Introduction
Various types of stem cell have been used in the
treatment of diseases of the nervous system, including injury
(1), stroke (2) and neurodegenerative diseases
(3). Bone marrow stem cell therapy
has offered promise in the treatment of diseases and injuries of
the brain and spinal cord and previous studies have demonstrated
functional recovery following a stroke and in Parkinson’s disease
using stem cell transplantation (4,5).
However, non-invasive methods are required for monitoring
transplanted cells following the successful transplantation of
cells in clinical therapy. Conventional methods require
histological analysis in vitro, which cannot be used for the
continuous investigation of transplanted cells in vivo
(6). However, magnetic resonance
(MR) scanners can be used for detecting the migration of implanted
stem cells. In order to use MR imaging (MRI) to trace stem cells in
the brain, incorporation of MRI contrast agents (CAs) into the
cells of interest is required. Two main classes of CA are used for
this purpose: Paramagnetic substances, which include T1-shortening
CAs, including gadolinium (Gd) chelates (7,8), and
superparamagnetic particles (T2-shortening CAs) (9–12).
Due to the advantage of having a high sensitivity
for cell tracking, T2 CAs have been widely used for the labeling of
numerous types of cell (13–18).
However, there are several disadvantages in using T2 CAs for cell
tracking, associated with the interpretation of images. Firstly, T2
CAs create signal loss, which may be mistaken for physiological
conditions, including hemorrhage, blood flow or pockets of air
(19–21) or areas containing high levels of
endogenous iron, including the liver, spleen or tumors, including
melanoma.
Compared with T2 CAs, Gd-based T1 CAs may be more
suitable for cell labeling, due to their higher signal (22). Gd-DTPA has been used to
successfully label various types of stem cell, including embryonic
and neuronal stem cells (23).
Compared with iron oxides, the major drawback of T1 CAs, with
respect to cell labeling, is their lower sensitivity. Novel large
macromolecular Gd-based CAs, gadolinium rhodamine dextran (24), nanoparticles of gadolinium oxide
(25) and gadofullerenes (26) have been identified as T1 CAs, which
possess higher relaxivities and improved efficacy in labeling stem
cells compared with those of small molecular T1 agents.
In the present study, a basic Gd-DTPA-based cell
labeling technique was investigated using an effective transfection
reagent with low toxicity to label mesenchymal stem cells prior to
imaging. Due to the paramagnetism of the labeling agents, the stem
cells were detected using MRI. In addition, the effect of labeling
on cellular viability, proliferation and differentiation was
determined.
Materials and methods
Isolation, cultivation and identification
of MSCs
MSCs were isolated and expanded from the bilateral
femora of adolescent male Sprague-Dawley (SD) rats weighing between
150 and 200 g, as previously described (27). The rats were supplied by the
Shantou University Medical College Laboratory Animal Center, and
their age was 7–8 weeks. Briefly, the bilateral femora and tibia
were harvested and the marrow was flushed out using a syringe
filled with Dulbecco’s modified Eagle’s medium (DMEM)/F12 (Gibco,
NY, USA) containing 10% fetal bovine serum. The bone marrow was
plated into 25-cm2 culture flasks and cultured in an
atmosphere of 5% CO2 at 37°C for 48 h. The supernatant
containing non-adherent cells was then removed and fresh medium was
added. When the cells reached ~80–90% confluence, they were
passaged two to three times by repeated trypsinization (0.25%
trypsin/0.02% EDTA) (Beyotime Biotechnology Institute, Haimen,
China) for 2–3 min and subsequent replating. The MSCs were
identified and characterized by the absence of staining for CD45
(type: PE-CD45), a surface marker of hematopoietic stem, and
positive staining for CD29 and CD45 (BD Biosciences, Franklin
Lakes, NJ, USA). All experimental and animal handling procedures
were approved by the Animal Care and Use Committee of Shantou
University (Shantou, China).
Cell labeling
Gd-DTPA (Magnevist®; Bayer HealthCare
Pharmaceuticals, Montville, NJ, USA) is the standard clinically
used MR CA, which has a molecular weight of 938 Da. The effectene
transfection reagent (Qiagen, Hercules, CA, USA), which is a
non-liposomal lipid transfection reagent, was used to transfect
Gd-DTPA into the MSCs. As a result of their negative charge, when
mixed with effectene, the Gd-DTPA particles were encapsulated with
the cationic lipids, which were then efficiently transferred into
cells. For cell labeling, single-cell suspensions of
1×106 MSCs were prepared. Gd-DTPA (30 μl; 0.5 mol/l) and
effectene (25 μl) were added to the MSCs in a 25-cm2
tissue culture flask containing 5 ml DMEM/F12 medium according to
the manufacturer’s instructions and were incubated for 30 min
(28). During labeling, the MSCs
(1×106 cells) were cultured in tissue culture flasks.
Subsequently, the effectene/Gd-DTPA mixture (55 μl) was added
directly to the DMEM/F12 culture medium for 24 h incubation under
standard cell culture conditions (37°C, 5% CO2). As a
negative control, either 30 μl 0.5 M Gd-DTPA or 25 μl effectene
were added to the culture medium of 1×106 cells instead
of the 55 μl effectene/Gd-DTPA mixture. Cells were incubated under
identical conditions for 4 h in a standard cell culture incubator.
