Introduction
Human cytomegalovirus (HCMV) is a ubiquitous
betaherpesvirus that has established a widespread and life-long
latent infection in the majority of the global human population.
HCMV infection is asymptomatic in healthy individuals, however, in
immunosuppressed individuals, HCMV primary infection or
reactivation usually causes serious side-effects, including HCMV
pneumonia, hepatitis, encephalitis (1–3) and
in certain cases, mortality.
HCMV has the largest genome of any characterized
human virus, which is comprised of a DNA double helix of >236 kb
and 160 predicted open reading frames (ORFs), including unique long
(UL) and unique short (US) regions (4–7).
During extensive passage in vitro, a 15 kb sequence in the
UL/b′ region (UL133-UL151) was deleted from the widely used HCMV
laboratory strain AD169 compared with the low passage Toledo strain
(8). In recent studies, the UL/b′
region has been demonstrated to be important for the dissemination,
latency and virulence of HCMV in human hosts (9–12).
The UL142 ORF, located within the UL/b′ region, is 921 bp in length
and is predicted to encode a protein, presumably an important
component in the inhibition of natural killer (NK) cell killing and
the innate defense against HCMV (13).
It is well established that HCMV has developed
effective approaches for hijacking and manipulating the host
cellular processes to contribute to the viral replication and
spread, primarily through interactions with host proteins. However,
the identities of the host proteins that interact with the UL142
gene product (pUL142) in the infected cells remain unknown. The aim
of the current study was to use a yeast two-hybrid screening system
to identify cellular proteins that interact with pUL142 and to
verify this interaction using a glutathione S transferase (GST)
pull-down assay and then detect their co-localization in
transfected embryonic kidney 293 (HEK-293) cells.
Materials and methods
Cells
HEK-293 cells (American Type Culture Collection,
Manassas, VA, USA) were cultured in Dulbecco’s modified Eagle’s
medium (HyClone, Logan, UT, USA), supplemented with 10% fetal
bovine serum (HyClone), 100 U/ml penicillin and 100 mg/ml
streptomycin (Boehringer-Ingelheim, Ingelheim am Rhein, Germany).
The HEK-293 cells were maintained at 37°C in 5% CO2.
Yeast two-hybrid screening
A yeast two-hybrid screening system (Matchmaker GAL4
Two-Hybrid System 3; Clontech Laboratories, Inc., Mountainview, CA,
USA) was used to identify pUL142 interacting proteins from a human
fetal brain cDNA library (pACT2-cDNA; Clontech Laboratories, Inc.).
The full ORF sequence of the UL142 gene was amplified by polymerase
chain reaction (PCR) using HCMV H strain DNA (GenBank no. GQ981646;
Shenyang, China), and inserted into the BamHI sites of a pGBKT7
vector (Clontech Laboratories, Inc.) generating a plasmid
(pGBKT7-UL142) that expresses a UL142 fusion protein with the
binding domain (BD) to be used as a bait plasmid for the two-hybrid
screening. The constructed plasmid was confirmed by DNA sequencing
using the dideoxy chain-termination method carried out by the
Beijing branch of the Invitrogen Life Technologies company
(Invitrogen Life Technologies, Carlsbad, CA, USA). The pGBKT7-UL142
and pACT2-cDNA plasmids were sequentially transformed into the
AH109 yeast host strain (Clontech Laboratories, Inc.) by
electroporation (Bio-Rad, Hercules, CA, USA) according to the
protocol provided by the manufacturer. The transformants were
selected by seeding the yeast onto plates containing X-a-Gal
(#8061-1, Clontech Laboratories, Inc) and minimal synthetic dropout
(SD) medium (Clontech Laboratories, Inc) lacking adenine (Ade),
histidine (His), tryptophan (Trp) and leucine (Leu), and incubated
for 3–7 days at 30°C. According to the chromogenic reaction of
α-galactosidase activity, colonies that turned blue were retained
and the positive results were confirmed by repeat assays. Inserts
of the selected clones were sequenced by the dideoxy
chain-termination method and analyzed by the Blast network service
at the National Center for Biotechnology Information (http://www.ncbi.nlm.gov/blast).
In vitro translation reactions
Biotinylated pUL142 was expressed using the
pGBKT7-UL142 plasmids (1 μg) and a TNT T7 Quick Coupled
Transcription/Translation system (Promega, Madison, WI, USA)
according to the manufacturer’s instructions. A 2-μl aliquot of the
biotin-containing translation products was separated directly on a
sodium dodecyl sulfate-polyacrylamide (SDS-PAGE) gel (10%) and
transferred onto a 0.2 μm polyvinylidene difluoride (PVDF) membrane
(Millipore, Temecula, CA, USA). The biotinylated pUL142 (55 kDa)
that reacted to streptavidin-horseradish peroxidase
(streptavidin-HRP, 1:10,000; Promega) was visualized using an
enhanced chemiluminescence western blotting detection system
(Bio-Rad).
