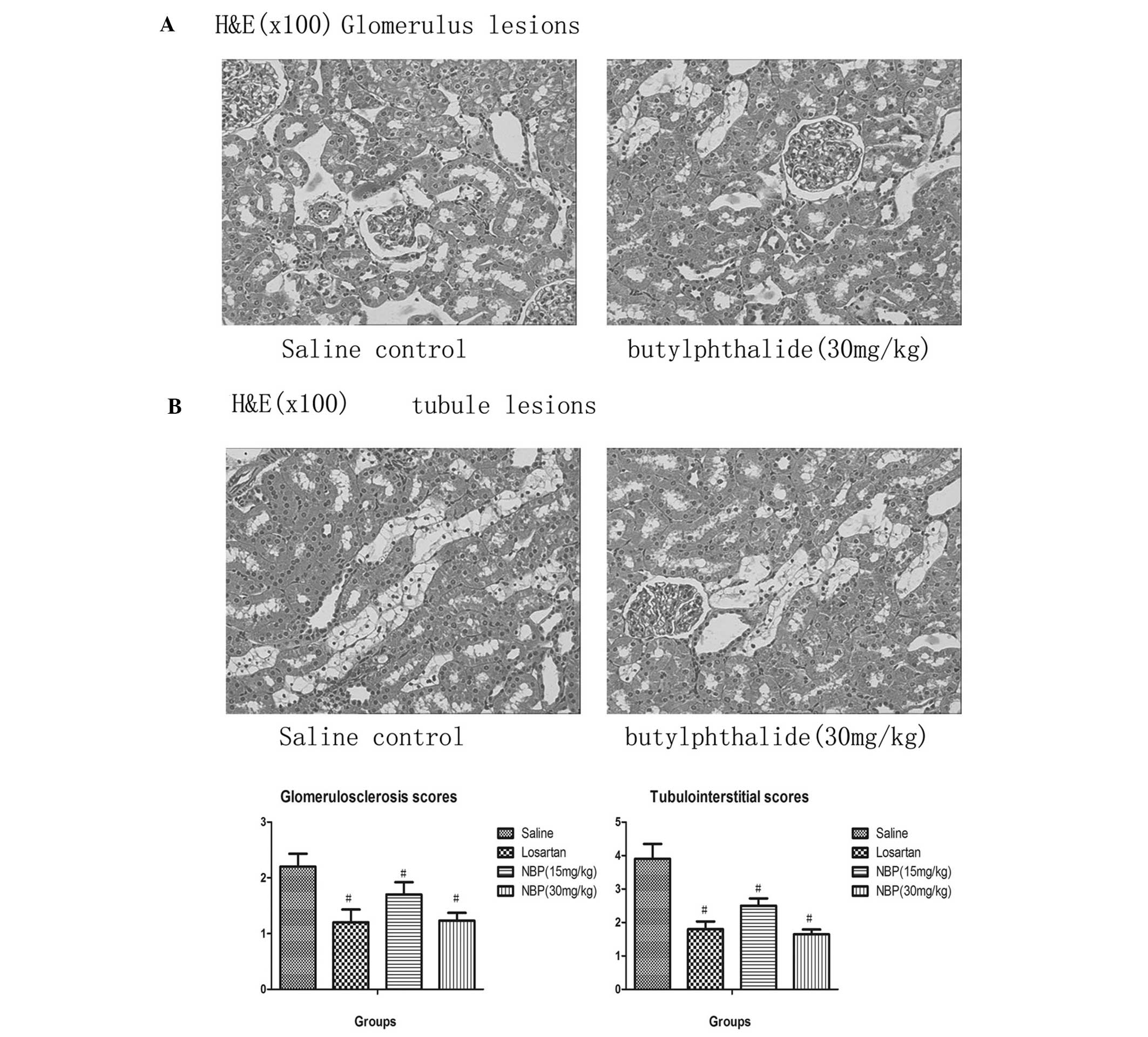
Benefits of NBP on the histopathology of
the kidneys
Histopathological findings in SHRs in the saline
control and 30 mg/kg NBP groups are shown in Fig. 2. In the absence of drug treatment,
SHRs exhibited greater degrees of glomerulosclerosis and apparent
histological abnormalities in the interstitium-tubule areas,
including interstitium infiltration of inflammatory cells,
fibrosis, tubular dilatation and protein casts.