A total of 1×106 unlabeled cells were used as a blank
control, which were incubated in pure culture medium without the
addition of any labeling agents.
Cellular viability and proliferation
Prior to labeling (0 h) and at 24, 48 and 72 h
following the initial labeling procedures, the cell viability was
determined using trypan blue exclusion assays. Cellular
proliferation was determined using an MTT assay, as previously
described (29). In brief,
1×104 cells/well were cultured in flat-bottomed 96-well
plates. Subsequently, 50% of the MSCs were labeled and the
remaining cells remained unlabeled and served as a control.
Following labeling, the cells were washed twice using
phosphate-buffered saline. Following this, 200 μl complete culture
medium was added to each well and the plate was incubated in a
CO2 incubator at 37°C and 5% CO2. Following
12, 24 and 48 h of incubation, 10 samples of the labeled cells and
10 samples of the unlabeled cells were selected for measurement at
each time-point. For measurement, 20 μl MTT (Beyotime Biotechnology
Institute, Haimen, China) was added at a final concentration of 5
mg/ml in medium and the cells were incubated for 4 h at 37°C and 5%
CO2. Following incubation, 150 μl dimethylsulfoxide
(DMSO; Sigma, St. Louis, MO, USA) was added and the absorbance of
the blue formazan product, formed by the enzymatic reduction of MTT
by the living cells, was measured at a wavelength of 570 nm
(Bio-Tek ELX800, Vermont, USA), with 750 nm as a reference.
Cell labeling efficacy and electron
microscopy
Labeling efficiency was measured using
spectrophotometry, in which the quantity of Gd-DTPA particles in
the cells was determined using a spectrometer (Zeeman Z-8200;
Hitachi, Tokyo, Japan), as previously described (30). Electron microscopy (CM-10; Philips
Scientifics, Eindhoven, The Netherlands) was performed at 60–80 kV.
The cells were evaluated for any structural changes as a result of
the labeling procedure and for the presence and localization of
intracellular CA particles.
In vitro MRI
The labeled and unlabeled MSCs were trypsinized,
centrifuged and resuspended using 20 μl culture medium in 1.5-ml
eppendorf tubes (800r/m, 3 min). The total number of cells in each
tube was 5×105. MRI of these tubes was performed at 7.0
T (Agilent 7T MRI; Agilent Technologies, Inc., Santa Clara, CA,
USA). The MR pulse sequences included T1-weighted (repetition time
TR=400 ms; Echo time TE=9 ms) two-dimensional fast spin echo
sequences with a 128×128 matrix, four acquisitions, a slice
thickness of 1 mm and a 30×30-mm field of view as well as
T2-weighted sequences (TR=2,600 ms; TR=100 ms). In addition, for
measurements of T1, a T1 map was obtained with a mixed
inversion-recovery spin echo sequence, which was initiated with an
inversion-recovery experiment followed by a spin echo experiment
(TR/TE=10,000 ms/10 ms) to obtain the T1 map. This sequence was
performed with a field of view of 30×30 mm, a matrix of 256×128
pixels and a section thickness of 1.5 mm. The signal intensities of
the cell pellets were observed and T1 maps were calculated using
image software without further modification, as described
previously (31). Each in
vitro MSC MRI procedure was repeated eight times.
Animal brain ischemia/reperfusion
model
Adult male SD rats weighing between 250 and 300 g
were used to prepare a conventional ischemia model. As described
previously (32), ligations of the
common carotid artery and external carotid arteries were performed
and a suture was placed through the carotid artery into the
internal carotid artery.
Gd-MSC transplantation
Following successful construction of the ischemic
model, chloral hydrate (Yulonghaizao Company, Qingdao, China) was
used to anesthetize the SD rats (3 ml/kg; intraperitoneally). The
SD rats were then administered with an injection of
1×106 (10 μl each) Gd-MSCs stereotactically. The
injection site was in the cortical area, adjacent to the right
middle cerebral artery, at a depth of ~3.5–5.0 mm. The coordinates
were 1–l.5 mm posterior to the bregma and 2–2.5 mm lateral to the
midline. None of the rats received immunosuppressants.
Gd-MSC observation using MRI
In order to examine the migration of Gd-MSCs in
vivo, 7-Tesla MRI was used to image the cells 1,3, 5 and 7 days
after the Gd-MSC injection, using T1-weighted fast spin echo
sequences (light/dark cycle 10/14 h, 22–26°C, diet 30–40 g/d, water
60–70 ml/d). Chloral hydrate 1.5ml/kg was used to anesthetize mice
intraperitoneally. The scanning parameters were TR/TE 400/9 ms and
an average of four. Each image was confirmed by two
professionals.
