Introduction
Abnormal lipid metabolism is associated with
numerous diseases, including hepatic adipose infiltration,
pancreatitis, obesity and diabetes (1–3). In
addition, abnormal intravascular lipid metabolism is a major
promoter of atherosclerosis, which may deteriorate into coronary
disease and cerebral arterial thrombosis. Zebrafish (Danio
rerio), with transparent embryos, external fertilization,
clutch size and moderate generation time has functioned as an
important vertebrate model (4,5).
There have been numerous lipid studies focused on the alimentary
system, saccus vitellinus, angiogenesis and the nervous system
(6,7). Conversely, the study of zebrafish
intravascular lipid metabolism has been limited, although studies
have proven that zebrafish have similar digestive physiology and
lipid metabolism to that of humans and the treatment of zebrafish
with antihyperlipidemic drugs triggers similar responses to those
observed in humans (8–11). Therefore, the establishment of a
standardized platform for antihyperlipidemic drug screening in a
zebrafish system is important.
Lipophilic dyes, including Oil Red O, Sudan Black B
and Nile red, are tools used for the visualization of lipids in
cells (12). The intravascular
lipids of embryonic zebrafish are dyed by Oil Red O. Schlegel and
Stainier (13) reported that the
vasculature of zebrafish no longer had stainable neutral lipids six
days post fertilization (dpf). Therefore, the changes in
intravascular lipids among 1–8-dpf zebrafish were observed using
Oil Red O staining in the present study.
There are numerous varieties of fluorescent sterol
analogs commercially available; a novel boron-dipyrromethene
(BODIPY)-tagged cholesterol analog was created with a modified
fluorophore linker and this analog was able to be incorporated into
cholesterol-rich regions of cells (14). Stoletov et al (11) discovered the deposition of red
fluorescent cholesterol in vessel walls following feeding 5-dpf
zebrafish a diet containing a fluorescent probe (CholEsteryl BODIPY
576/589-C11) for ten consecutive days. This method was used in
order to study intravascular cholesterol metabolism. As CholEsteryl
BODIPY 576/589-C11 has been out of production since May 2012, it
was necessary to identify a novel fluorescent probe and develop an
analogous experimental protocol.
In the present study, the intravascular lipid
metabolism of embryonic zebrafish was investigated using Oil Red O
and CholEsteryl BODIPY 542/563 C11. Furthermore, the effects of a
high-fat diet (HFD) on adult zebrafish intravascular lipid
metabolism were studied. It was hypothesized that the optimized
protocol developed in the present study may be used to investigate
the gene functions associated with zebrafish lipid metabolism and
to screen novel lipid-lowering drugs.
Materials and methods
Zebrafish
The zebrafish used in the present study were
obtained from the Shanghai Research Center for Model Organisms
(Shanghai, China), and the facilities for housing these animals
were accredited by the Association for Assessment and Accreditation
of Laboratory Animal Care International (Frederick, MD, USA).
Zebrafish [AB strain and fli1:enhanced green fluorescent protein
(EGFP) strain] were raised and maintained in pH 7.0
reverse-osmosis-purified water under a 14-h light/10-h dark
photoperiod at 28°C. Embryos were raised in 15-cm Petri dishes
following fertilization and adolescent fish were housed in 2 l
tanks with ~10 fish per tank. A total of 30 7-dpf fasting wild-type
zebrafish were incubated in breeding water containing 0.1% egg yolk
for 48 h. Wild-type zebrafish (3 dpf, n=30) were incubated in
breeding water containing 0.1 ppm CholEsteryl BODIPY 542/563 C11
(Invitrogen Life Technologies, Carlsbad, CA, USA) for 24 h. One
group of 30 7-dpf zebrafish were incubated in water containing the
fluorescent probe CholEsteryl BODIPY 542/563 C11 and fed a
high-cholesterol diet (HCD) for 10 d. Another two groups of 7-dpf
zebrafish (n=30/group) were incubated in regular breeding water and
fed with a regular, or HCD containing CholEsteryl BODIPY 542/563
C11 for 10 d. A total of 100 male zebrafish (age, 13 weeks) were
randomly divided into two groups and fed a regular diet thrice
daily (control group), or a HCD twice daily and a high-fat egg-yolk
diet once daily (HCD group) for seven weeks. Animal experiments
were approved by the Institutional Animal Care and Use Committee of
Shanghai Research Center for Model Organisms (SRCMO) (Shanghai,
China) and performed in accordance with their ethical standards.