NBP treatment over 20 weeks resulted in significant
alleviation of glomerulosclerosis (P<0.05). At doses of 15 and
30 mg/kg, reduction in glomeruloscerosis scores was significant
compared with the control group (P<0.05). Furthermore, at each
dose, NBP significantly reduced interstitium infiltration of
inflammatory cells, fibrosis, tubular dilatation and protein casts
in SHRs, which was observed in the histopathological sections and
was objectively calculated using the tubulointerstitial scoring
system (P<0.05).
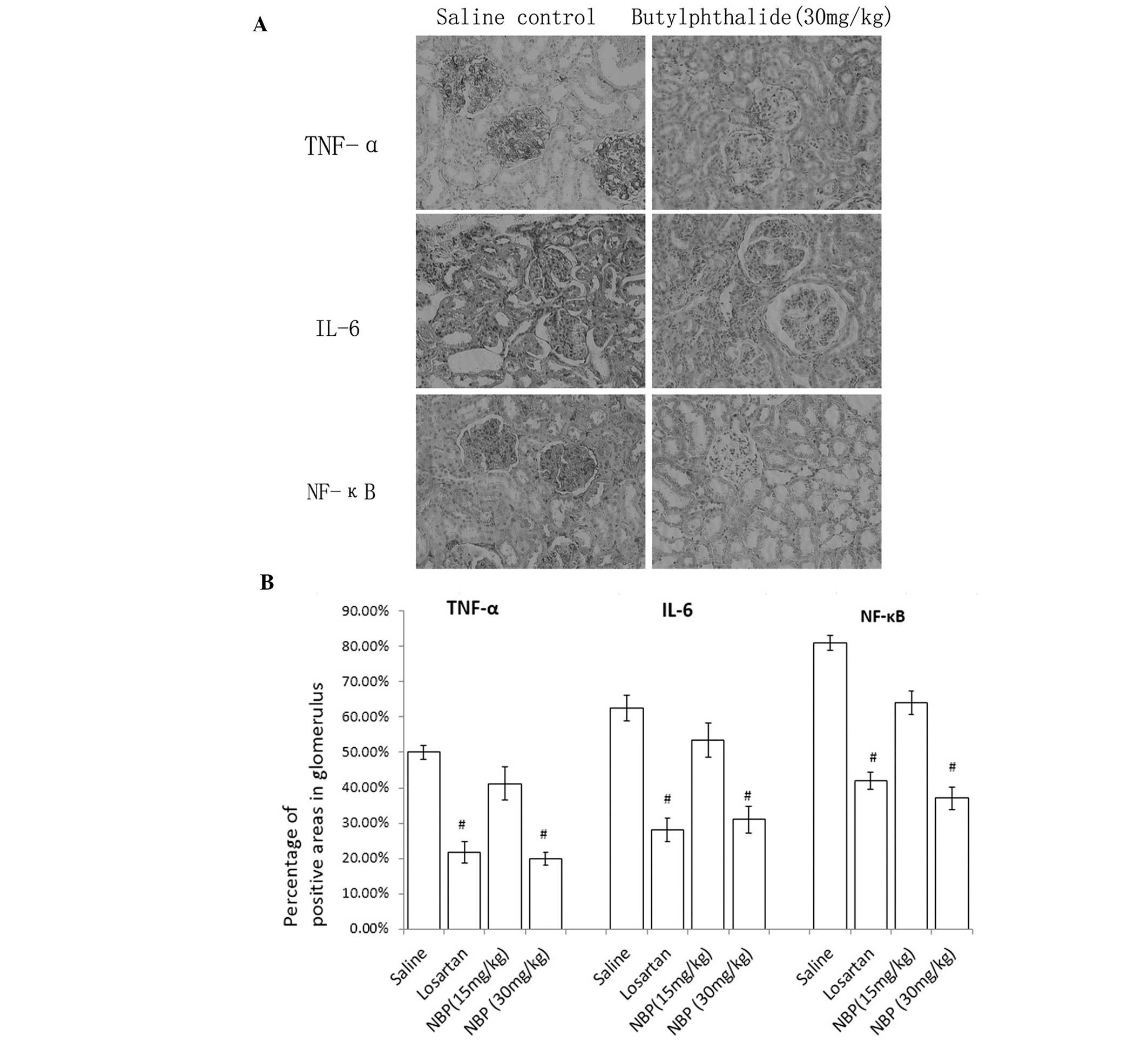
Expression of TNF-α, IL-6 and NF-κB by
IHC
As shown in Fig. 3,
immunohistochemistry demonstrated positive staining of TNF-α, IL-6
and NF-κB in glomeruli and tubules of all the groups. NBP decreased
the expression of these cytokines and chemokines in glomeruli and
tubules in a dose-dependent manner compared with the saline control
group.
