Introduction
In North America, prostate cancer is currently the
most commonly diagnosed noncutaneous cancer in males (1). Its incidence and mortality rate are
increasing in China (2). Although
the majority of males with prostate cancer may be successfully
treated with surgery or radiation therapy, 20–40% of cases will
biochemically recur within 10 years of treatment. This risk of
recurrence is elevated to ~50% in males with locally advanced
disease, a condition that is primarily managed by radiation
therapy. Thus, new technologies that improve the therapeutic index
of radiation therapy for local disease have the opportunity to
significantly impact on the morbidity and mortality rates of
prostate cancer (3).
Higher doses of radiation may be used to improve the
outcome of radiation therapy. When high doses (>7,200 cGy) of
radiation therapy were applied, the 5-year survival rate increased
from 41 to 75% in patients with unfavorable tumors, including those
with T3-stage lesions, initial prostate-specific antigen levels
greater than 10 ng/ml, or a biopsy Gleason score greater than 7
(4). However, the use of high-dose
radiation treatment is limited due to serious side effects and late
complications, including erectile dysfunction, bladder
inflammation, urethral stricture and radiation proctitis, caused by
a high dose of radiation toxicity (5). Moreover, hormone-independent prostate
cancer is resistant to radiation therapy compared with
hormone-dependent prostate cancer. Therefore, it is critical to
increase the radiation sensitivity of prostate cancer cells to
minimize the radiation toxicity by lowering effective therapeutic
doses and increase the radiation therapy benefit.
Nuclear factor-kappa B (NF-κB) proteins are a group
of transcriptional regulators that activate the expression of genes
involved in diverse biological processes, including inflammation
and cell growth, differentiation and apoptosis (6). Activation of NF-κB signaling is
considered to be a significant mechanism in the development of
prostate cancer. Insights obtained from the study of NF-κB
functions in cancer have provided a mechanistic link between
inflammation and tumorigenesis, as well as valuable information
regarding NF-κB-mediated cytoprotection against cancer
therapeutics. Consequently, NF-κB is being considered as a target
for anticancer therapy (7,8). All subunits of the NF-κB family,
RelA, p50, RelB, p52 and c-Rel, are expressed in prostate tissues,
and all but c-Rel are detectable in the nucleus of prostatic cells
as well as other cancerous tissues (5). This suggests that both the canonical
(RelA and p50) and non-canonical (RelB and p52) NF-κB subunits may
be activated in prostate cancer cells, whereas c-Rel remains
inactive (9). Upon radiation, the
NF-κB pathway is activated by radiation-induced reactive oxygen
species (9,10). The level of nuclear RelB has been
shown to correlate with prostate cancer patient Gleason scores,
suggesting that RelB may be associated with prostate cancer
progression. In numerous types of cancer, the RelA-based classic
pathway is considered to play a significant role in response to
chemotherapeutics and radiotherapeutics (9). However, in prostate cancer, RelB may
contribute to the radioresistance of high-risk prostate cancers
(11). Therefore, targeting RelB
activation may prove to be a valid strategy to defeat aggressive
and radiation-resistant prostate cancer.
In the present study, it was demonstrated that RelB
inhibition by specific RelB siRNA prior to radiotherapy
significantly increased the radiation sensitivity of RM-1
cells.
Materials and methods
RelB expression in prostate samples
Specimens were acquired from tissue from radical
prostatectomy surgery and transurethral resection of the prostate,
and normal human prostate was acquired from donors following
cardiac mortality. The 71 prostate samples were collected between
January 2012 and December 2013 at Renmin Hospital of Wuhan
Univeristy (Wuhan, China). The age range of the patients was
between 45 and 77 years old. All the specimens were collected
following the provision of informed consent from the patient and
their family. The study was approved by the ethics committee of
Renmin Hospital of Wuhan University, China. Paraffin-embedded
sections of normal human prostate (n=18), benign prostate
hyperplasia (BPH; n=29) and prostate cancer (n=24; T1cN0M0-T3a
N0M0) were obtained from Renmin Hospital of Wuhan University. The
prostate cancer specimens were from the androgen-independent stage
of cancer. The specimens were stained with rabbit anti-human RelB
monoclonal antibody (Santa Cruz Biotechnology, Inc., Santa Cruz,
CA, USA) and developed with 3,3-diaminobenzidine. All slides were
reviewed by one pathologist who was blinded to the study. Samples
were scored based on RelB expression intensity as 0 (negative), 1
(weak), 2 (moderate) or 3 (strong).
Cell culture
The mouse prostate cancer cell line RM-1, a highly
aggressive and androgen-independent murine prostatic cancer cell
line, was purchased from the Chinese Academy of Sciences (Beijing,
China) and maintained in Dulbecco’s modified Eagle’s medium
(Hyclone, Logan, UT, USA) supplemented with 10% fetal bovine serum,
2 mM L-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin,
at 37°C in a humidified atmosphere containing 5%
CO2.