GST pull-down assay
The Snapin sequence of the pACT2-Snapin, which was
identified by yeast two-hybrid screening, was obtained by digestion
with restriction endonucleases EcoRI and XhoI (Takara Bio, Inc.,
Dalian, China), and inserted into the GST-tagged pGEX-4T-2 vector
(Pharmacia Biotech, Inc., Piscataway, NJ, USA), designated as the
GST-Snapin fusion protein expression plasmid. The GST pull-down
experiment was performed according to the manufacturer’s
instructions (MagneGST™ Pull-Down System; Promega). The GST-Snapin
fusion proteins (43 kDa) were expressed and extracted from the BL21
Escherichia coli strain (Tiangen, Beijing, China)
transfected with the GST-Snapin fusion protein expression plasmid.
GST-Snapin fusion proteins (200 μl) were incubated with MagneGST™
particles, which allow for the capture of a GST-labeled protein and
protein complex, for 30 min at room temperature on a rotating
platform. Following three washes of GST-Snapin fusion proteins, the
particles were resuspended in 20 μl GST binding/wash buffer.
Biotinylated UL142 proteins (80 μl) were added to the resuspended
particles to a final volume of 800 μl GST binding/wash buffer.
Following incubation at 4°C for 1.5 h on a rotating platform, the
reaction mixture was recovered by a magnetic stand (Promega). The
prepared products were analyzed, using western blotting to detect
the GST-Snapin using a mouse anti-GST monoclonal antibody (Pierce,
Logan, UT, USA), and detecting the captured biotinylated UL142
proteins as mentioned above.
Immunofluorescence assay
The full-length UL142 coding sequence was obtained
by PCR from HCMV H strain DNA. EcoRI and KpnI sites (underlined)
were incorporated into the 5′ ends of the amplicon with the
following primers: forward, 5′-CCGGAATTCACG GATTGAATGGGCGTGTT-3′,
and reverse 5′-CGGGGTACCTTACTGACCGCGCCATAC-3′,
respectively. The PCR products were inserted into the mammalian
expression vector enhanced green fluorescent protein (GFP) plasmid
(pEGFP-N1) (BD Biosciences, Franklin Lakes, NJ, USA) following
digestion with the suitable restriction endonuclease and ligation
by T4 DNA ligase (Promega), resulting in the pUL142-GFP
plasmid.
The Snapin coding sequence from pACT2-Snapin was
cloned into the pDsRed-C1 vector (BD Biosciences) with the EcoRI
(introduced by the forward primer, 5′-CCGGAATTCTGCGGGGGCTGGTTCCGCCGC-3′)
and KpnI (by the reverse primer, 5′-CGGGGTACCTTATTTGCCTGGGGAGCCA-3′)
sites, to produce the gene-rating plasmid pDsRed-Snapin. All
constructs were confirmed by sequencing and sequence analysis
(Invitrogen Life Technologies).
HEK-293 cells at 75% confluence were co-transfected
with 1 μg pUL142-GFP and 1 μg pDsRed-Snapin using X-tremeGENE HP
DNA Transfection Reagent (Roche, Mannheim, Germany) for a 24 h
transfection period, which was subsequently replaced by normal
growth media. The cells were analyzed using a TCS SP2 Nikon laser
scanning confocal microscope (Nikon Eclipse C1 Plus, Tokyo, Japan)
with 488-nm and 543-nm excitation beams at 48 h
post-transfection.
Results
Snapin was screened as a binding protein
of HCMV pUL142 with a yeast two-hybrid assay
Putative pUL142 binding proteins were identified
from a human fetal brain cDNA library by yeast two-hybrid assay.
Results of autonomous activation tests carried out using the
UL142-BD fusion protein (pGBKT7-UL142) showed no autoactivation was
caused by pUL142 in this system.
Among the positive clones identified from the cDNA
library, four were confirmed by sequencing to contain the cDNA
sequences of Snapin. Two of these contained the complete coding
sequence of Snapin with more than 99% nucleotide identity. Yeast
cells expressing AD-Snapin and BD-UL142 fusion proteins grew well
on the SD medium lacking Ade/Trp/Leu/His, and turned blue in a
further chromogenic reaction. These results strongly indicated that
pUL142 binds to the Snapin protein in yeast.