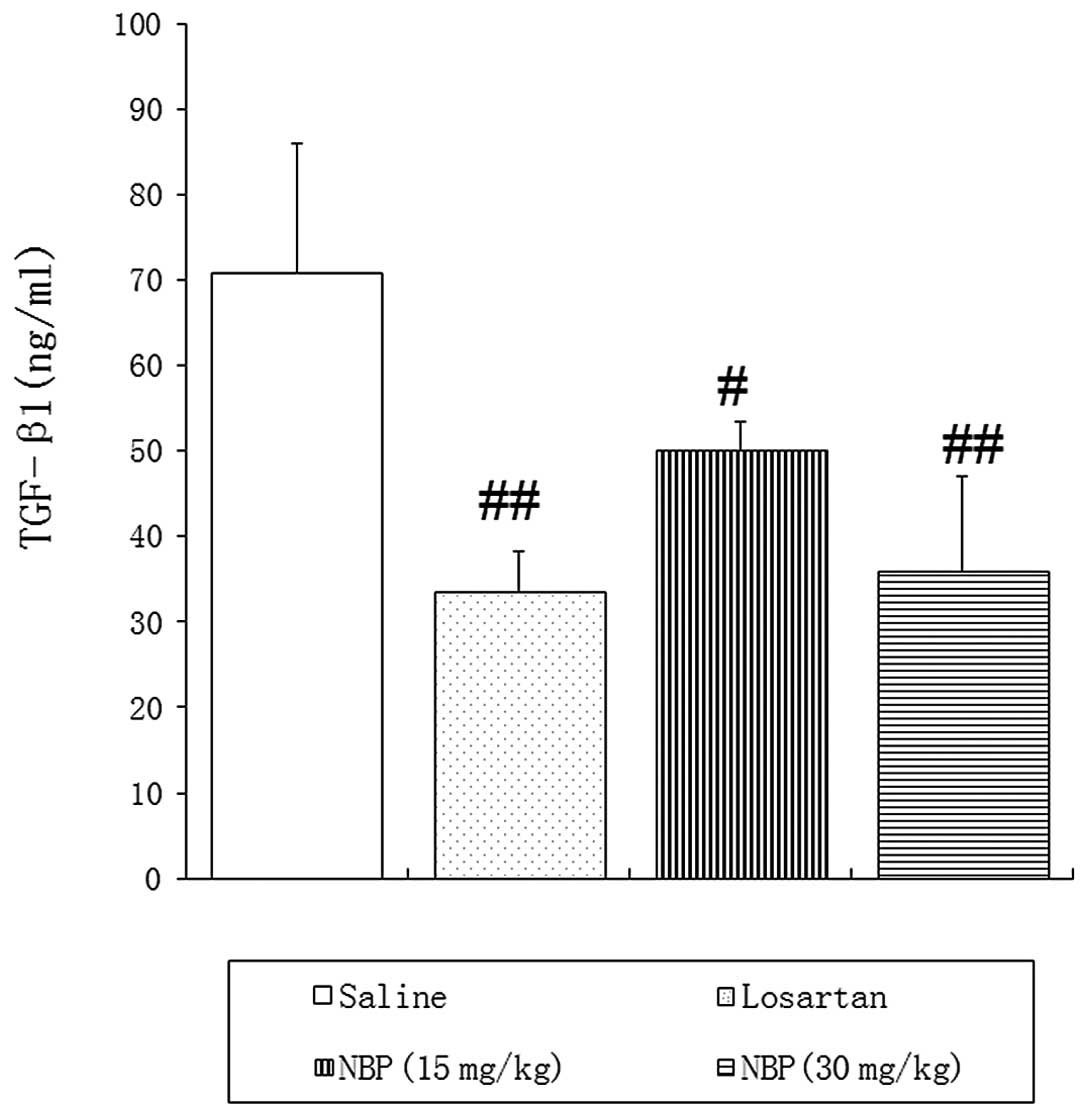
TGF-β1 protein contents in blood and
renal tissues
ELISA and western blotting confirmed high expression
of TGF-β1 in peripheral blood and renal tissues of SHRs treated
with saline, suggesting that TGF-β1 may be involved in the
development of fibrosis in hypertensive nephropathy.
As shown in Fig. 4,
the blood concentration of TGF-β1 was significantly reduced in NBP-
and losartan-treated rats, compared with saline control animals.
Western blotting showed that the expression of TGF-β1 in kidney