Construction of recombinant lentivirus
vectors
Five types of shRNA sequence targeting the mRNA
sequence of RelB (accession number, M83380) were designed and
evaluated for specificity and knockdown efficiency. The most
effective shRNA for RelB targeting was 5′-TGTCGTCAGGATCT
GCTTCTTCAAGAGAGAAGCAGATCCTGACGACATTTT TTC-3′ and
3′-ACAGCAGTCCTAGACGAAGAAGTTCTCTC TTCGTCTAGGACTGCTGTAAAAAAGAGCT-5′.
shRNA was designed, chemically synthesized and purified by
polyacrylamide gel electrophoresis (PAGE) in the absence of RNase
contamination, according to the manufacturer’s instructions
(R&S, Shanghai, China). The lentiviral work vector plentilox3.7
was used to recombine the shuttle plasmid. The shRNAs were cloned
to plentilox3.7, which was linearized by restriction endonucleases
HindIII and KspAI. All constructs were verified by
sequencing. The recombinant lentiviral vectors containing
RelB-specific siRNA were designated as Lenti-siRNA-RelB. A vector
containing siRNA targeting luciferase, designated as Lenti-siRNA,
was used as a control. To produce the lentivirus, the recombinant
work vector and package plasmids (pVSVG, pRSV-REV and pMDLg/pRRE)
were cotransfected to 293T cells using Lipofectamine 2000
(Invitrogen Life Technologies, Carlsbad, CA, USA). The culture
medium was collected 48 h later, concentrated by
ultracentrifugation, aliquoted and stored at −80°C for later use.
Vector titers were determined by measuring GFP expression in 293T
cells transduced with serial dilutions of vectors, and transducing
units (TU)/ml were calculated as the number of cells infected, as
follows: percentage of EGFP+ cells/10 × dilution of
vector. The vector titers were 1×108 TU/ml. The RM-1
cells were transfected at a multiplicity of infection (MOI) of 100.
Based on the results of fluorescence microscopy and flow cytometry,
the infection efficiency reaches up to 95% with an MOI of 100
(12).
Radiation treatment of RM-1 cells
The cells were divided into four groups for the
radiation therapy analysis. In the control group, RM-1 cells were
treated with phosphate-buffered saline (PBS). In the radiation
therapy group (RT group), RM-1 cells were treated with PBS and
exposed to radiation at a single dose of 6 Gy. In the siRNA
luciferase and radiation therapy group (siRNA control+RT group),
RM-1 cells were transfected with siRNA targeting luciferase at an
MOI of 100 and, after 72 h, cells were exposed to radiation at a
single dose of 6 Gy. In the siRNA RelB and radiation therapy group
(siRNA RelB+RT group), RM-1 cells were transfected with siRNA RelB
at an MOI of 100, and after 72 h cells were exposed to radiation at
a single dose of 6 Gy.
RM-1 cells in six-well plates were irradiated using
a 6-MeV linear accelerator at room temperature. Doses were
administered at 2.25 Gy/min. The distance between the radiation
source and the cells was 100 cm. Following radiation therapy, the
cells were incubated in normal medium for 48 h and used for
subsequent experiments. In the clonogenic survival assay, each cell
group received a single dose of 0, 2, 4, 6 or 8 Gy per treatment
following infection with lentivirus.
Real-time polymerase chain reaction
(PCR)
Total RNA (1 μg) was isolated from RM-1 cells with
TRIzol reagent and reverse-transcribed using oligo-dT primers with
reverse transcription reagents (Toyobo Corporation, Osaka, Japan).
Reverse-transcribed RNA was amplified with SYBR-Green PCR Master
mix (Toyobo Corporation) plus 0.4 μM gene-specific upstream and
downstream primers on a 7500 Fast Realtime cycler (Applied
Biosystems, Inc., Foster City, CA, USA). PCR conditions were as
follows: 40 cycles of denaturation at 95°C for 15 sec, annealing at
55°C for 15 sec, and extension at 72°C for 45 sec. The primer
sequences were as follows: Forward, CCGTGAAAAGATGACCCAG and
reverse, TAGCCACGCTCGGTCAGG for β-actin; forward,
GACATACCGTGGTGTTCAGC and reverse, GTCCTCGTAGGGTGGCGTT for RelB.
Data were analyzed by relative quantification using the
2−ΔΔCT method (13).
Western blot analysis
Proteins from the cytosol and nucleus were isolated
from RM-1 cells. Protein samples were separated using 10% SDS-PAGE
and transferred onto a nitrocellulose membrane. RelB protein was
detected with a primary rabbit anti-mouse RelB monoclonal antibody
(1:1,000; Invitrogen Life Technologies) and a horseradish
peroxidase-conjugated goat anti-rabbit secondary polyclonal
antibody (KPL, Gaithersburg, MD, USA). The protein signals were
visualized with an enhanced chemiluminescence reaction system.
β-actin (Santa Cruz Biotechnology, Inc.) was used as an internal
control.
DNA binding ability of NF-κB
subunits
The DNA-binding capacity of NF-κB subunits in RM-1
nuclear extracts was measured using the Nuclear Extract™ and
TransAM™ NF-κB kits (Active Motif, Carlsbad, CA, USA) according to
the manufacturer’s instructions. Briefly, an oligonucleotide
containing the NF-κB consensus binding site (5′-GGGACTTTCC-3′) was
immobilized in 96-well plates, and nuclear extracts containing
activated NF-κB were added. Binding of NF-κB to the
oligonucleotides was detected using a rabbit anti-mouse primary
monoclonal antibody (Invitrogen Life Technologies), which accesses
an epitope on NF-κB subunits when NF-κB binds its target DNA. The
addition of secondary goat anti-rabbit polyclonal antibody (KPL)
conjugated to horseradish peroxidase was used to detect the NF-κB.