Results
MSC culture and identification
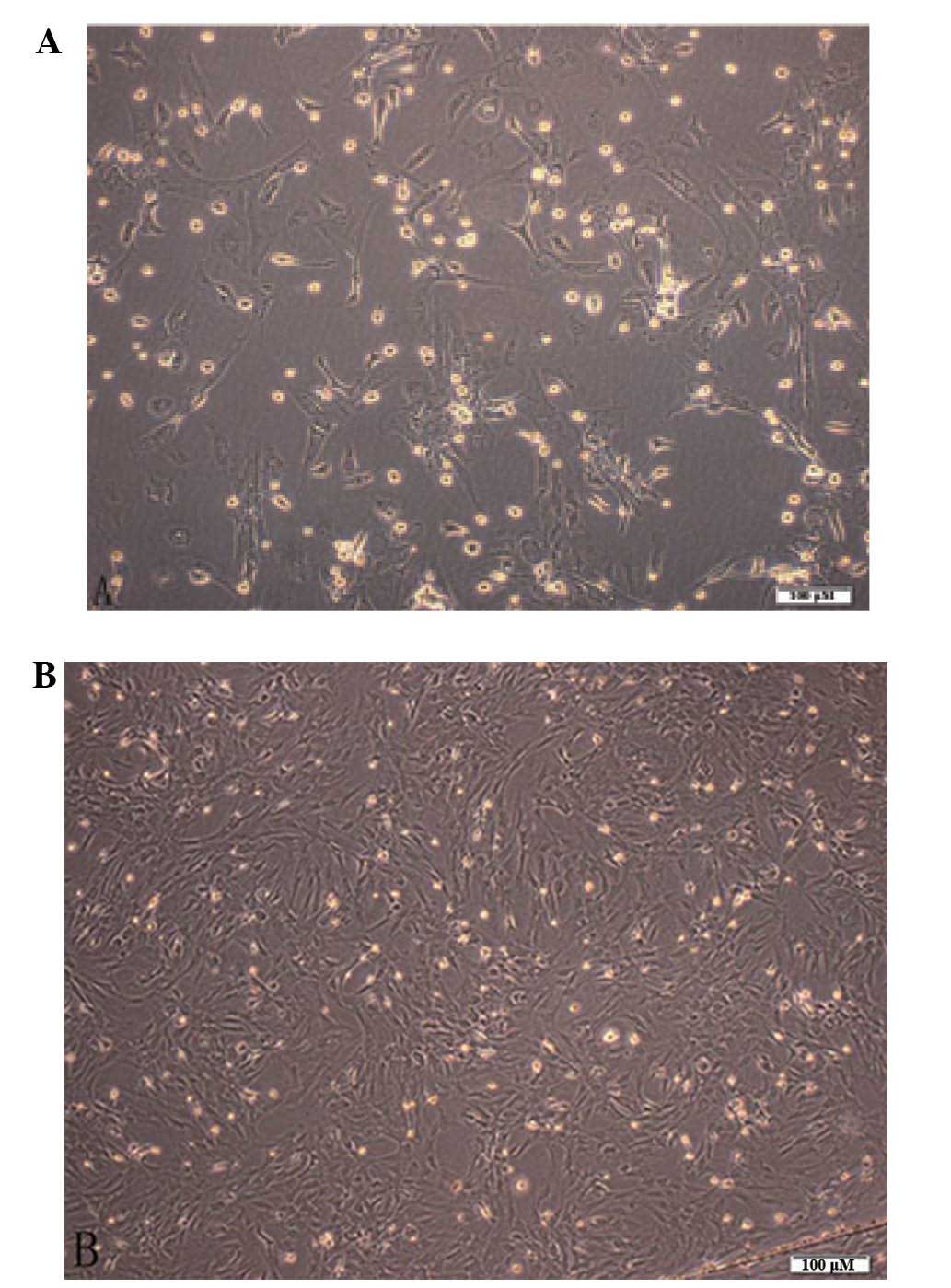
The primary bone marrow stromal cells (BMSCs) were
seeded onto petri dishes following spherical suspension in culture
medium for 10 h for adherence. After 48 h of adherence, the cells
had a stretched appearance with short spindles, triangular centered
nuclei and marked refraction, rapidly demonstrating colony of
amplification (Fig. 1). The
expression of cell surface antigens was detected using flow
cytometry. With proceeding incubation time, gradual necrosis was
observed in the suspended hematopoietic cells; however, replacing
the medium led to the growth of evenly distributed cells with a
fusiform fibrotic shape. The expression of cell surface antigens
was detected using flow cytometry. The flow cytometeric analysis of
the BMSC surface antigens revealed CD29, CD45 and CD90 at 84.69,
0.72 and 90.28%, respectively (Fig.
2).
Cell labeling
Gd-DTPA is a small soluble molecule. Following
mixing with effectene, numerous Gd-DTPA particles with a negative
charge were observed, which were initially condensed together and
were subsequently coated with cationic lipids and transferred into
cells. The internalized Gd-DTPA particles were mainly aggregated
together. Following labeling, the Gd-DTPA particles were clearly
visible inside the cytoplasm and accentuated around the cellular
apparatus. The high-density particles measured ~50 μm in diameter
under the electron microscope (Fig.
3). Using atomic emission spectrophotometry, the
effectene-mediated Gd-DTPA labeling efficiency was determined to be
86%.