tissues was also reduced by NBP treatment, in a dose-dependent
manner (Fig. 5). These findings
suggested that NBP may slow renal fibrosis via modulation of TGF-β1
signaling pathways.
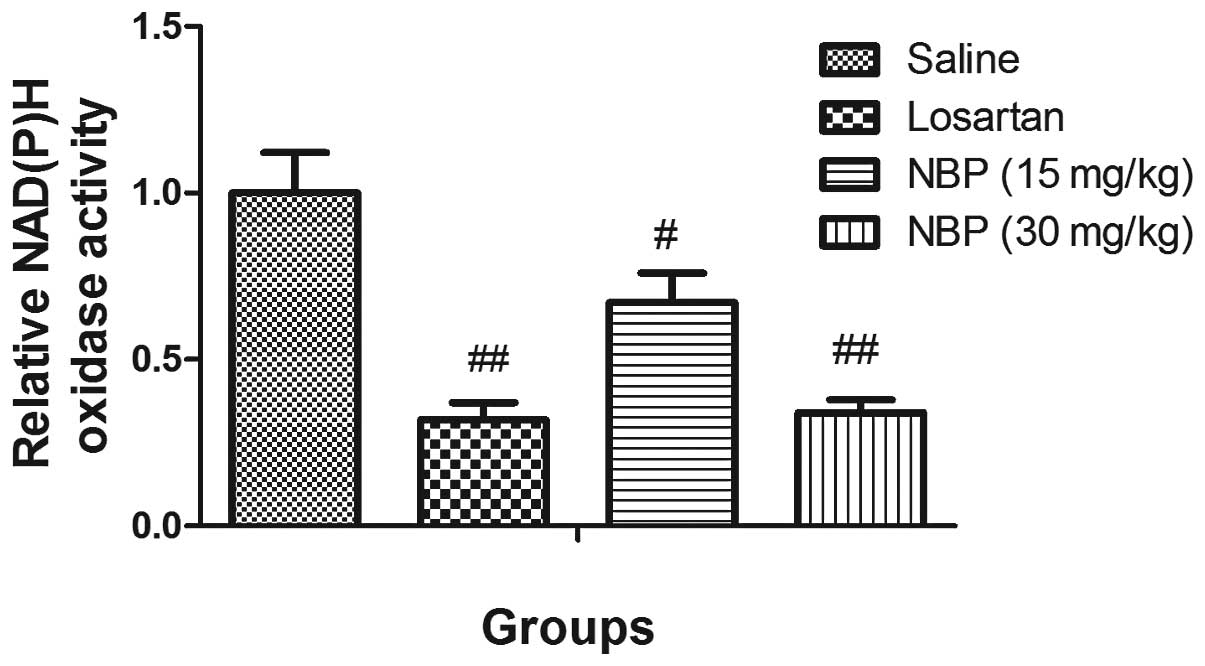
NBP treatment reduced NAD(P)H oxidase
activity in isolated glomeruli
The NAD(P)H oxidase activity in isolated glomeruli
of the three treatment groups was significantly lower than the
saline SHR group, which suggested NBP and losartan may reduce the
oxidative stress in the glomeruli of hypertensive rats (Fig. 6).