Interaction between HCMV pUL142 and
Snapin in vitro was confirmed by GST pull-down assay
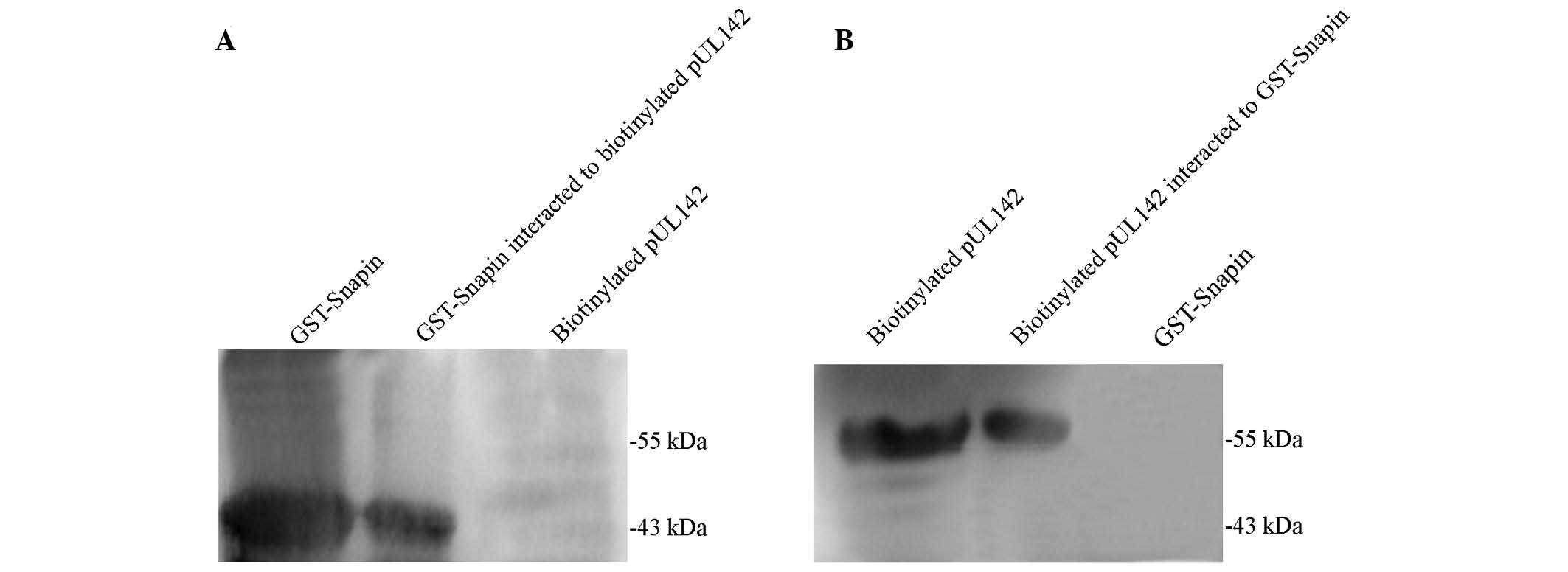
GST pull-down experiments were performed to verify
the direct interaction between pUL142 and Snapin. Consistent with
the yeast two-hybrid assay results, pUL142 was specifically bound
to GST-Snapin. As shown in Fig. 1,
GST-Snapin (Fig. 1A, lane 1) and
biotinylated pUL142 (Fig. 1B, lane
1) were highly expressed in the transformed BL21 and the TNT
system, respectively. Following the incubation of the two expressed
proteins with MagneGST™particles, GST-Snapin and pUL142 were
detected in the recovered proteins (Fig. 1A, lane 2 and Fig. 1B, lane 2, respectively). In
contrast, no corresponding band was observed in the control lanes
(Fig. 1A, lane 3, and Fig. 1B, lane 3). This result confirmed
that pUL142 was able to be captured by GST-Snapin, and that there
was a direct interation between Snapin and pUL142 in this in
vitro experiment.
Colocalization of HCMV pUL142 and Snapin
was detected by immunofluorescence assay in the HEK-293 cells
To detect whether pUL142 had the same localization
as that of Snapin, the plasmids pUL142-GFP and pDsRed-Snapin were
co-transfected into HEK-293 cells and their expressed proteins were
detected by immunofluorescence assay. As shown in Fig. 2, the fusion proteins expressed by
pDsRed-Snapin and pUL142-GFP were broadly expressed and spatially
colocalized in the transfected HEK-293 cells, indicating that they
could form a complex in the transient expression system.