Effect of labeling on the biological
behavior of the cells
Evaluation of cell viability by trypan blue
exclusion assessment revealed an initial transient reduction in the
number of cells. No significant difference was observed in the cell
viability between the unlabeled and labeled cells (P>0.05) 24,
48 and 72 h after labeling (Fig.
4). In the MTT-based proliferation assay, no statistically
significant decrease was observed in the proliferation of the
labeled cells compared with that of the unlabeled MSCs (P>0.05)
12, 24 and 48 h after labeling (Table
I).
 | Table IResults of the cell viability MTT
assay. |
Table I
Results of the cell viability MTT
assay.
| 12 h | 24 h | 48 h |
|---|
|
|
|
|
|---|
| Group | Labeled | Unlabeled | Labeled | Unlabeled | Labeled | Unlabeled |
|---|
| 1 | 0.293 | 0.256 | 0.516 | 0.491 | 0.458 | 0.606 |
| 2 | 0.370 | 0.408 | 0.591 | 0.546 | 0.286 | 0.246 |
| 3 | 0.389 | 0.353 | 0.631 | 0.609 | 0.392 | 0.439 |
| 4 | 0.362 | 0.388 | 0.440 | 0.516 | 0.374 | 0.347 |
| 5 | 0.295 | 0.353 | 0.561 | 0.559 | 0.348 | 0.380 |
| 6 | 0.381 | 0.349 | 0.622 | 0.588 | 0.293 | 0.433 |
| 7 | 0.257 | 0.262 | 0.437 | 0.515 | 0.286 | 0.249 |
In vitro and in vivo MRI
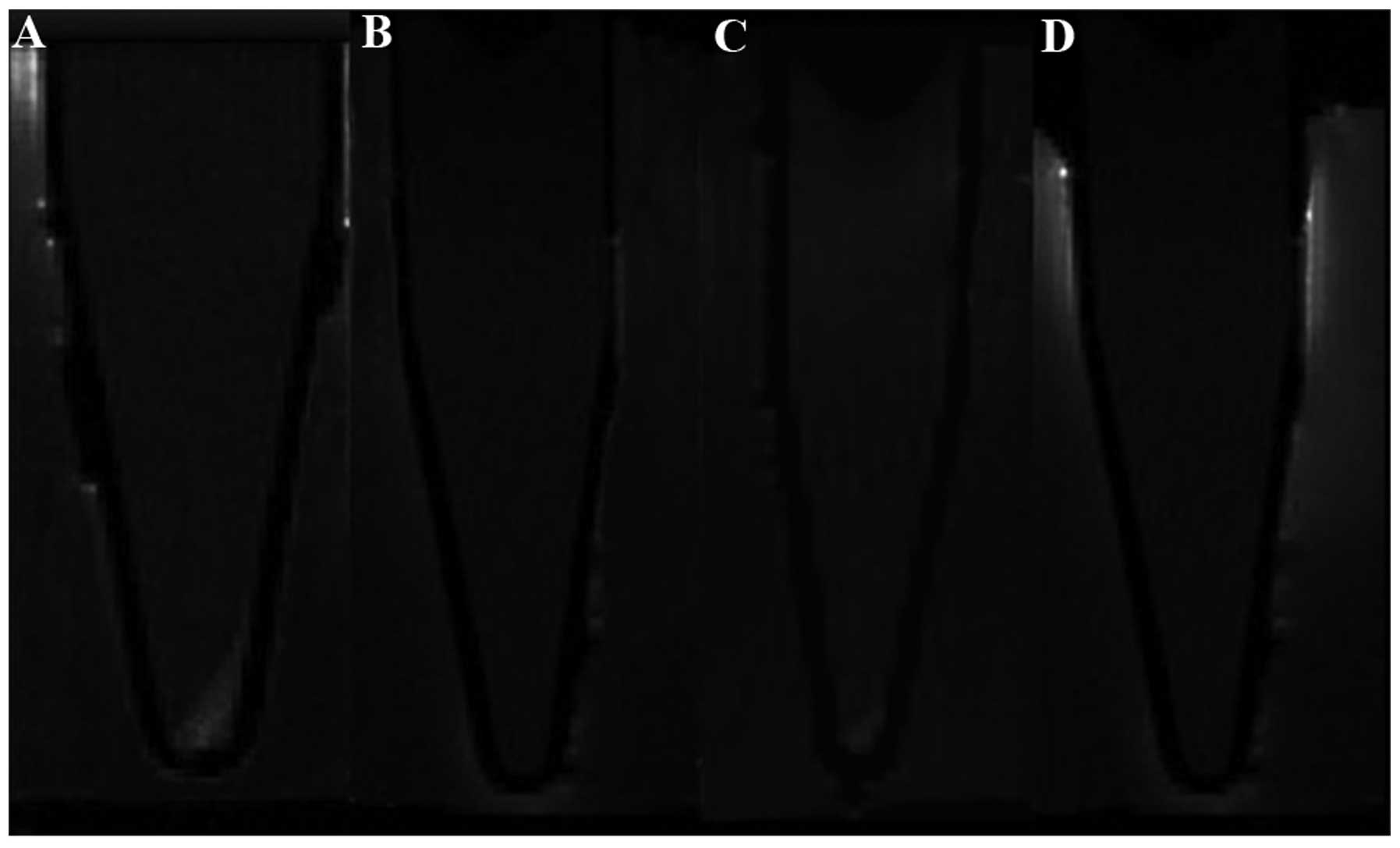
As shown in Fig. 5,
the labeled cells (Fig. 5A)
demonstrated markedly higher signal intensity in T1-weighted
imaging compared with the unlabeled cells (Fig. 5B) and the cells incubated with