Discussion
NBP has shown beneficial effects in the treatment of
hypertension, stroke and cerebral ischemia in clinical practice
(13). It was hypothesized that
NBP may also be effective in the treatment of hypertensive
nephropathy. The current study used SHRs as an animal model in
order to test this hypothesis.
In the present study, SHRs aged 16 weeks were used.
At the end of the 20 week treatment period, all rats progressed to
chronic renal dysfunction, with low creatinine clearance, high UAE
and high BUN levels. NBP significantly attenuated renal dysfunction
and improved these parameters of renal function, as demonstrated by
biochemical analysis. NBP treatment at 30 mg/kg dosage had a
similar effect on improving biochemical parameters of renal
function as losartan treatment at 10 mg/kg dosage.
Notably, histopathological analysis showed that NBP
significantly alleviated injuries in glomeruli and proximal
tubules. The glomerulosclerosis scores in the NBP-treated SHRs were
significantly lower than those in the control SHRs. The degree of
tubulointerstitial fibrosis and other tubular-interstitium injuries
(interstitium infiltration, interstitium fiborosis, tubular
dilatation and tubule-interstitium protein casts) was also
significantly reduced after NBP treatment (P<0.05). These
results suggest the therapeutic potential of NBP in the treatment
of hypertensive nephropathy.
TGF-β1 and its receptors are important in end-stage
renal fibrosis(22,29). Their over-activation is known to
enhance the progression of renal fibrosis. In the present study,
ELISAs and western blotting demonstrated significantly attenuated
expression of TGF-β1 protein in the blood and kidney tissues of
NBP-treated SHRs, suggesting one possible mechanism underlying NBP
suppression of hypertensive nephropathy.
In terms of mechanisms underlying the renal
protective effect of NBP in hypertensive nephropathy, it was
hypothesized that the blood pressure lowering effect of this
compound was one of the most significant. The results demonstrated
that NBP decreased the SAP and DAP significantly in SHRs during
long-term treatment. Thus, reducing BP is an important mechanism by
which NBP may slow the progression of hypertensive nephropathy.
This has also been demonstrated in previous studies (6,7).
Inflammatory cytokines have been shown to be pivotal
in the pathogenesis of hypertension associated with end-stage renal
disease (30,31). The present study provides strong
evidence for an anti-inflammatory effect of NBP in the kidney. The
results suggest that NBP may exert such a role by inducing
normalization of the levels of pro-inflammatory cytokines (TNF-α
and IL-6) together with a reduction in levels of the transcription
factor NF-κB. It was shown that NBP inhibited the NF-κB pathway
together with a downregulation of the expression of several
proinflammatory cytokine genes. These included IL-6, which causes
alterations in endothelial permeability, induction of mesangial
cell proliferation and increased fibronectin expression in chronic
nephropathy (32,33). Proinflammatory mediators and
increasing oxidative stress are important in the pathogenesis of
hypertensive nephrology (34).
Increasing oxidative stress is considered to
contribute to progress of hypertensive nephropathy in the
glomeruli. NAD(P)H oxidase is a major source of reactive oxygen
species. Oxidative stress depletes NO and causes endothelial
dysfunction, which initiates arteriosclerosis (35). In the current study, NBP
significantly decreased NADP(H) oxidase activity in the glomeruli,
which may protect endothelial function and thus offer a significant
clinical benefit.