The distribution and regeneration of blood vessels were observed
using fli1:EGFP zebrafish, in which blood vessel endothelial cells
express EGFP.
Drug preparations
A stock solution of CholEsteryl BODIPY 542/563 C11
was produced by dissolving 1 mg CholEsteryl BODIPY 542/563 C11 in 2
ml chloroform (Sinopharm Chemical Reagent Co., Ltd., Shanghai,
China) (stored at −20°C). Subsequently, 20 μl stock solution was
transferred into Eppendorf tubes (EP). Once the chloroform was
completely evaporated, 10 ppm solution was made by dissolving the
powder into dimethyl sulfoxide (DMSO; Sangon Biotech Co., Ltd.,
Shanghai, China). The final concentrations of CholEsteryl BODIPY
542/563 C11 and DMSO in breeding water were 0.1 ppm and 0.1% (v/v),
respectively.
A working solution of the narcotic drug
3-aminobenzoic acid ethylester methanesulfate (MS-222; 200 ppm) was
prepared by dissolving 20 mg MS-222 (Fluka, Sigma-Aldrich, St.
Louis, MO, USA) in 100 ml double distilled water (SRCMO).
A 0.5% (w/v) stock solution of Oil Red O was
prepared by adding solid Oil Red O (Sigma-Aldrich) to isopropanol
(Sinopharm Chemical Reagent Co., Ltd.). The working solution (0.3%,
w/v) was produced by diluting the stock solution with double
distilled water.
Diet preparations and feeding
systems
To prepare the HCD, regular zebrafish diet (3% crude
fat; SLRC Laboratory Animal Co., Ltd., Shanghai, China) was ground
into powder and soaked in chloroform containing cholesterol (SLRC
Laboratory Animal Co., Ltd.). Following evaporation of the
chloroform, the HCD was ready (4% cholesterol, w/w).
Chicken’s egg yolk powder (Shanghai Yuanye
Biotechnology Co., Ltd., Shanghai, China) was used for the HFD and
7-dpf zebrafish were also incubated in a water system containing
0.1% (g/ml) egg yolk for 48 h. The breeding water was replaced with
freshly prepared 0.1% (g/ml) egg yolk solution every 4 h. Following
48 h of incubation, the zebrafish were washed in breeding water and
subsequently stained with Oil Red O.
CholEsteryl BODIPY 542/563 C11 was dissolved in
chloroform, in which regular zebrafish diet or HCD was soaked.
Following complete evaporation of the chloroform, a diet containing
1/100,000 (w/w) fluorescent probe was produced.
Oil Red O staining
Oil Red O staining was performed according to the
protocol described in a study by Schlombs et al (15). Briefly, following anesthesia with
MS-222 (200 ppm), zebrafish were fixed in 4% paraformaldehyde for
12 h at 4°C and washed three times in 1X phosphate-buffered saline.
Fish were subsequently preincubated in 60% isopropanol for 30 min
and dyed with fresh 0.3% Oil Red O for 3 h. Samples were ready for
microscopic observation following three washes with 60%
isopropanol. A Nikon SMZ1500 (Nikon Corporation, Tokyo, Japan) was
used for observations and capturing images.
Administration of CholEsteryl BODIPY
542/563 C11
Wild-type zebrafish (3 dpf) were incubated in
breeding water containing 0.1 ppm CholEsteryl BODIPY 542/563 C11
for 24 h to determine the efficiency of CholEsteryl BODIPY 542/563
C11 intravascular lipid staining. Two experimental methods were
subsequently used to administer CholEsteryl BODIPY 542/563 C11 to
the zebrafish. In one group, 7-dpf wild-type zebrafish were kept in
breeding water containing 0.1 ppm CholEsteryl BODIPY 542/563 C11
and fed with a HCD (twice/day) for 10 d. In the second system,
7-dpf wild-type zebrafish were kept in regular breeding water and
fed with a regular diet (control group, n=30) or a HCD (HCD group,
n=30) supplemented with 1/100,000 (w/w) CholEsteryl BODIPY 542/563
C11 for 10 d. A Nikon ECLIPSE TE2000-E microscope (Nikon
Corporation, Tokyo, Japan) was used for observations and capturing
images. Images were analyzed using NIS-Elements BR 3.1 software
(Nikon Corporation, Tokyo, Japan) to calculate the intra-aortic
fluorescence intensity.