The specificity of this assay was confirmed by incubations in the
presence of an excess of non-immobilized consensus oligonucleotide
as a competitor, and blank controls, in which PBS replaced the
nuclear extracts. 3,3′,5,5′-Tetramethylbenzidine was added, and
absorbance was detected at 655 nm.
Measurement of apoptosis by flow
cytometry
RM-1 cells were collected, resuspended in binding
buffer and stained with Annexin V-FITC and propidium iodide
(Nanjing KeyGen Biotech Co., Ltd., Nanjing, China). After washing
the samples three times in PBS, the cells were analyzed by flow
cytometry.
Clonogenic survival assay
Following radiation exposure, RM-1 cells from each
well of the six-well plates were trypsinized and grown in
triplicate in 60-mm culture dishes with various densities for 14
days. After the cells were fixed in methanol/acetic acid (3:1) for
30 min, cell clones were counted under a microscope (>50
cells/clone). Plating efficiency (PE) was calculated by dividing
the average number of colonies per plate by the amount of cells
plated, and then multiplying by 100. Survival fractions (SFs) were
calculated using the following formula: SF = colony number/(cell
number cultured × PE). A one-hit multi-target model was fitted to
the cell survival curve [SF=1−1 (1−e−KD)N,
SF=1−(1−e−D/D0)N (D0=1/K)] to
determine the quasi-threshold dose (Dq), mean lethal
dose (D0), 2 Gy survival fraction (SF2), N
value and sensitization enhancement ratio.
Statistical analysis
Statistical analysis was performed using one-way
analysis of variance for multiple group comparison using SPSS 11.5
software (SPSS, Inc., Chicago, IL, USA). Data are presented as the
mean ± standard deviation. P<0.05 was considered to indicate a
statistically significant difference.
Results
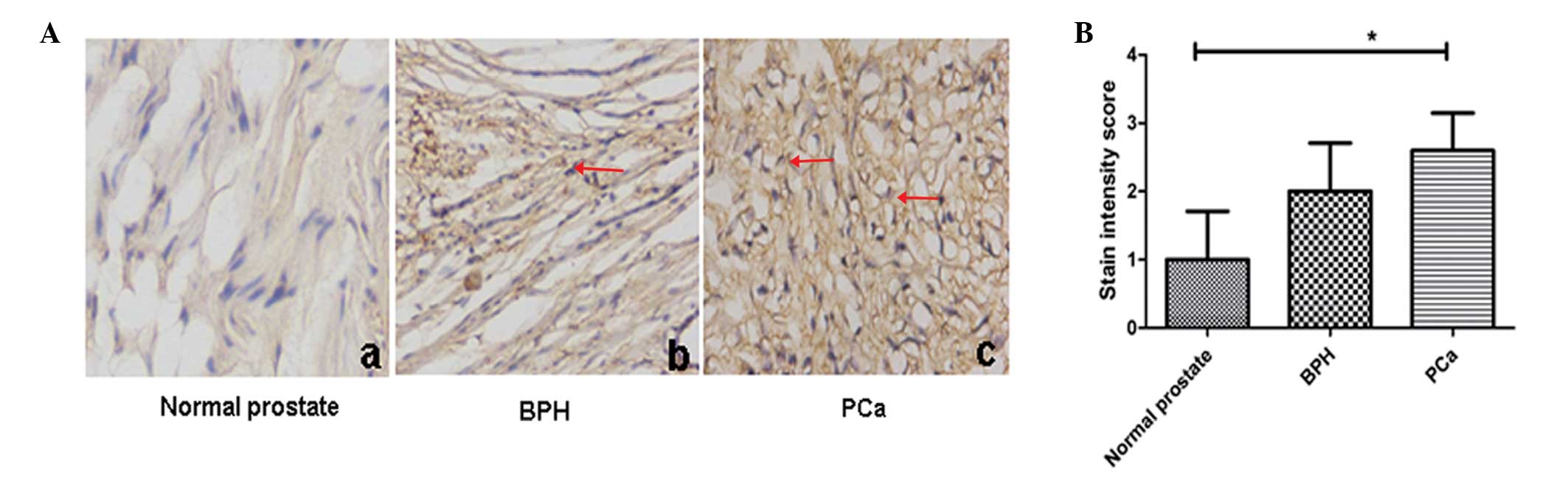
RelB expression is upregulated in
prostate cancer
Paraffin-embedded sections of normal human prostate
(n=18), BPH (n=29) and prostate cancer (n=24) were examined and
scored for RelB expression levels. All prostate cancer specimens,
which were from the androgen-independent stage of cancer, revealed
an upregulation of RelB compared with BPH and normal prostate
tissue. The average score of RelB expression in prostate cancer
samples was significantly higher than that in normal prostate and
BPH (Fig. 1). This finding is
consistent with previous studies (14).