In conclusion, the present study showed that chronic
administration of NBP normalizes SAP and DAP, improves proteinuria
and ameliorates fibrosis in the kidney of SHRs. These effects were
associated with a reduction in renal fibrosis and oxidative stress,
and decreased levels of TNF-α, IL-6 and NF-κB. This study provides
strong evidence for a protective role of NBP in the kidney.
Acknowledgements
The authors would like to acknowledge the support
provided by the Tianjin Medical University General Hospital,
Tianjin, China.
References
|
1
|
Azegami T, Sasamura H, Hayashi K and Itoh
H: Vaccination against the angiotensin type 1 receptor for the
prevention of L-NAME-induced nephropathy. Hypertens Res.
35:492–499. 2012. View Article : Google Scholar
|
|
2
|
Hill GS: Hypertensive nephrosclerosis.
Curr Opin Nephrol Hypertens. 17:266–270. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang S, Li H, Li Y, et al: Nicousamide
normalizes renovascular hypertension in two-kidney one-clip
hypertensive rats. Biomed Rep. 1:89–92. 2013.PubMed/NCBI
|
|
4
|
Zhang S, Li Y, Li H, et al: Renal
protective effect of nicousamide on hypertensive nephropathy in
spontaneously hypertensive rats. Biomed Rep. 1:34–40.
2013.PubMed/NCBI
|
|
5
|
Iseki K: Factors influencing the
development of end-stage renal disease. Clin Exp Nephrol. 9:5–14.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Klag MJ, Whelton PK, Randall BL, et al:
Blood pressure and end-stage renal disease in men. N Engl J Med.
334:13–18. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tozawa M, Iseki K, Iseki C, Kinjo K,
Ikemiya Y and Takishita S: Blood pressure predicts risk of
developing end-stage renal disease in men and women. Hypertension.
41:1341–1345. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen Y, Lipkowitz MS, Salem RM, et al:
Progression of chronic kidney disease: Adrenergic genetic influence
on glomerular filtration rate decline in hypertensive
nephrosclerosis. Am J Nephrol. 32:23–30. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang G, Lai FM, Kwan BC, et al: Expression
of ACE and ACE2 in patients with hypertensive nephrosclerosis.
Kidney Blood Press Res. 34:141–149. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Moghadam MH, Imenshahidi M and Mohajeri
SA: Antihypertensive effect of celery seed on rat blood pressure in
chronic administration. J Med Food. 16:558–563. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang L, Lü L, Chan WM, et al: Effects of
DL-3-n-butylphthalide on vascular dementia and angiogenesis.
Neurochem Res. 37:911–919. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liao SJ, Lin JW, Pei Z, et al: Enhanced
angiogenesis with dl-3n-butylphthalide treatment after focal
cerebral ischemia in RHRSP. Brain Res. 1289:69–78. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chong ZZ and Feng YP:
dl-3-n-butylphthalide attenuates reperfusion-induced blood-brain
barrier damage after focal cerebral ischemia in rats. Zhongguo Yao
Li Xue Bao. 20:696–700. 1999.
|
|
14
|
Cui LY, Zhu YC, Gao S, et al: Ninety-day
administration of dl-3-n-butylphthalide for acute ischemic stroke:
a randomized, double-blind trial. Chinese Med J (Engl).
126:3405–3410. 2013.
|
|
15
|
Li L, Zhang B, Tao Y, et al:
DL-3-n-butylphthalide protects endothelial cells against
oxidative/nitrosative stress, mitochondrial damage and subsequent
cell death after oxygen glucose deprivation in vitro. Brain Res.
1290:91–101. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu CL, Liao SJ, Zeng JS, et al:
dl-3n-butylphthalide prevents stroke via improvement of cerebral
microvessels in RHRSP. J Neurol Sci. 260:106–113. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tian D, Ling S, Chen G, et al:
Hypertensive nephropathy treatment by heart-protecting musk pill: a
study of anti-inflammatory therapy for target organ damage of
hypertension. Int J Gen Med. 4:131–139. 2011.PubMed/NCBI
|
|
18
|
Sun L, Ke Y, Zhu CY, et al: Inflammatory
reaction versus endogenous peroxisome proliferator-activated
receptors expression, re-exploring secondary organ complications of
spontaneously hypertensive rats. Chin Med J (Engl). 121:2305–2311.