Detection of adult zebrafish blood
lipids
A total of 100 male zebrafish (age, 13 weeks) were
randomly divided into control and HFD groups. In the control group,
the zebrafish were fed a regular diet thrice daily for seven weeks.
The zebrafish in the HFD group were fed a HCD (twice daily) and a
high-fat egg-yolk diet (once daily, in between the HCD feeds) for
seven weeks. Any remaining feed was removed 30 min following each
administration.
Zebrafish were deeply anesthetized with MS-222 (200
ppm) and 5 μl blood was drawn from the artery of each fish to
obtain 1 μl serum following centrifugation at 2,500 × g for 10 min
at 4°C. A total of 30 zebrafish were randomly selected from each
group and the collected serum of five zebrafish was combined as one
sample for detection. Total cholesterol and triglycerides in serum
were measured using the Siemens Advia 2400 automatic analyzer
(Siemens Healthcare, Erlangen, Germany) (11).
Statistical analysis
SPSS 11.5 (SPSS, Inc., Chicago, IL, USA) was used
for data analysis. Values are expressed as the mean ± standard
deviation. Student’s t-test was used to compare the data between
two groups, and data among more than two groups were compared using
analysis of variance. χ2 analysis and Fisher’s exact
test were used accordingly for comparison of rates. P<0.05 was
considered to indicate a statistically significant difference
between values.
Results
Oil Red O staining of 1–8-dpf fasting
zebrafish
The Oil Red O-stained samples of 1–8-dpf fasting
wild-type (AB strain) zebrafish and the 1–8-dpf fli1:EGFP zebrafish
were prepared and observed under a fluorescence microscope
(Fig. 1A and B). Intravascular
lipid levels were reduced with increasing days post-fertilization
due to the absorption of the yolk sac. Due to the incomplete blood
vessel development, no clear Oil Red O signal for intravascular
lipids was detected in 1-dpf zebrafish. Lipid signals were
significant in 2–5-dpf zebrafish and comparison to age-matched
fli1:EGFP zebrafish indicated that the detected lipids were
intravascular. The lipid signal became markedly weaker in 6-dpf
zebrafish. Aside from the swim bladder, no lipid signal was
detected in the blood vessels of zebrafish older than seven dpf, as
the yolk sac had been completely absorbed. These results
demonstrated that Oil Red O staining was able to indicate