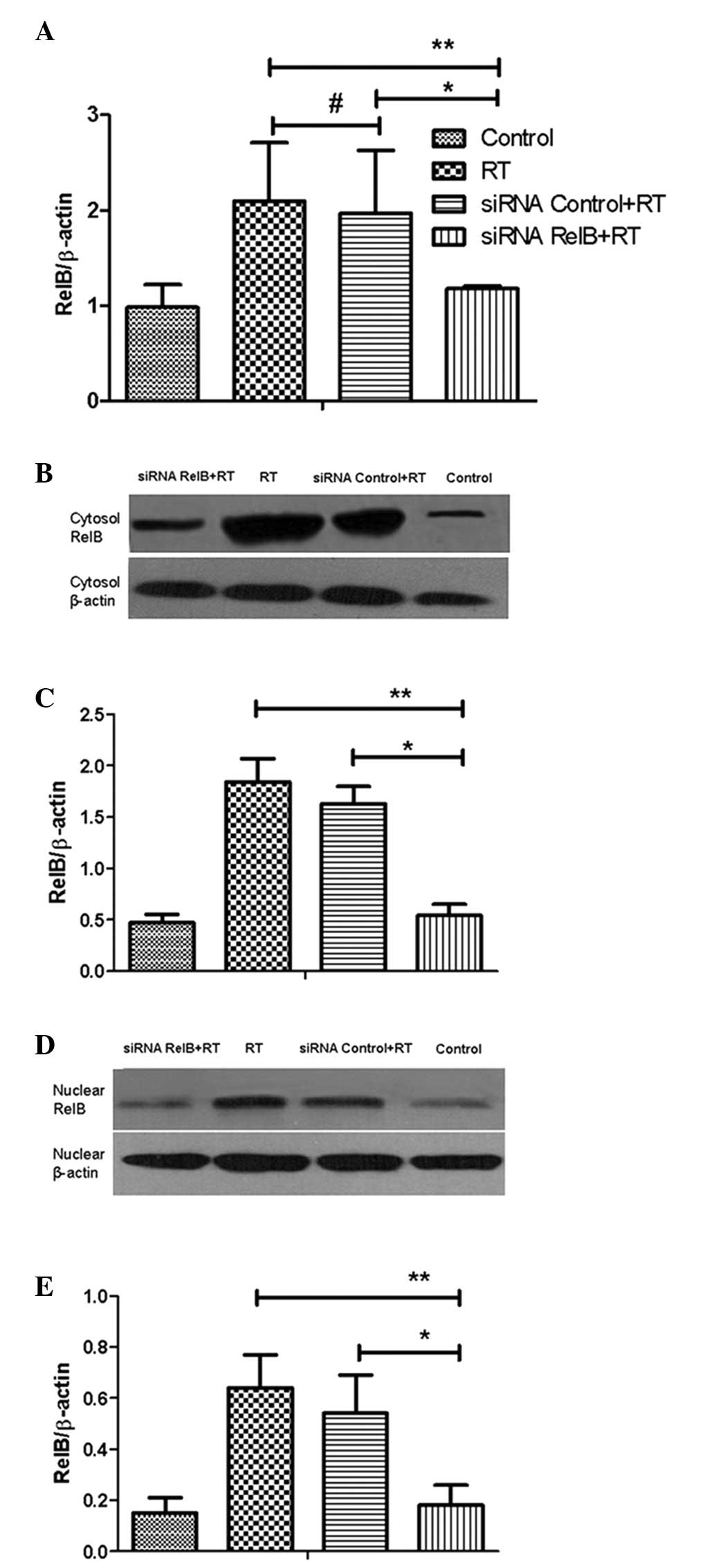
siRNA RelB suppresses activation of NF-κB
upon radiation of RM-1 cells
To more closely examine the required function and
mechanisms of RelB in cells, RM-1 cells were first transfected with
the appropriate siRNAs and then treated with radiation therapy. As
shown in Fig. 2A, the mRNA
expression of RelB in the siRNA RelB+RT group was reduced by ~40%
compared with the siRNA control+RT group and the RT group (t-test;
P<0.05).
To further examine the reduced RelB expression in
the siRNA RelB+RT group, western blot analysis was performed on the
cytosolic and nuclear cell fractions. The results revealed that
RelB protein levels were 71.8% lower in the cytosolic fractions
(Fig. 2B) and 70.4% lower in the
nuclear fractions (Fig. 2D) in the
siRNA RelB+RT group compared with those of the siRNA control+RT
group, which had similar RelB protein levels to the RT group.
This finding demonstrated that RelB siRNA
effectively inhibited the induction of RelB expression in RM-1
cells in response to radiation therapy.
RelB siRNA reduces RelB DNA binding
Next, the DNA-binding ability of NF-κB subunits was
measured in RM-1 nuclear extracts (Fig. 3). No significant differences in the
binding abilities of p50, p52, RelA (p65) or c-Rel were observed
among the four treated cell groups. However, there was a marked
decrease in the RelB DNA binding capacity in the siRNA RelB+RT
group. These results revealed that RelB siRNA transfection reduced
RelB binding to DNA, but had no effect on the DNA binding activity
of p50, p52, RelA (p65) and c-Rel.