Discussion
Interactions between virus and host proteins may be
an important method for viruses to establish a suitable environment
for replication and dissemination. Understanding the potential
interactions between viral proteins and those between viral and
human proteins is important for elucidating the mechanisms of
infection and developing novel strategies for the treatment and
prevention of herpesvirus latency and infection (14–16).
As a novel HCMV encoded major histocompatibility complex (MHC)
class I-related molecule, the UL142 protein contains an MHC class I
Antigen (MICA) recognition domain (17,18),
which is able to downregulate the expression levels of the NKG2D
ligand, leading to protection from NK cytotoxicity and inhibition
of NK cell-mediated lysis (19–22).
Snapin, expressed in a number of types of cells,
including adipocytes and neuronal cells (23–26),
has been established to be associated with the soluble
N-ethylmaleimide-sensitive factor attachment protein receptor
(SNARE) complex (27,28). SNAREs are ubiquitous proteins that
direct vesicular trafficking and exocytosis, and mediate the
release of neurotransmitters in neurons (29). Zhou et al (30), have revealed that Snapin has a
critical role in coordinating dynein-driven retrograde transport
and late endosomal-lysosomal trafficking, thus maintaining
efficient autophagy-lysosomal function. In addition, a number of
studies have demonstrated that Snapin may regulate HCMV genomic DNA
synthesis by modulating the cellular distribution of viral helicase
(25,31–33).
In the present study, a direct interaction between
pUL142 and Snapin was confirmed in vitro. Furthermore,
Snapin and pUL142 were demonstrated to be highly colocalized in
cotransfected HEK-293 cells. These results indicate that the
interaction of pUL142 with the host protein Snapin may occur in
vivo and influence vesicular trafficking and exocytosis by
increasing the formation of the virus releasable pool and synaptic
transmission. However, the biological functions of the interaction
between pUL142 and Snapin in vivo are still in question and
details of this aspect require further investigation.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (nos. 30672248, 81171580,
81171581, 81201274 and 81371788) and the Specialized Research Fund
for the Doctoral Program of Higher Education (no.: 20112104110012)
and the Outstanding Scientific Fund of Shengjing Hospital and the
Natural Science Foundation of Liaoning Province, China (no.
201202283).
References
|
1
|
Malm G and Engman ML: Congenital
cytomegalovirus infections. Semin Fetal Neonatal Med. 12:154–159.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fishman JA and Rubin RH: Infection in
organ-transplant recipients. New Engl J Med. 338:1741–1751. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Alford CA, Stagno S, Pass RF and Britt WJ:
Congenital and perinatal cytomegalovirus infections. Rev Infect
Dis. 12:745–753. 1990. View Article : Google Scholar
|
|
4
|
Dolan A, Cunningham C, Hector RD, et al:
Genetic content of wild-type human cytomegalovirus. J Gen Virol.
85:1301–1312. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Murphy E, Yu D, Grimwood J, et al: Coding
potential of laboratory and clinical strains of human
cytomegalovirus. Proc Natl Acad Sci USA. 100:14976–14981. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chee MS, Bankier AT, Beck S, et al:
Analysis of the protein-coding content of the sequence of human
cytomegalovirus strain AD169. Curr Top Microbiol Immunol.
154:125–169. 1990.PubMed/NCBI
|
|
7
|
Davison AJ, Dolan A, Akter P, et al: The
human cytomegalovirus genome revisited: comparison with the
chimpanzee cytomegalovirus genome. J Gen Virol. 84:17–28. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cha TA, Tom E, Kemble GW, Duke GM,
Mocarski ES and Spaete RR: Human cytomegalovirus clinical isolates
carry at least 19 genes not found in laboratory strains. J Virol.
70:78–83. 1996.PubMed/NCBI
|
|
9
|
Varnum SM, Streblow DN, Monroe ME, et al:
Identification of proteins in human cytomegalovirus (HCMV)
particles: the HCMV proteome. J Virol. 78:10960–10966. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Grainger L, Cicchini L, Rak M, Petrucelli
A, Fitzgerald KD, Semler BL and Goodrum F: Stress-inducible
alternative translation initiation of human cytomegalovirus latency
protein pUL138. J Virol. 84:9472–9486. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Umashankar M, Petrucelli A, Cicchini L, et
al: A novel human cytomegalovirus locus modulates cell
type-specific outcomes of infection. PLoS Pathog. 7:e10024442011.