intravascular lipid levels in vivo.
Effects of HFD on the intravascular lipid
levels of zebrafish
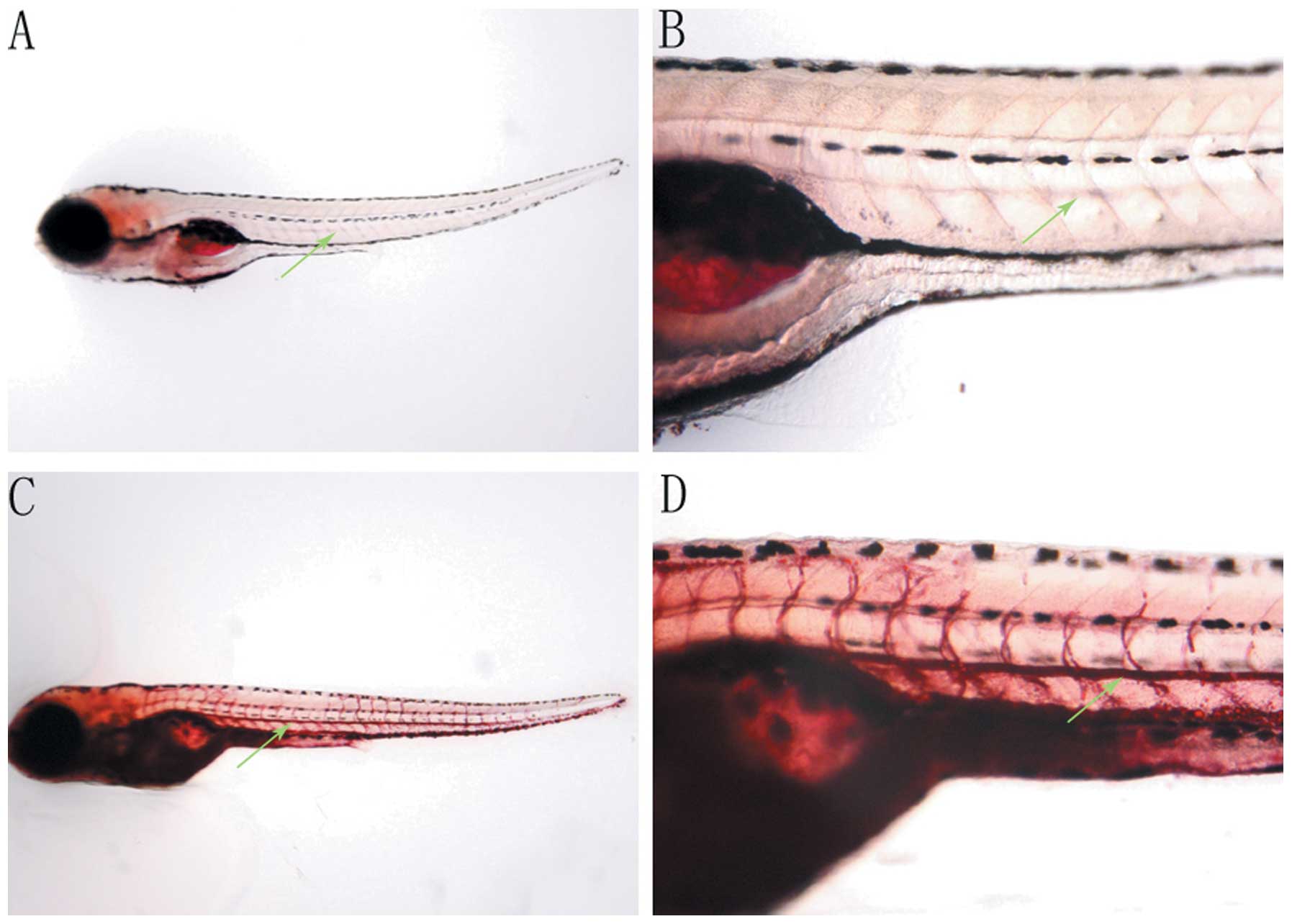
Fasting 7-dpf zebrafish incubated in breeding water
containing 0.1% (g/ml) egg yolk, as a HFD, for 48 h were stained
with Oil Red O and compared with the fasting zebrafish at 9-dpf
(Fig. 2). The Oil Red O signal for
intravascular lipids was not detected in fasting 9-dpf zebrafish
(Fig. 2A and B), while the
intravascular lipid levels of zebrafish incubated in 0.1% egg yolk
for 48 h were significantly increased, demonstrated by the greater
intensity of Oil Red O staining observed (Fig. 2C and D).
Staining for intravascular cholesterol of
embryonic zebrafish
The 3-dpf wild-type zebrafish incubated in breeding
water containing 0.1 ppm CholEsteryl BODIPY 542/563 C11 for 24 h
were observed under a fluorescence microscope. The distribution of
red fluorescence observed in these wild-type zebrafish was
analogous to that of the blood vessel distribution observed in
4-dpf fli1:EGFP zebrafish (Fig.
3A–D). Therefore, zebrafish intravascular cholesterol was able
to be stained by CholEsteryl BODIPY 542/563 C11.
Effects of HCD on zebrafish intravascular
cholesterol
Wild-type zebrafish (7-dpf) were incubated in
breeding water containing 0.1 ppm CholEsteryl BODIPY 542/563 C11
and fed with the HCD (twice/day) for 10 d. However, it was
difficult to accurately calculate intravascular fluorescence
intensity as not only the blood vessels, but additionally other
areas of the zebrafish expressed red fluorescence (Fig. 3E).
Amongst the 7-dpf wild-type zebrafish kept in
regular breeding water and fed a regular diet (n=30, control group)
or the HCD (n=30, HCD group) containing CholEsteryl BODIPY 542/563
C11 for 10 d, two zebrafish died in each group and there was no
significant difference in mortality between the two groups
(P=1.000). The remaining zebrafish were used for observation of
intravascular fluorescence intensity by fluorescence microscopy.
The intravascular fluorescence intensity of zebrafish in the HCD
group was markedly higher than that of those in the control group
(Fig. 3F and G). Furthermore,
there was a significant difference between the intra-aortic
fluorescence intensity of the HCD group and that of the control
group (2.06±0.14 vs. 1.00±0.23, P<0.05; Fig. 3H).