either Gd-DTPA (Fig. 5C) or
effectene alone (Fig. 5D). This
signal feature was consistent with the presence of Gd-DTPA
particles inside the cytoplasm. Subsequently, the increased signal
intensity of T1-weighted imaging of the labeled cells was evaluated
and the T1 values of the labeled cells were further measured. In
T1-weighted imaging, the signal intensities of the labeled cells,
the cells with Gd-DTPA, the cells with effectene and the unlabeled
cells were 1067±34, 635±16, 603±19 and 598±25, respectively. The
signal intensity of the labeled cells was increased significantly
compared with that of the negative control cells (P<0.001) and
the signal intensity of the labeled cells was 1.48 times higher
than that of the unlabeled cells. The T1 values of the labeled
cells, the cells with Gd-DTPA, the cells with effectene and the
unlabeled cells were 886±269, 2,086±58, 2,182±280 and 2,185±200 ms,
respectively. The T1 value of the labeled cells was decreased
significantly compared with those of the negative controls and the
unlabeled cells (P<0.001). The T1 values of the labeled cells
were 2.46 times lower than those of the unlabeled cells. In terms
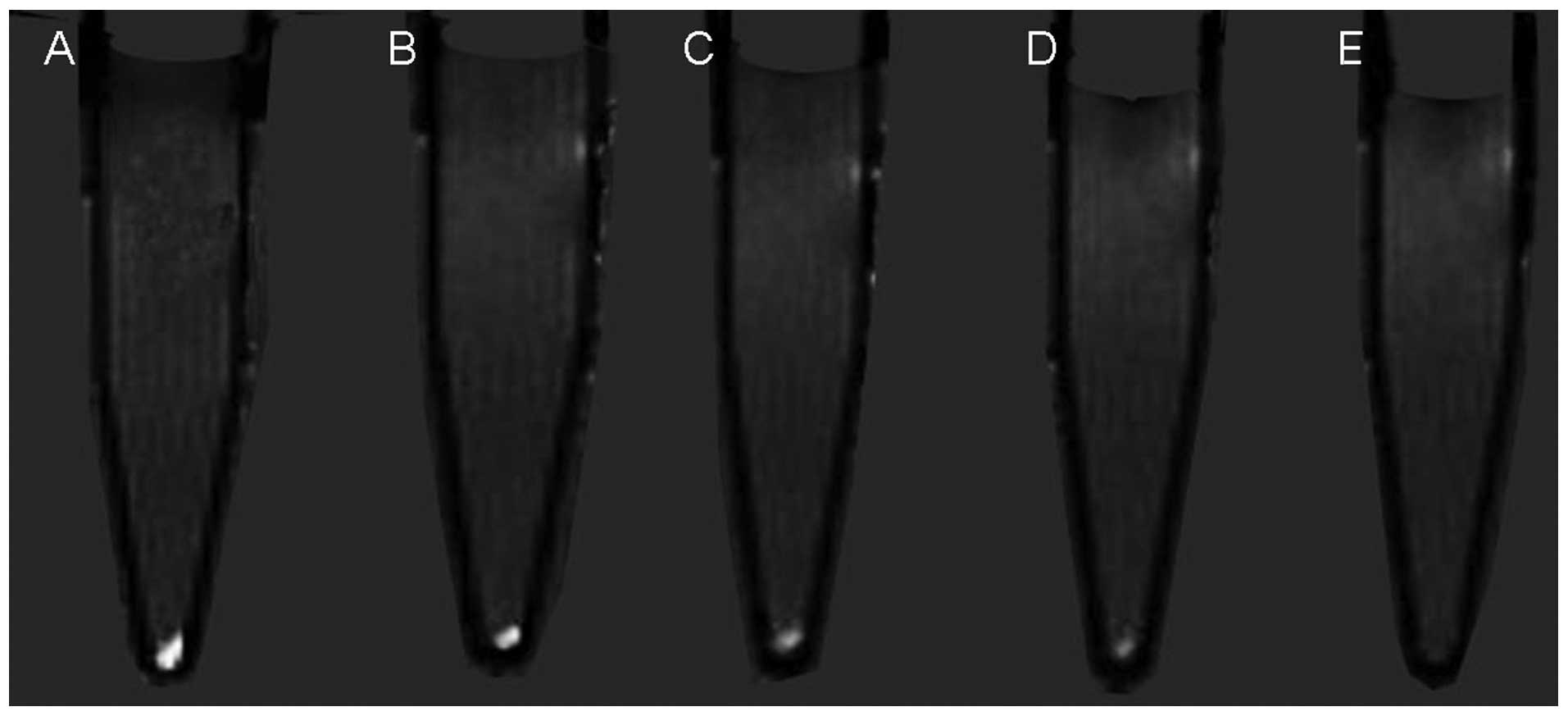
of cell proliferation, the T1-weighted image signal intensity of
the BMSCs reduced gradually and, at the fifth generation, the
signal intensity was similar to that observed in the unlabeled
cells (Fig. 6).