View Article : Google Scholar
|
|
12
|
Wang YP, Qi Y, Huang YJ, et al:
Identification of immediate early gene X-1 as a cellular target
gene of hcmv-mir-UL148D. Int J Mol Med. 31:959–966. 2013.PubMed/NCBI
|
|
13
|
Wills MR, Ashiru O, Reeves MB, et al:
Human cytomegalovirus encodes an MHC class I-like molecule (UL142)
that functions to inhibit NK cell lysis. J Immunol. 175:7457–7465.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pei Y, Fu W, Yang E, et al: A Hsp40
chaperone protein interacts with and modulates the cellular
distribution of the primase protein of human cytomegalovirus. PLoS
Pathog. 8:e10029682012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Paulus C, Krauss S and Nevels M: A human
cytomegalovirus antagonist of type I IFN-dependent signal
transducer and activator of transcription signaling. Proc Natl Acad
Sci USA. 103:3840–3845. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cristea IM, Moorman NJ, Terhune SS, et al:
Human cytomegalovirus pUL83 stimulates activity of the viral
immediate-early promoter through its interaction with the cellular
IFI16 protein. J Virol. 84:7803–7814. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bauer S, Groh V, Wu J, Steinle A, Phillips
JH, Lanier LL and Spies T: Activation of NK cells and T cells by
NKG2D, a receptor for stress-inducible MICA. Science. 285:727–729.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu J, Song Y, Bakker AB, Bauer S, Spies T,
Lanier LL and Phillips JH: An activating immunoreceptor complex
formed by NKG2D and DAP10. Science. 285:730–732. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chalupny NJ, Rein-Weston A, Dosch S and
Cosman D: Down-regulation of the NKG2D ligand MICA by the human
cytomegalovirus glycoprotein UL142. Biochem Biophys Res Commun.
346:175–181. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zou Y, Bresnahan W, Taylor RT and Stastny
P: Effect of human cytomegalovirus on expression of MHC class
I-related chains A. J Immunol. 174:3098–3104. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Beck S and Barrell BG: Human
cytomegalovirus encodes a glycoprotein homologous to MHC class I
antigens. Nature. 331:269–272. 1988. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Novotny J, Rigoutsos I, Coleman D and
Shenk T: In silico structural and functional analysis of the human
cytomegalovirus (HHV5) Genome. J Mol Biol. 310:1151–1166. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bao Y, Lopez JA, James DE and Hunziker W:
Snapin interacts with the Exo70 subunit of the exocyst and
modulates GLUT4 trafficking. J Biol Chem. 283:324–331. 2008.
View Article : Google Scholar
|
|
24
|
Buxton P, Zhang XM, Walsh B, Sriratana A,
Schenberg I, Manickam E and Rowe T: Identification and
characterization of Snapin as a ubiquitously expressed
SNARE-binding protein that interacts with SNAP23 in non-neuronal
cells. Biochem J. 375:433–440. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Luo J, Chen J, Yang E, et al: Modulation
of the cellular distribution of human cytomegalovirus helicase by
cellular factor snapin. J Virol. 87:10628–10640. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Barnard EC, Brown G and Stow ND: Deletion
mutants of the herpes simplex virus type 1 UL8 protein: effect on
DNA synthesis and ability to interact with and influence the
intracellular localization of the UL5 and UL52 proteins. Virology.
237:97–106. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Söllner TH: Regulated exocytosis and SNARE
function. Mol Membr Biol. 20:209–220. 2003. View Article : Google Scholar
|
|
28
|
Weber T, Zemelman BV, McNew JA, et al:
SNAREpins: minimal machinery for membrane fusion. Cell. 92:759–772.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Penfold ME and Mocarski ES: Formation of
cytomegalovirus DNA replication compartments defined by
localization of viral proteins and DNA synthesis. Virology.
239:46–61. 1997. View Article : Google Scholar
|
|
30
|
Zhou B, Zhu YB, Lin L, Cai Q and Sheng ZH:
Snapin deficiency is associated with developmental defects of the
central nervous system. Biosci Rep. 31:151–158. 2011. View Article : Google Scholar
|
|
31
|
Wu CA, Nelson NJ, McGeoch DJ and Challberg
MD: Identification of herpes simplex virus type 1 genes required
for origin-dependent DNA synthesis. J Virol. 62:435–443.
1988.PubMed/NCBI
|
|
32
|
Suzuki F, Morishima S, Tanaka T and
Muramatsu I: Snapin, a new regulator of receptor signaling,
augments alpha1A-adrenocept-or-operatedcalcium influx through
TRPC6. J Biol Chem. 282:29563–29573. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Snyder DA, Kelly ML and Woodbury DJ: SNARE
complex regulation by phosphorylation. Cell Biochem Biophys.
45:111–123. 2006. View Article : Google Scholar : PubMed/NCBI
|