Effects of HFD on serum total cholesterol
and triglycerides in zebrafish
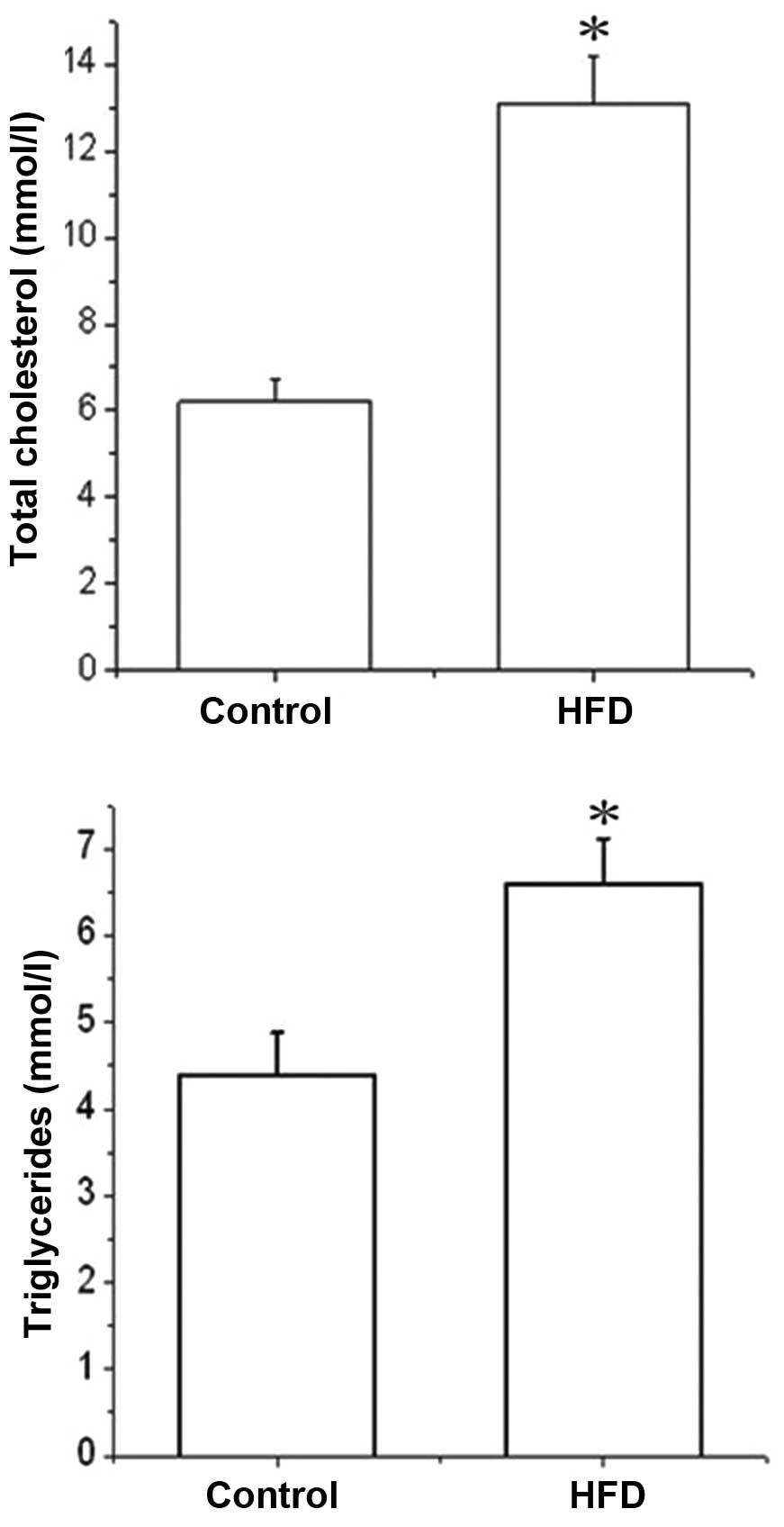
All zebrafish in the control and HFD groups
survived. In each group, 30 zebrafish were randomly selected for
detection of the serum total cholesterol and triglycerides
(Fig. 4). The serum total
cholesterol of zebrafish in the HFD group was significantly higher
than that in the control group (13.1±1.10 vs. 6.20±0.50 mmol,
P<0.05). Furthermore, serum triglyceride levels were also
markedly increased in the HFD group compared to those of the
control group (6.60±0.52 vs. 4.38±0.50 mmol; P<0.05).