2008.
|
|
19
|
Koshikawa S, Nishikimi T, Inaba C, et al:
Fasudil, a Rho-kinase inhibitor, reverses L-NAME exacerbated severe
nephrosclerosis in spontaneously hypertensive rats. J Hypertens.
26:1837–1848. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Alfie J, Aparicio LS and Waisman GD:
Current strategies to achieve further cardiac and renal protection
through enhanced renin-angiotensin-aldosterone system inhibition.
Rev Recent Clin Trials. 6:134–146. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Berl T: Review: renal protection by
inhibition of the renin-angiotensin-aldosterone system. J Renin
Angiotensin Aldosterone Syst. 10:1–8. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang S, Xin H, Li Y, et al: Skimmin, a
coumarin from Hydrangea paniculata, slows down the progression of
membranous glomerulonephritis by anti-inflammatory effects and
inhibiting immune complex deposition. Evid Based Complement
Alternat Med. 8192962013.PubMed/NCBI
|
|
23
|
Tapia E, Sanchez-Lozada LG, Soto V, et al:
Sildenafil treatment prevents glomerular hypertension and
hyperfiltration in rats with renal ablation. Kidney Blood Press
Res. 35:273–280. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pörsti I, Fan M, Kööbi P, et al: High
calcium diet down-regulates kidney angiotensin-converting enzyme in
experimental renal failure. Kidney Int. 66:2155–2166. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li P, Ma LL, Xie RJ, et al: Treatment of
5/6 nephrectomy rats with sulodexide: a novel therapy for chronic
renal failure. Acta Pharmacol Sin. 33:644–651. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Giani JF, Muñoz MC, Pons RA, et al:
Angiotensin-(1–7) reduces proteinuria and diminishes structural
damage in renal tissue of stroke-prone spontaneously hypertensive
rats. Am J Physiol Renal Physiol. 300:F272–F282. 2011. View Article : Google Scholar
|
|
27
|
Tomohiro T, Kumai T, Sato T, et al:
Hypertension aggravates glomerular dysfunction with oxidative
stress in a rat model of diabetic nephropathy. Life Sci.
80:1364–1372. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li L, Yang R, Sun K, et al: Cerebroside-A
provides potent neuroprotection after cerebral ischaemia through
reducing glutamate release and Ca2+ influx of NMDA
receptors. Int J Neuropsychopharmacol. 15:497–507. 2012. View Article : Google Scholar
|
|
29
|
Liu Y: Renal fibrosis: new insights into
the pathogenesis and therapeutics. Kidney Int. 69:213–217. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tomino Y, Hagiwara S and Gohda T: AGE-RAGE
interaction and oxidative stress in obesity-related renal
dysfunction. Kidney Int. 80:133–135. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Koyner JL, Sher Ali R and Murray PT:
Antioxidants. Do they have a place in the prevention or therapy of
acute kidney injury? Nephron Exp Nephrol. 109:e109–e117. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Semedo P, Correa-Costa M, Antonio Cenedeze
M, et al: Mesenchymal stem cells attenuate renal fibrosis through
immune modulation and remodeling properties in a rat remnant kidney
model. Stem Cells. 27:3063–3073. 2009.PubMed/NCBI
|
|
33
|
Azuma H, Nadeau K, Takada M, Mackenzie HS
and Tilney NL: Cellular and molecular predictors of chronic renal
dysfunction after initial ischemia/reperfusion injury of a single
kidney. Transplantation. 64:190–197. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Navarro-González JF and Mora-Fernández C:
The role of inflammatory cytokines in diabetic nephropathy. J Am
Soc Nephrol. 19:433–442. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cervenka L and Heller J: Comparison of the
effects of a low-protein diet with the effects of a converting
enzyme inhibitor on the progression of renal insufficiency in
hypertensive rats. Renal Fail. 18:173–180. 1996. View Article : Google Scholar
|