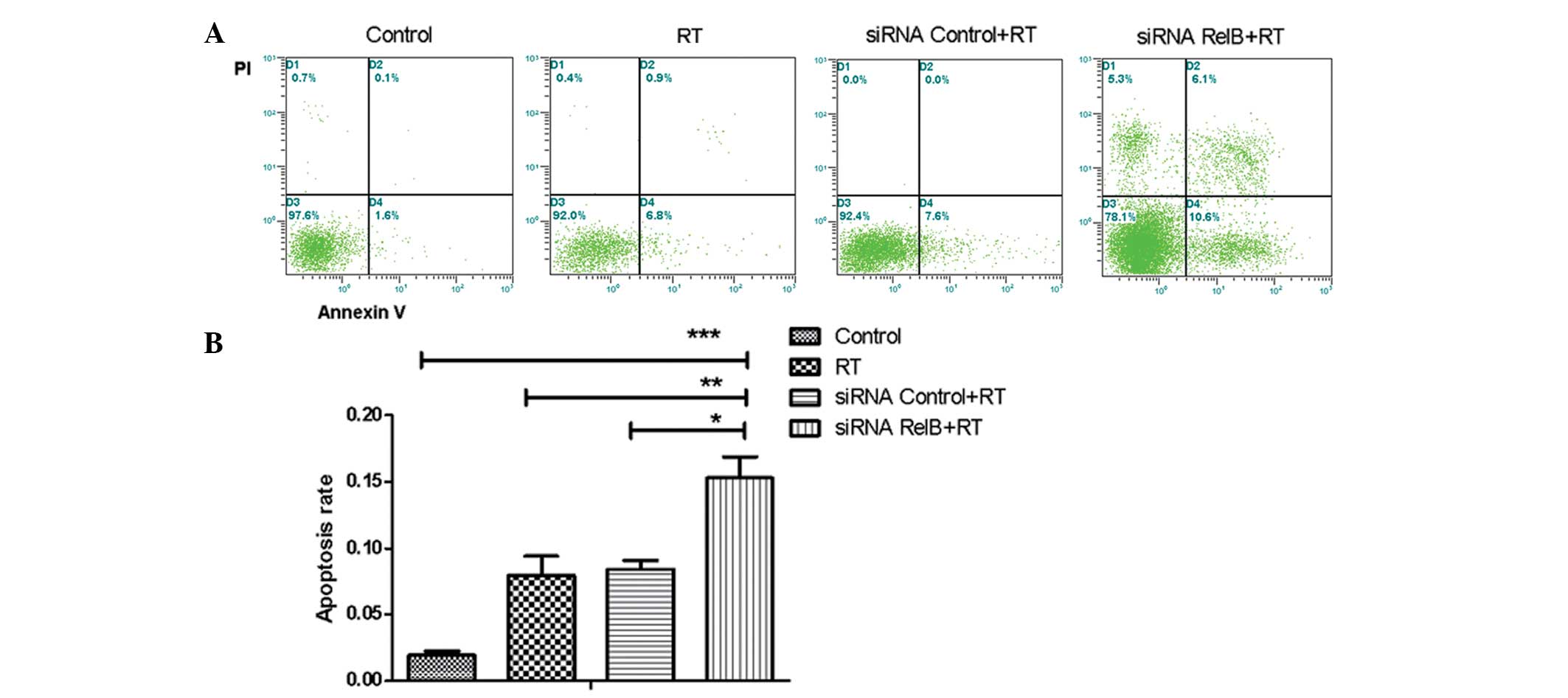
RelB siRNA augments radiation
therapy-induced apoptosis in RM-1 cells
Next, the effects of radiation therapy on inducing
apoptosis were examined in the various treated cell groups. As
shown in Fig. 4, the siRNA
control+RT group and the RT group demonstrated increased apoptosis
in response to radiation compared with untreated cells. Notably,
the siRNA RelB+RT group had a notably higher apoptotic rate than
the siRNA control+RT group and the RT group (P<0.05). This
indicates that silencing of RelB by siRNA increases the levels of
apoptosis induced by radiation therapy.
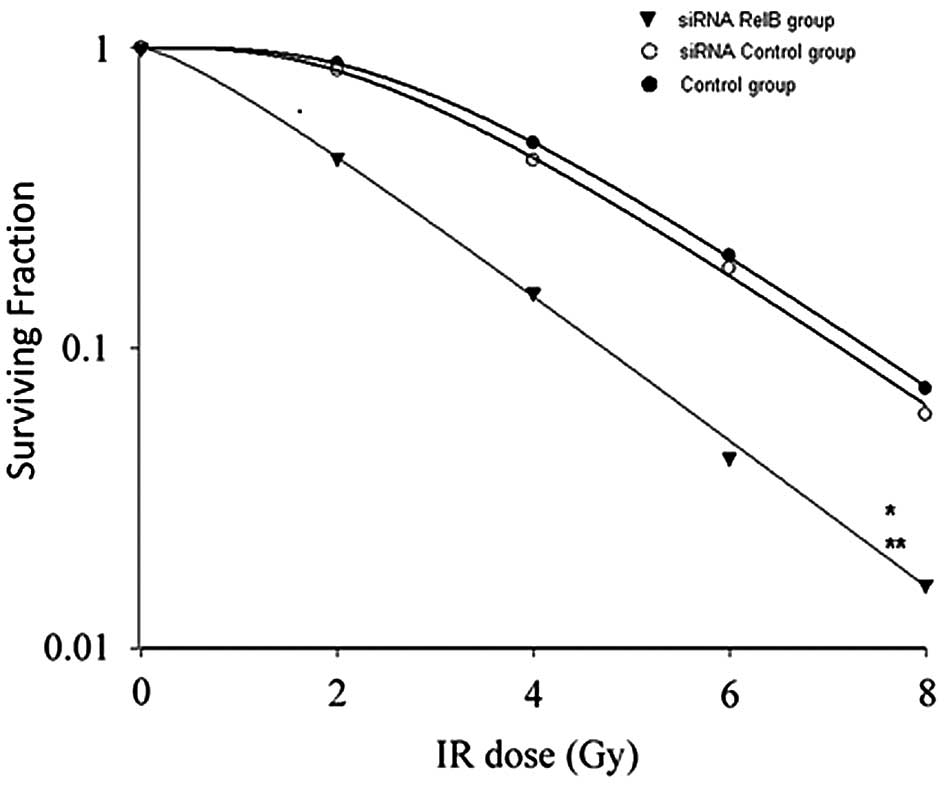
Pretreatment with RelB siRNA increases
the radiosensitivity of RM-1 cells
Next, the radiosensitivity of the RM-1 experimental
cell groups treated with varying Gy dosages was examined. As shown
in Fig. 5, the surviving fraction
values from the siRNA RelB+RT group (0.8, 0.11, 0.06 and 0.02) were
less than those of the siRNA control+RT group (0.95, 0.8, 0.12 and
0.09) at dosages of 2, 4, 6 and 8 Gy, indicating that RM-1 cells
transfected with RelB siRNA were more sensitive to
radiation-induced cell death compared with controls.