Examination of the migration of labeled cells in the
in vivo model revealed points of high signal, which became
more marked with increasing time. The scope, which was a high
signal area in basal ganglia in T1-weighted, also increased
gradually with time in the MRI (Fig.
7). The unlabeled control BMSCs lacked these points of
increased signal intensity.
Discussion
The development of tissue replacement by stem cell
transplant or transgene therapy provides a promising treatment
strategy for various diseases (3).
In several mouse and primate models of diseases of the central
nervous system, including Parkinson’s disease, Alzheimer’s disease,
spinal cord injury and ischemic stroke, the use of exogenous BMSCs
in cellular replacement therapy has achieved initial successes
(33–35). However, the distribution and
migration of stem cells in vivo has been confined to tissue
analysis in vitro and extensive clinical application is
difficult (36).
MRI provides a potent and versatile tool for
non-invasive investigation. By obtaining useful information in
preclinical settings, this approach generates data whose
interpretation provides more informative and reliable results
(8). 7T MRI have a higher spatial
resolution, magnetic field intensity and tissue resolution and
therefore a shorter imaging time, which is of advantage compared
with other imaging techniques which include some low field
intensity MRI, such as 0.3T, 1.0T, 1,5T, and 3,0T.
An understanding of the migratory behavior of stem
cells following transplantation into the host environment using a
non-invasive, non-toxic labeling technique in living subjects is
required. Previous studies have labeled different cell populations
in humans, including lymphocytes, monocytes, progenitor cells and
embryonic cells, using iron-containing MR CAs, including
superparamagnetic iron oxide or ultrasmall paramagnetic iron oxide
(37,38). However, the incorporated particles
induce signal loss due to the effects of magnetic susceptibility
and, at a site of primary or secondary hemorrhage, including
hemorrhagic transformation of an ischemic stroke or tumor, the
physiologically iron-loaded erythrocytes induce the same loss of
signal as that observed in the iron-labeled stem cells. Thus, it is
not possible to distinguish between labeled stem cells which have
migrated and hemorrhage (39). In
addition, iron may be toxic upon excessive accumulation (40,41).
A T1-weighted positive stem cell-labeled imaging technique has been
previously studied in vitro using an established gadolinium
chelate, Gd-DTPA, and uptake transfection agents, including
lipofectin, to promote cellular uptake compared with that of other
imaging agents (36).
Gd-DTPA is a widely used MR CA, which has been
approved by the U.S. Food and Drug Administration (6). Gd-DTPA is hydrophilic and is
therefore not taken up spontaneously by cells. This limitation was
overcome in the present study, by the incorporation of Gd-DTPA into
cationic liposomes, which enabled efficient uptake into cells by
binding to anionic moieties in the cell membrane. In the present
study, the transfection agent effectene was used to induce the
migration of Gd-DTPA into stem cells as it has a lower toxicity and
is more effective for transfection into primary cells than calcium
phosphate, liposome or viral vectors (42,43).
No difference was observed in the cell viability or proliferation
between the labeled and unlabeled MSCs. Therefore, labeling rat
MSCs with Gd-DTPA particles did not alter the cell viability or
proliferation. The Gd-DTPA labeling efficiency, determined using
spectrometry, was high and, indicating that Gd-DTPA labeling was
successfully achieved in the BMSCs. Electron microscopy also
confirmed the presence of Gd-DTPA particles inside the cytoplasm.
Examination of the longevity of the CA revealed that it was
retained up to the fifth generation following labeling in
vitro. However, a major disadvantage of using Gd-DTPA was its
relatively high threshold, which can be detected using MRI
(44). Despite this relatively
high threshold, the observed high MRI signal intensity of labeled
stem cells, including those treated with Gd-DPTA, renders these
labeling techniques suitable to be investigated for use in clinical
applications (45).
In conclusion, the present study demonstrated a
readily available strategy to magnetically label MSCs. This
approach did not require any novel synthesis and therefore provided
a simple and straightforward method of magnetically labeling stem
cells to track their extent of migration in vivo and in
vitro following implantation. In addition, as the presented
labeling method uses a clinical standard agent, this may facilitate
the introduction of MR monitoring of the biodistribution of
magnetically labeled cells in a clinical setting. Therefore, use of
the presented labeling method for MRI tracing may become a powerful
tool in monitoring the transformation and distribution mechanism of
transplanted cells in vivo.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (no. 30930027).
References
|
1
|
Eftekharpour E, Karimi-Abdolrezaee S and
Fehlings MG: Current status of experimental cell replacement
approaches to spinal cord injury. Neurosurg Focus. 24:E192008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang ZG, Jiang Q, Zhang R, et al:
Magnetic resonance imaging and neurosphere therapy of stroke in
rat. Ann Neurol. 53:259–263. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Walczak P and Bulte JW: The role of
noninvasive cellular imaging in developing cell-based therapies for
neurodegenerative disorders. Neurodegener Dis. 4:306–313. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kurozumi K, Nakamura K, Tamiya T, et al:
Mesenchymal stem cells that produce neurotrophic factors reduce
ischemic damage in the rat middle cerebral artery occlusion model.