Discussion
At present, organism-based models are widely used in
small-chemical compound discovery and such animal-based screening
models make compound-screening feasible prior to the elucidation of
detailed mechanisms of the signaling pathways involved (16–18).
For an animal-based model, the animal should be small, low-cost,
compatible with simple culture conditions and suitable for
high-throughput screening (19).
Zebrafish meet these criteria, are able to generate large clutches
of eggs and quickly develop most organs, including the blood
vessels. Furthermore, numerous gastrointestinal organs, including
the intestines, liver, gallbladder, exocrine and endocrine
pancreas, as well as the specialized cell types associated with
lipid absorption and processing in larval zebrafish were found to
be analogous with those of humans (13,20,21).
Therefore, the zebrafish was selected as an experimental model to
establish the methods for investigating intravascular lipid levels
in the present study.
Convenient and effective methods for intravascular
lipid-detection in zebrafish are essential for antihyperlipidemic
drug screening. Oil Red O is a low-cost, reproducible and neutral
fat-staining agent and is useful in high-throughput research
(22). Clifton et al
(23) analyzed the effects of
certain novel compounds on lipid metabolism in the digestive tract
of zebrafish using Oil Red O staining. However, there were few
studies investigating the intravascular lipid metabolism of
zebrafish using Oil Red O. In the present study, Oil Red O-stained
samples of 1–8-dpf zebrafish were prepared and the staining results
demonstrated that Oil Red O was able to effectively indicate
intravascular lipids in vivo. Fluorescent probes are also
useful for dynamic monitoring of zebrafish lipids in vivo
(11). Two methods for
incorporating the fluorescent probe CholEsteryl BODIPY 542/563 C11
were established. In one group, CholEsteryl BODIPY 542/563 C11 was
added into the zebrafish breeding system and in the other,
CholEsteryl BODIPY 542/563 C11 was incorporated into the fish diet.
When the 3-dpf zebrafish were incubated in breeding water
containing 0.1 ppm CholEsteryl BODIPY 542/563 C11 for 24 h, the
intravascular cholesterol was stained by CholEsteryl BODIPY 542/563
C11. A previous study has indicated that zebrafish older than 7-dpf
must be fed with the HCD for ≥10 d in order to achieve a marked
increase in the intravascular cholesterol (11). However, this increased incubation
time in breeding water containing CholEsteryl BODIPY 542/563 C11
resulted in not only blood vessels but also other areas of the
zebrafish emitting strong red fluorescence, which made accurate
quantification of intravascular fluorescence intensity difficult.
In the second system, where CholEsteryl BODIPY 542/563 C11 was
incorporated into the fish diet, the fluorescence was concentrated
in the blood vessels.
The fli1:EGFP transgenic zebrafish expressed an
endothelial-specific EGFP that enabled detailed visualization of
blood vessels in vivo (24). Therefore, fli1:EGFP zebrafish,
which exhibited the distribution of blood vessels, were used for
comparison, to ensure that the substances stained with Oil Red O or
labeled by CholEsteryl BODIPY 542/563 C11 were intravascular
lipids. Zebrafish are optically transparent until 30-dpf, which
enables temporal observations of fluorescent probes and dyes in the
live animal. Oil Red O staining and fluorescence labeling methods
were able to indirectly indicate the relative amount of
intravascular lipids, whereas the blood of adult zebrafish could be
used to accurately quantify blood lipids. Therefore, blood was
collected from male zebrafish aged 13 weeks, and the serum total
cholesterol and triglycerides were analyzed in order to validate
the accuracy of Oil Red O staining and fluorescence labeling. The
results supported those indicated by Oil Red O and CholEsteryl
BODIPY 542/563 C11 staining. The use of adult zebrafish for drug
screening is unsuitable due to the fact that such experiments using
adult zebrafish can be time-consuming and expensive (25).
Since zebrafish eggs and embryos are highly
permeable, compounds are able to be added directly to the breeding
water and the accessibility of compounds to the embryos is
therefore high. Moreover, the use of zebrafish would be convenient
for high-throughput screening, as the eggs and larvae are small
enough to be screened in 96-well plates. Conventionally, chemical
libraries are stored as stock solutions in DMSO. Previous studies
have demonstrated that zebrafish embryos are tolerant to a range of
DMSO concentrations and that 1% DMSO is compatible with normal
development, indicating that compounds are able to be screened at a
wide range of concentrations (26,27).