An increased D0 value indicates a higher
cell radiation resistance, while the Dq value represents the cell’s
ability to recover from sub-lethal damage. The values of
D0 (1.68) and Dq (0.60) of the siRNA RelB+RT group were
significantly lower than those of the RT group (1.02, 3.08, 0.89,
4.97) and siRNA control+RT group (1.93, 2.76, 0.84, 4.17),
indicating that RM-1 cells transfected with RelB siRNA have a lower
radiation resistance and a weakened damage recovery ability.
Discussion
Radiation therapy is in the front-line for the
treatment of localized prostate cancer. However, a significant
percentage of patients have radiation-resistant disease (15). Cellular hallmarks of cancer include
self-sufficiency in growth, loss of growth inhibitory mechanisms,
evasion of apoptosis, sustained angiogenesis, immortality and
invasion/metastasis (16). All of
these mechanisms are known to be affected in some way via the
activation of NF-κB signaling (14,17).
Traditional research on the mechanisms underlying
the effects of radiation has only focused on intracellular damage
and repair processes with the influence of radiation treatment on
the cellular microenvironment (18). Recently, standard concepts of
radiobiology have been based on the assumption that double-strand
DNA breaks are the most significant aspects of tumor cell death via
free radical formation (19).
Studies in radiation biology have acquired new insights in
understanding the effects of ionizing radiation on various levels,
including immune inflammatory reactions, transmembrane signaling
pathways, genomic instability and apoptosis (20). Cellular signaling in response to
ionizing radiation involves the complex modulation of various
proteins and compensatory stimuli that have not yet been fully
characterized (21). Certain
findings have revealed that these survival signaling mechanisms
have provided cells with a cytoprotective response capability,
which allows cells to accelerate proliferation, improve repair
functions and resist apoptosis after being exposed to ionizing
radiation (22–23). In the setting of clinically
relevant doses of ionizing radiation with fractionated schedules,
there has been concern that prostate cancer cells may be able to
adapt to repeated stressful exposures, possibly resulting in the
evolution of a more aggressive phenotype.
A number of studies have reported altered expression
of NF-κB-related proteins in human cancers (24–26).
Several studies have shown that the NF-κB pathway is a significant
factor in radiation resistance, and the classical (canonical)
pathway is believed to confer protection of prostate cancer cells
from ionizing radiation. Although inhibition of the classic pathway
may be a useful approach for the enhancement of radiotherapies and
chemotherapies, it may also sensitize normal cells to treatment
with radiotherapies and chemotherapies.
It is well recognized that NF-κB activity promotes
radiation resistance, although it is uncertain which NF-κB family
member functions in the radiation resistance. Studies on the
classic pathway have revealed that suppression of p65 and ablation
of IKKβ increased the chemosensitivity and radiosensitivity of
certain cancer cells. However, as the classic pathway is also known
to play a significant role in protecting normal tissues against
chemotherapies and radiotherapies (11), the overall benefits from
suppression of RelA in the sensitization of cancer treatments
remain to be investigated.
The alternative pathway, which is involved in
prostate cancer aggressiveness, has also been shown to be
significant in radiation resistance in prostate cancer (11). The alternative NF-κB pathway
component RelB protects prostate cancer cells from the detrimental
effects of ionizing radiation in part by stimulating expression of
the mitochondria-localized antioxidant enzyme manganese superoxide
dismutase (MnSOD). Studies have demonstrated that RelB is uniquely
expressed at a high level in prostate cancer (Fig. 1) and correlates with high Gleason
scores (11,31). Blocking RelB activation suppresses
MnSOD expression and sensitizes prostate cancer cells to radiation
(15). In the present study,
pretreatment with RelB siRNA inhibited RelB expression in the
cytosol and nucleus even upon stimulation of radiotherapy (Fig. 2), confirming the blocking of the
alternative pathway.