Mol Ther. 11:96–104. 2005. View Article : Google Scholar
|
|
5
|
Satake K, Lou J and Lenke LG: Migration of
mesenchymal stem cells through cerebrospinal fluid into injured
spinal cord tissue. Spine (Phila Pa). 1976. 29:1971–1979. 2004.
View Article : Google Scholar
|
|
6
|
Liu Y, He ZJ, Xu B, et al: Evaluation of
cell tracking effects for transplanted mesenchymal stem cells with
jetPEI/Gd-DTPA complexes in animal models of hemorrhagic spinal
cord injury. Brain Res. 1391:24–35. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Frank J, Anderson S, Kalsih H, et al:
Methods for magnetically labeling stem and other cells for
detection by in vivo magnetic resonance imaging. Cytotherapy.
6:621–625. 2004. View Article : Google Scholar
|
|
8
|
Modo M, Hoehn M and Bulte J: Cellular MR
imaging. Mol Imaging. 4:143–164. 2004.
|
|
9
|
Bulte J, Zhang SC, Van Gelderen P, et al:
Neurotransplantation of magnetically labeled oligodendrocyte
progenitors: magnetic resonance tracking of cell migration and
myelination. Proceedings of the Natl Acad Sci USA. 96:15256–15261.
1999. View Article : Google Scholar
|
|
10
|
Josephson L, Tung C-H, Moore A and
Weissleder R: High-efficiency intracellular magnetic labeling with
novel superparamagnetic-Tat peptide conjugates. Bioconj Chem.
10:186–191. 1999. View Article : Google Scholar
|
|
11
|
Meincke M, Schlorf T, Kossel E, Jansen O,
Glueer CC and Mentlein R: Iron oxide-loaded liposomes for MR
imaging. Front Biosci. 13:40022008. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ris F, Lepetit-Coiffe M, Meda P, et al:
Assessment of human islet labeling with clinical grade iron
nanoparticles prior to transplantation for graft monitoring by MRI.
Cell Transplant. 19:1573–1585. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Arbab AS, Liu W and Frank JA: Cellular
magnetic resonance imaging: current status and future prospects.
Expert Rev Med Devices. 3:427–439. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu YL, Ye Q, Foley LM, et al: In situ
labeling of immune cells with iron oxide particles: an approach to
detect organ rejection by cellular MRI. Proc Natl Acad Sci USA.
103:1852–1857. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schafer R, Ayturan M, Bantleon R, et al:
The use of clinically approved small particles of iron oxide (SPIO)
for labeling of mesenchymal stem cells aggravates clinical symptoms
in experimental autoimmune encephalomyelitis and influences their
in vivo distribution. Cell Transplant. 17:923–941. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
So PW, Kalber T, Hunt D, et al: Efficient
and rapid labeling of transplanted cell populations with
superparamagnetic iron oxide nanoparticles using cell surface
chemical biotinylation for in vivo monitoring by MRI. Cell
Transplant. 19:419–429. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Suzuki Y, Zhang S, Kundu P, Yeung AC,
Robbins RC and Yang PC: In vitro comparison of the biological
effects of three transfection methods for magnetically labeling
mouse embryonic stem cells with ferumoxides. Magn Reson Med.
57:1173–1179. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
van Tiel ST, Wielopolski PA, Houston GC,
Krestin GP and Bernsen MR: Variations in labeling protocol
influence incorporation, distribution and retention of iron oxide
nanoparticles into human umbilical vein endothelial cells. Contrast
Media Mol Imaging. 5:247–257. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jara H, Yu B, Caruthers S, Melhem E and
Yucel E: Voxel sensitivity function description of flow-induced
signal loss in MR imaging: Implications for black-blood MR
angiography with turbo spin-echo sequences. Magnetic Reson Med.
41:575–590. 1999. View Article : Google Scholar
|
|
20
|
Reichenbach JR, Venkatesan R, Yablonskiy
DA, Thompson MR, Lai S and Haacke EM: Theory and application of
static field inhomogeneity effects in gradient-echo imaging.
Journal of Magn Reson Imaging. 7:266–279. 1997. View Article : Google Scholar
|
|
21
|
Van Den Bos EJ, Baks T, Moelker AD, et al:
Magnetic resonance imaging of haemorrhage within reperfused
myocardial infarcts: possible interference with iron oxide-labelled
cell tracking? Eur Heart J. 27:1620–1626. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kraitchman DL, Gilson WD and Lorenz CH:
Stem cell therapy: MRI guidance and monitoring. J Magn Reson
Imaging. 27:299–310. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rudelius M, Daldrup-Link HE, Heinzmann U,
et al: Highly efficient paramagnetic labelling of embryonic and
neuronal stem cells. Eur J Nucl Med Mol Imaging. 30:1038–1044.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Modo M, Cash D, Mellodew K, et al:
Tracking transplanted stem cell migration using bifunctional,
contrast agent-enhanced, magnetic resonance imaging. Neuroimage.
