Introduction
Certain liver diseases lead to hepatocyte damage
that can progress to liver failure, for which a transplant may be
the patients’ only treatment option. However, transplant organs are
a highly limited resource, and transplant rejection remains to be
an unresolved problem. The replacement of hepatocytes by stem cells
or stem cell-stimulated endogenous and exogenous regeneration are
the major goals of liver-directed cell therapy (1–4).
Mature proliferating hepatocytes have the potential to be used as
hepatic cell replacement (5).
However, hepatocyte progenitors are still required under certain
circumstances, such as the impaired ability of differentiated
hepatocytes to further divide (6).
Studies have suggested that reservoirs of stem cells may reside in
organ tissues in order to promote self-repair and regeneration
(7). However, adult stem cells
have been suggested to be more plastic than once believed (8). Adult stem cells can be isolated from
human lipoaspirates and differentiate toward osteogenic,
adipogenic, neurogenic, myogenic and chondrogenic lineages
(9). While stem cells derived from
different tissues have shown promise for therapeutic applications,
Lagasse et al (10)
demonstrated that transplanted purified hematopoietic stem cells
could give rise to hepatocytes and restore liver function in
fumarylacetoacetate hydrolase-deficient mice. In humans, female
recipients of male bone marrow (BM) were found to have hepatocytes
that contained the Y chromosome (11), implying that hepatocytes could be
derived from BM cells (12).
Several studies have indicated that transplanted BM cells adopt the
phenotype of hepatocytes and restore liver function by cell fusion
rather than differentiation (13,14).
Kern et al (3) and Wagner
et al (15) compared
mesenchymal stem cells (MSCs) derived from human adipose and bone
marrow with respect to morphology, the success rate of isolating
MSCs, colony frequency, expansion potential, multiple
differentiation capacity and immune phenotype. They showed that
adipose tissue-derived mesenchymal stem cells (AT-MSCs) had similar
characteristics to those of BM mesenchymal stem cells (BMSCs).
Adipose tissue contains stem cells similar to BMSCs; these cells
could be isolated from cosmetic liposuctions and grown easily under
standard tissue culture conditions (3). The multi-lineage differentiation
capacity of AT-MSCs cells has been confirmed (3,9,15).
The aim of the present study was to investigate
whether AT-MSCs had the potential to differentiate into functional
hepatocytes in vitro and in vivo, in order to provide
a novel stem cell therapy for the treatment of liver diseases.
Materials and methods
All procedures were performed according to
manufacturer’s instruction unless noted otherwise.
Isolation and culture of MSCs from
adipose tissue
Human adipose tissue was collected following
liposuction surgery performed at Zhongshan Hospital affiliated to
Xiamen University (Xiamen, China). Written informed consent and
approval were obtained from the patients or their family, and the
Human Research Ethical Committee of Zhongshan Hospital Xiamen
University (Xiamen, China). The lipoaspirates (10–20 ml) were
washed twice with an equal volume of phosphate-buffered saline
(PBS; Digestive Diseases Institute of Xiamen University, Xiamen,
China) and digested with 0.075% collagenase I (Sigma, St. Louis,
MO, USA). Red Blood Cell Lysis Buffer (Beyotime, Haimen, China) was
used to eliminate erythrocytes; cells were passed through a 40-μm
mesh filter, and suspended in low-glucose Dulbecco’s modified
Eagle’s medium (DMEM-LG; Gibco-BLR, Carlsbad, CA, USA) supplemented
with 10% fetal bovine serum (FBS) and 100 U/ml
penicillin/streptomycin (Gibco-BLR). The resuspended stromal
vascular fraction (SVF) cells were plated at a density of
5×105/cm2 in a 100-mm culture dish (Corning;
Corning, NY, USA). The fibroblastoid adherent cells were designated
AT-MSCs. Cells were harvested at 90% confluence using Trypsin-EDTA
(Gibco-BLR), and designated as passage 0 (P0). Cells at P3–P5 were
used for the subsequent experiments unless otherwise stated.
Growth kinetics of AT-MSCs
To determine the growth kinetics of cultured
AT-MSCs, 60-mm culture dishes were seeded with 1×105
cultured AT-MSCs (P3–P5). At several time-points (between days 2
and 12) following seeding, cells from duplicate dishes were
harvested and counted. AT-MSC numbers were plotted against the
number of days in culture, and the exponential growth phase of the
cells was determined.
Measurement of AT-MSC proliferation
For the cell proliferation assay, 2×103
viable AT-MSC were seeded in each triplicate well in a 96-well
plate. AT-MSCs proliferation was measured using a cell counting kit
8 (CCK-8; Beyotime). The plates were placed in a humidified
incubator until the cells adhered to the plate; then 10 μl of the
CCK-8 solution was added to each well and plates were incubated for
another 2 h at 37°C prior to reading the absorbance at 450 nm using
a microplate reader (550; Bio-rad Laboratories, Inc., Shanghai,
China). The assay was repeated every day at the same time for one
week.