The RelB expression level was shown to be increased
in radioresistant PC-3 cells, even though all five subunits of
NF-κB were expressed in PC-3 cells not treated with radiation
(28). Upon pretreatment with the
RelB inhibitor 1α,25-dihyroxyvitamin D3, PC-3 cells
remained radiosensitive following traditional treatment (29). Therefore, valid methods to inhibit
the alternative pathway needed to be further developed. A further
study indicated that ionizing radiation enhanced the DNA binding
activity of RelB more than that of RelA in PC-3 cells, indicating
that increased RelB nuclear import may be significant in
radioresistance development in prostate cancer cells (30). In the current study, the DNA
binding activity of RelB was inhibited in the siRNA RelB+RT group,
but the binding of other subunits was not affected (Fig. 3). Together, this suggests that the
sensitization of prostate cancer to radiotherapy is increased by
RelB gene silencing via the inhibition of RelB nuclear import and
the reduction of RelB DNA binding activity.
Inhibiting RelB in aggressive androgen-independent
PC-3 cells significantly reduces the incidence and growth rate of
tumors. One study demonstrated that stable expression of RelB in
androgen-responsive LNCaP tumors increased the circulating IL-8
levels. The decrease in tumorigenicity coincided with a reduction
in the NF-κB target interleukin-8 (IL-8). Consistent with this
finding, downregulation of RelB via siRNA also reduced tumor growth
and decreased levels of IL-8 (31). The blockage of p50:RelA dimer
nuclear translocation by ablating expression or SN50-mediated
inhibition of IKKβ significantly suppressed tumorigenesis and
improved therapeutic efficacy in several cancer types (29). The prevention of p52:RelB nuclear
translocation by expression of p100M and treatment with SN52 and
1α,25-dihydroxyvitamin D3 dramatically enhanced the
radiosensitization effect (15,29,30).
The prevention of RelB nuclear translocation decreased the
tumorigenicity of prostate cancer cells. Together, these results
suggest that the inhibition of RelB nuclear translocation
suppresses the tumorigenesis and radioresistance of prostate
cancer. In the present study, siRNA RelB efficiently enhanced the
radiosensitivity of aggressive prostate cancer cells at low
ionizing radiation doses with a limited effect on the growth of
normal prostate cells, providing a potential therapeutic approach
for the treatment of prostate cancer with high Gleason scores. In
line with the observations of others, this study demonstrated that
the RM-1 cell survival fraction number and other survival
parameters following ionizing radiation treatment were lower in
siRNA RelB RM-1 cells compared with those of siRNA control RM-1
cells (Fig. 5). Furthermore, the
apoptotic rate was much higher in the siRNA RelB+RT group compared
with that of the siRNA control+RT group (Fig. 4).
The blockage of RelB expression by siRNA RelB in the
radiotherapy of prostate cancer is capable of inhibiting RelB
expression in the cytosol and nucleus. Treatment with siRNA RelB
also decreased the RelB DNA binding capacity and augmented the
apoptotic rate of RM-1 cells. The clonogenic survival assay
revealed that it significantly enhanced the sensitization of
prostate cancer to radiotherapy. Overall, the findings from this
study provide a foundation for possible future study of
radioresistance development.
The inhibition of the NF-κB alternative pathway via
RelB silencing may provide a unique opportunity for selective
sensitization of prostate cancer to radiotherapy.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 30671993), the Hubei
Provincial Natural Science Foundation of China (grant no.
2011CDB483) and the Fundamental Research Funds for the Central
Universities (grant no. 201130202020010).
References
|
1
|
Brawley OW: Prostate cancer epidemiology
in the United States. World J Urol. 30:195–200. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shao Q, Ouyang J, Fan Y, et al: Prostate
cancer in the senior men from rural areas in east district of
China: contemporary management and 5-year outcomes at
multi-institutional collaboration. Cancer Lett. 315:170–177. 2012.
View Article : Google Scholar
|
|
3
|
Fitzgerald TJ, Wang T, Goel HL, et al:
Prostate carcinoma and radiation therapy: therapeutic treatment
resistance and strategies for targeted therapeutic intervention.
Expert Rev Anticancer Ther. 8:967–974. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Criswell T, Leskov K, Miyamoto S, et al:
Transcription factors activated in mammalian cells after clinically
relevant doses of ionizing radiation. Oncogene. 22:5813–5827. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Seo SI, Song SY, Kang MR, et al:
Immunohistochemical analysis of NF-kappaB signaling proteins
IKKepsilon, p50/p105, p52/p100 and RelA in prostate cancers. APMIS.
117:623–628. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Perkins ND and Gilmore TD: Good cop, bad
cop: the different faces of NF-κB. Cell Death Differ. 13:759–772.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Enzler T, Sano Y, Choo MK, et al:
Cell-selective inhibition of NF-κB signaling improves therapeutic
index in a melanoma chemotherapy model. Cancer Discov. 1:496–507.
2011. View Article : Google Scholar
|
|
8
|
Umezawa K: Possible role of peritoneal
NF-κB in peripheral inflammation and cancer: lessons from the
inhibitor DHMEQ. Biomed Pharmacother. 65:252–259. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lessard L, Begin LR, Gleave ME, et al:
Nuclear localisation of nuclear factor-kappaB transcription factors
in prostate cancer: an immunohistochemical study. Br J Cancer.