17:803–811. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Klasson A, Ahrén M, Hellqvist E, et al:
Positive MRI contrast enhancement in THP-1 cells with
Gd2O3 nanoparticles. Contrast Media Mol
Imaging. 3:106–111. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sitharaman B, Tran LA, Pham QP, et al:
Gadofullerenes as nanoscale magnetic labels for cellular MRI.
Contrast Media Molr Imaging. 2:139–146. 2007. View Article : Google Scholar
|
|
27
|
Okamoto T, Aoyama T, Nakayama T, et al:
Clonal heterogeneity in differentiation potential of immortalized
human mesenchymal stem cells. Biochem Biophys Res Commun.
295:354–361. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shen J, Cheng LN, Zhong XM, Duan XH, Guo
RM and Hong GB: Efficient in vitro labeling rabbit neural stem cell
with paramagnetic Gd-DTPA and fluorescent substance. Eur J Radiol.
75:397–405. 2010. View Article : Google Scholar
|
|
29
|
Denizot F and Lang R: Rapid colorimetric
assay for cell growth and survival: modifications to the
tetrazolium dye procedure giving improved sensitivity and
reliability. J Immunol Methods. 89:271–277. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ward R, Wilmet S, Legssyer R and Crichton
R: The influence of iron homoeostasis on macrophage function.
Biochem Soc Trans. 30:762–765. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Engström M, Klasson A, Pedersen H,
Vahlberg C, Käll P-O and Uvdal K: High proton relaxivity for
gadolinium oxide nanoparticles. MAGMA. 19:180–186. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gutiérrez-Fernández M, Rodríguez-Frutos B,
Alvarez-Grech J, et al: Functional recovery after hematic
administration of allogenic mesenchymal stem cells in acute
ischemic stroke in rats. Neuroscience. 175:394–405. 2011.
View Article : Google Scholar
|
|
33
|
Cho H, Choi YK, Lee DH, et al: Effects of
magnetic nanoparticle-incorporated human bone marrow-derived
mesenchymal stem cells exposed to pulsed electromagnetic fields on
injured rat spinal cord. Biotechnolo Appl Biochem. 60:596–602.
2013. View
Article : Google Scholar
|
|
34
|
Barry F and Murphy M: Mesenchymal stem
cells in joint disease and repair. Nat Rev Rheumatol. 9:584–594.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
van Velthoven CT, Sheldon RA, Kavelaars A,
et al: Mesenchymal stem cell transplantation attenuates brain
injury after neonatal stroke. Stroke. 44:1426–1432. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Guenoun J, Koning GA, Doeswijk G, et al:
Cationic Gd-DTPA liposomes for highly efficient labeling of
mesenchymal stem cells and cell tracking with MRI. Cell Transplant.
21:191–205. 2012. View Article : Google Scholar
|
|
37
|
Sun R, Dittrich J, Le-Huu M, et al:
Physical and biological characterization of superparamagnetic iron
oxide-and ultrasmall superparamagnetic iron oxide-labeled cells: a
comparison. Invest Radiol. 40:504–513. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hoehn M, Küstermann E, Blunk J, et al:
Monitoring of implanted stem cell migration in vivo: a highly
resolved in vivo magnetic resonance imaging investigation of
experimental stroke in rat. Proc Natl Acad Sci USA. 99:16267–16272.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Drey F, Choi Y, Neef K, et al: Noninvasive
in vivo tracking of mesenchymal stem cells and evaluation of cell
therapeutic effects in a murine model using a clinical 3.0 T MRI.
Cell Transplant. 22:1971–1980. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Crichton RR, Wilmet S, Legssyer R and Ward
RJ: Molecular and cellular mechanisms of iron homeostasis and
toxicity in mammalian cells. J Inorg Biochem. 91:9–18. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rosenberg JT, Sellgren KL, Sachi-Kocher A,
et al: Magnetic resonance contrast and biological effects of
intracellular superparamagnetic iron oxides on human mesenchymal
stem cells with long-term culture and hypoxic exposure.
Cytotherapy. 15:307–322. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
van den Bos EJ, Wagner A, Mahrholdt H, et
al: Improved efficacy of stem cell labeling for magnetic resonance
imaging studies by the use of cationic liposomes. Cell Transplant.
12:743–756. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Daldrup-Link HE, Rudelius M, Oostendorp
RA, et al: Targeting of Hematopoietic Progenitor Cells with MR
Contrast Agents. Radiology. 228:760–767. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Shyu W-C, Chen C-P, Lin S-Z, Lee Y-J and
Li H: Efficient tracking of non-iron-labeled mesenchymal stem cells
with serial MRI in chronic stroke rats. Stroke. 38:367–374. 2007.
View Article : Google Scholar
|
|
45
|
Kim T, Momin E, Choi J, et al: Mesoporous
silica-coated hollow manganese oxide nanoparticles as positive T 1
contrast agents for labeling and MRI tracking of adipose-derived
mesenchymal stem cells. J Am Chem Soc. 133:2955–2961. 2011.
View Article : Google Scholar : PubMed/NCBI
|