For lipid detection and imaging, in vivo fluorescent signals
may be detected by automatic fluorescence microscopy in
high-throughput screening (28).
Future studies may focus on the development of lower
vertebrate models of human diseases, for example mutant zebrafish
carrying mutations in the lipid metabolism pathway (29–31).
The effects of expressional changes in genes associated with
regulation of intravascular lipids induced by Morpholino- or
messenger RNA injections may be investigated using Oil Red O
staining or breeding water containing CholEsteryl BODIPY 542/563
C11. Alternatively, Oil Red O may be used to analyze drug efficacy
in 7-dpf zebrafish fed with an HFD following 48 h of incubation in
breeding water containing drugs. Drug efficacy may also be studied
in 7-dpf zebrafish fed with an HFD labeled with CholEsteryl BODIPY
542/563 C11 using a fluorescence microscope following incubation in
breeding water containing drugs for 10 d. The hypolipidemic effects
of screened drugs may be validated in adult zebrafish kept in a
medicinal bath and fed an HFD.
In conclusion, the methods of Oil Red O staining and
CholEsteryl BODIPY 542/563 C11 administered via breeding water or
diet were optimized for zebrafish intravascular lipid metabolism
research. These methods may be applied to novel lipid-regulating
drug screening and investigations into genes associated with lipid
regulation.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 30971436, 31201010,
and 81270376), and the Foundation of Shanghai Ninth People’s
Hospital (grant no. 2013A02).
References
|
1
|
Joffe BI, Panz VR and Raal FJ: From
lipodystrophy syndromes to diabetes mellitus (Review). Lancet.
357:1379–1381. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McNeely MJ, Edwards KL, Marcovina SM,
Brunzell JD, Motulsky AG and Austin MA: Lipoprotein and
apolipoprotein abnormalities in familial combined hyperlipidemia: a
20-year prospective study. Atherosclerosis. 159:471–481. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Watanabe S, Yaginuma R, Ikejima K and
Miyazaki A: Liver diseases and metabolic syndrome (Review). J
Gastroenterol. 43:509–518. 2008. View Article : Google Scholar
|
|
4
|
Rocke J, Lees J, Packham I and Chico T:
The zebrafish as a novel tool for cardiovascular drug discovery
(Review). Recent Pat Cardiovasc Drug Discov. 4:1–5. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hölttä-Vuori M1, Salo VT, Nyberg L,
Brackmann C, Enejder A, Panula P and Ikonen E: Zebrafish: gaining
popularity in lipid research (Review). Biochem J. 429:235–242.
2010. View Article : Google Scholar
|
|
6
|
Schirmer H, Pereira TC, Rico EP, Rosemberg
DB, Bonan CD, Bogo MR and Souto AA: Modulatory effect of
resveratrol on SIRT1, SIRT3, SIRT4, PGC1α and NAMPT gene expression
profiles in wild-type adult zebrafish liver. Mol Biol Rep.
39:3281–3289. 2012. View Article : Google Scholar
|
|
7
|
Zhang Y, Hu G, Li S, Li ZH, Lam CO, Hong
SJ, Kwan YW, Chan SW, Leung GP and Lee SM: Pro-angiogenic activity
of astragaloside IV in HUVECs in vitro and zebrafish in vivo. Mol
Med Rep. 5:805–811. 2012.
|
|
8
|
Carten JD and Farber SA: A new model
system swims into focus: using the zebrafish to visualize
intestinal lipid metabolism in vivo. Clin Lipidol. 4:501–515. 2009.
View Article : Google Scholar
|
|
9
|
Hama K, Provost E, Baranowski TC,
Rubinstein AL, Anderson JL, Leach SD and Farber SA: In vivo imaging
of zebrafish digestive organ function using multiple quenched
fluorescent reporters. Am J Physiol Gastrointest Liver Physiol.
296:G445–G453. 2009. View Article : Google Scholar :
|
|
10
|
Farber SA, Pack M, Ho SY, Johnson ID,
Wagner DS, Dosch R, Mullins MC, Hendrickson HS, Hendrickson EK and
Halpern ME: Genetic analysis of digestive physiology using
fluorescent phospholipid reporters. Science. 292:1385–1388. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stoletov K, Fang L, Choi SH, Hartvigsen K,
Hansen LF, Hall C, Pattison J, Juliano J, Miller ER, Almazan F, et
al: Vascular lipid accumulation, lipoprotein oxidation, and
macrophage lipid uptake in hypercholesterolemic zebrafish. Cir Res.