93:1019–1023. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Murley JS, Kataoka Y and Cao D: Delayed
radioprotection by NFkappaB-mediated induction of Sod2 (MnSOD) in
SA-NH tumor cells after exposure to clinically used
thiol-containing drugs. Radiat Res. 162:536–546. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu Y, Josson S, Fang F, Oberley TD, et al:
RelB enhances prostate cancer growth: implications for the role of
the nuclear factor-kappaB alternative pathway in tumorigenicity.
Cancer Res. 69:3267–3271. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Heng-cheng TT, Liu XH, Kuang YU, et al:
Construction and identification of mouse RelB siRNA-expressing
lentiviral vectors. Sci Res Essays. 6:777–783. 2011.
|
|
13
|
Chendil D, Das A, Dey D, et al: Par-4, a
pro-apoptotic gene, inhibits radiation-induced NF kappa B activity
and Bcl-2 expression leading to induction of radiosensitivity in
human prostate cancer cells PC-3. Cancer Biol Ther. 1:152–160.
2002. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Okera M, Bae K, Bernstein E, et al:
Evaluation of nuclear factor kappaB and chemokine receptor CXCR4
co-expression in patients with prostate cancer in the Radiation
Therapy Oncology Group (RTOG) 8610. BJU Int. 108:E51–E58. 2011.
View Article : Google Scholar
|
|
15
|
Holley AK, Xu Y, St Clair DK and St Clair
WH: RelB regulates manganese superoxide dismutase gene and
resistance to ionizing radiation of prostate cancer cells. Ann N Y
Acad Sci. 1201:129–136. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: the next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Watson C, Miller DA, Chin-Sinex H, et al:
Suppression of NF-kappaB activity by parthenolide induces X-ray
sensitivity through inhibition of split-dose repair in TP53 null
prostate cancer cells. Radiat Res. 171:389–396. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bischoff P, Altmeyer A and Dumont F:
Radiosensitising agents for the radiotherapy of cancer: advances in
traditional and hypoxia targeted radiosensitisers. Expert Opin Ther
Pat. 19:643–662. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Carlson DJ, Yenice KM and Orton CG: Tumor
hypoxia is an important mechanism of radioresistance in
hypofractionated radiotherapy and must be considered in the
treatment planning process. Med Phys. 38:6347–6350. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yacoub A, Miller A, Caron RW, et al:
Radiotherapy-induced signal transduction. Endocr Relat Cancer.
13:S99–S114. 2006. View Article : Google Scholar
|
|
21
|
Koterba K, Beckman MJ and Gewirtz DA: A
switch between cytoprotective and cytotoxic autophagy in the
radiosensitization of breast tumor cells by chloroquine and vitamin
D. Horm Cancer. 2:272–285. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Klokov D, Criswell T, Leskov KS, Araki S,
Mayo L and Boothman DA: IR-inducible clusterin gene expression: a
protein with potential roles in ionizing radiation-induced adaptive
responses, genomic instability, and bystander effects. Mutat Res.
568:97–110. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Simon EL, Goel HL, Teider N, et al: High
dose fractionated ionizing radiation inhibits prostate cancer cell
adhesion and beta(1) integrin expression. Prostate. 64:83–91. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tang CH and Tsai CC: CCL2 increases MMP-9
expression and cell motility in human chondrosarcoma cells via the
Ras/Raf/MEK/ERK/NF-κB signaling pathway. Biochem Pharmacol.
83:335–344. 2012. View Article : Google Scholar
|
|
25
|
Zhu L, Zhu B, Yang L, Zhao X, Jiang H and
Ma F: RelB regulates Bcl-xl expression and the irradiation-induced
apoptosis of murine prostate cancer cells. Biomed Rep. 3:354–358.
2014.
|
|
26
|
Umemura N, Zhu J, Mburu YK, et al:
Defective NF-κB signaling in metastatic head and neck cancer cells
leads to enhanced apoptosis by double-stranded RNA. Cancer Res.
72:45–55. 2012. View Article : Google Scholar :
|
|
27
|
Pradhan M, Baumgarten SC, Bembinster LA,
et al: CBP mediates NF-κB-dependent histone acetylation and
estrogen receptor recruitment to an estrogen response element in
the BIRC3 promoter. Mol Cell Biol. 32:569–575. 2012. View Article : Google Scholar :
|
|
28
|
Josson S, Xu Y, Fang F, et al: RelB
regulates manganese superoxide dismutase gene and resistance to
ionizing radiation of prostate cancer cells. Oncogene.
25:1554–1559. 2006. View Article : Google Scholar
|
|
29
|
Xu Y, Fang F, St Clair DK, et al:
Suppression of RelB-mediated manganese superoxide dismutase
expression reveals a primary mechanism for radiosensitization
effect of 1alpha,25-dihydroxyvitamin D(3) in prostate cancer cells.
Mol Cancer Ther. 6:2048–2056. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xu Y, Fang F, St Clair DK, et al: SN52, a
novel nuclear factor-kappaB inhibitor, blocks nuclear import of
RelB:p52 dimer and sensitizes prostate cancer cells to ionizing
radiation. Mol Cancer Ther. 7:2367–2376. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Reuter S, Charlet J, Juncker T, et al:
Effect of curcumin on nuclear factor kappaB signaling pathways in
human chronic myelogenous K562 leukemia cells. Ann N Y Acad Sci.
1171:436–447. 2009. View Article : Google Scholar : PubMed/NCBI
|