104:952–960. 2009. View Article : Google Scholar
|
|
12
|
Kang OH, Kim SB, Seo YS, Joung DK, Mun SH,
Choi JG, Lee YM, Kang DG, Lee HS and Kwon DY: Curcumin decreases
oleic acid-induced lipid accumulation via AMPK phosphorylation in
hepatocarcinoma cells. Eur Rev Med Pharmacol Sci. 17:2578–2586.
2013.PubMed/NCBI
|
|
13
|
Schlegel A and Stainier DY: Microsomal
triglyceride transfer protein is required for yolk lipid
utilization and absorption of dietary lipids in zebrafish larvae.
Biochemistry. 45:15179–15187. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Marks DL, Bittman R and Pagano RE: Use of
Bodipy-labeled sphingolipid and cholesterol analogs to examine
membrane microdomains in cells. Histochem Cell Biol. 130:819–832.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schlombs K, Wagner T and Scheel J: Site-1
protease is required for cartilage development in zebrafish. Proc
Natl Acad Sci USA. 100:14024–14029. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Murphey RD, Stern HM, Straub CT and Zon
LI: A chemical genetic screen for cell cycle inhibitors in
zebrafish embryos. Chem Biol Drug Des. 68:213–219. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gosai SJ, Kwak JH, Luke CJ, Long OS, King
DE, Kovatch KJ, Johnston PA, Shun TY, Lazo JS, Perlmutter DH, et
al: Automated high-content live animal drug screening using C.
elegans expressing the aggregation prone serpin α1-antitrypsin Z.
PLoS One. 5:e154602010. View Article : Google Scholar
|
|
18
|
Wheeler GN and Brändli AW: Simple
vertebrate models for chemical genetics and drug discovery screens:
lessons from zebrafish and Xenopus. Dev Dyn. 238:1287–1308. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Peterson RT, Link BA, Dowling JE and
Schreiber SL: Small molecule developmental screens reveal the logic
and timing of vertebrate development. Proc Natl Acad Sci USA.
97:12965–12969. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lieschke GJ and Currie PD: Animal models
of human disease: zebrafish swim into view (Review). Nat Rev Genet.
8:353–367. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wallace KN, Akhter S, Smith EM, Lorent K
and Pack M: Intestinal growth and differentiation in zebrafish.
Mech Dev. 122:157–173. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mehlem A, Hagberg CE, Muhl L, Eriksson U
and Falkevall A: Imaging of neutral lipids by oil red O for
analyzing the metabolic status in health and disease. Nat Protoc.
8:1149–1154. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Clifton JD, Lucumi E, Myers MC, Napper A,
Hama K, Farber SA, Smith AB III, Huryn DM, Diamond SL and Pack M:
Identification of novel inhibitors of dietary lipid absorption
using zebrafish. PLoS One. 5:e123862010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lawson ND and Weinstein BM: In vivo
imaging of embryonic vascular development using transgenic
zebrafish. Dev Biol. 248:307–318. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Terriente J and Pujades C: Use of
zebrafish embryos for small molecule screening related to cancer.
Dev Dyn. 242:97–107. 2013. View Article : Google Scholar
|
|
26
|
Brändli AW: Prospects for the Xenopus
embryo model in therapeutics technologies. CHIMIA. 58:694–702.
2004. View Article : Google Scholar
|
|
27
|
Chan J and Serluca FC: Chemical approaches
to angiogenesis. Methods Cell Biol. 76:475–487. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Starkuviene V and Pepperkok R: The
potential of high-content high-throughput microscopy in drug
discovery. Br J Pharmacol. 152:62–71. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hong CC, Peterson QP, Hong J-Y and
Peterson RT: Artery/vein specification is governed by opposing
phosphatidylinositol-3 kinase and MAP kinase/ERK signaling. Curr
Biol. 16:1366–1372. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Peterson RT and Fishman MC: Discovery and
use of small molecules for probing biological processes in
zebrafish. Methods Cell Biol. 76:569–591. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Stern HM, Murphey RD, Shepard JL, Amatruda
JF, Straub CT, Pfaff KL, Weber G, Tallarico JA, King RW and Zon LI:
Small molecules that delay S phase suppress a zebrafish bmyb
mutant. Nat Chem Biol. 1:366–370. 2005. View Article : Google Scholar : PubMed/NCBI
|