Cell cycle analysis
AT-MSCs in the exponential growth phase were
detached with 0.25% trypsin-EDTA (Gibco-BRL), and the resultant
pelleted AT-MSCs (106) were gently suspended in 1 ml 70%
ethanol (Xiamen Chemical Company, Xiamen, China) and kept for at
least 6 h at −20°C. Cells were washed with PBS twice, incubated for
1 h at room temperature (RT) in the dark with 100 μl 2.5 mg/ml
propidium iodide (PI) (Sigma-Aldrich, Shanghai, China) and 1 mg/ml
RNase (Beyotime) in PBS. The cells were analyzed with a FACSscan
flow cytometer (Beckman Coulter, Brea, CA, USA) with a 488 nm
wavelength.
Transmission electron microscopy
Cells at 80–90% confluence were harvested with 0.25%
trypsin-EDTA and washed twice with cold PBS. Fixing solution (2.5%
glutaraldehyde; Xiamen Xinlongda Chemicals Company, Xiamen, China)
was added and the pellet was incubated for 4 h at 4°C. The cells
were washed after 2 h (or overnight) at 4°C with 0.1 M PBS three
times. The fixed samples, which could be stored stably for several
months, were sent to the electron microscopy department in the
School of Life Sciences at the Xiamen University for further
processing and analysis using the JEM-2100HC transmission electron
microscope (JEOL, Tokyo, Japan).
Flow cytometry
AT-MSCs (106) were trypsinized and
incubated with fluorescein isothiocyanate (FITC)-conjugated CD34,
CD45, CD90, phycoerythrin (PE) -conjugated human leukocyte antigen
(HLA)-DR, CD11b, CD29, CD105 (mouse, monoclonal, 1:200;
eBioscience, San Diego, CA, USA) or PE-conjugated CD3, CD73, CD117,
(mouse, monoclonal, 1:200; BD Biosciences, San Jose, CA, USA)
antibodies for 30 minutes at RT, followed by three washes. Staining
with unconjugated anti-programmed death ligand (PDL) 1 (rabbit,
polyclonal, 1:100; BD Biosciences), FITC-conjugated goat
anti-rabbit immunoglobulin G (IgG) antibody (Ab) (goat, polyclonal,
1:200; BD Biosciences) was used as a secondary antibody. The
fluorescent labeled cells were analyzed on a FACSscan flow
cytometer (Becton-Dickinson, Franklin Lakes, NJ, USA) using
CELLQuest Pro software (Becton-Dickinson).
Immunofluorescence assay (IFA)
Cultured cells were fixed with 4% paraformaldehyde
(Sigma) in PBS for 5 minutes at RT and permeabilized with 0.3%
Triton X-100 (Sigma) for 20 minutes. Cells were then incubated with
blocking solution consisting of PBS and 5% FBS at RT for 30
minutes. For immunofluorescence staining, primary antibodies
against albumin (ALB), (rat, polyclonal, 1:50; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA), α-fetoprotein (AFP)
(mouse, mono, 1:50; Wuhan Boster Biological Technology, Ltd.,
Wuhan, China) and pan cytokeratin (panCK; mouse, mono, 1:50;
Zhongshan Goldenbridge Biotechnology, Beijing, China) were used.
Following incubation with the primary antibody, cells were
incubated with FITC-labeled anti-rat, Dylight-conjugated anti-mouse
or FITC-labeled anti-mouse (rabbit, polyclonal, 1:200; Jackson
ImmunoResearch, West Grove, PA, USA) secondary antibodies for 1 h
at 37°C and stained with DAPI (Sigma) to identify the cell nuclei.
The cells were photographed with a structured illumination
fluorescence microscope (DM IL LED; Leica Microsystems, Tokyo,
Japan).
Hepatic differentiation protocols
To induce hepatogenic differentiation, AT-MSCs were
plated after three passages on 5-mm culture dishes coated with
collagen type I (Sigma) in expansion medium (DMEM-LG supplemented
with 10% FBS). Following reaching confluence, cells were washed
twice with PBS and cultured in basic hepatic differentiation medium
supplemented with 1X insulin-transferrin-selenium (ITS; Sigma),
10−8 M dexamethasone (Sigma), 20 ng/ml epidermal growth
factor (PeproTech EC, London, UK), 20 ng/ml fibroblast growth
factor (FGF; PeproTech EC), 40 ng/ml oncostatin M (OSM; Sigma) and
40 ng/ml hepatocyte growth factor (HGF) (PeproTech EC). After 2
weeks, the medium was replaced with hepatic differentiation medium
with an increased concentration of dexamethasone at 10−5
M (Fig. 1A) and/or 1 μM
trichostatin A (TSA; Sigma) (Fig.
1B). The differentiation medium was used with or without serum.
2 ml of differentiation medium was added to each 12-well culture
dish and changed twice a week. Hepatic differentiation was
identified by cell morphology, immunohistochemistry, reverse
transcription polymerase chain reaction (RT-PCR) analysis and
biochemical functions at different time-points. All assays were
performed with undifferentiated AT-MSCs as negative controls and
human primary hepatocytes or HepG2 cells (Life and Science College,
Xiamen University, Xiamen, China) as positive controls.
In vitro adipogenic differentiation
The adipogenic differentiation assay was performed
on AT-MSCs obtained between P3–P5 using the Human Mesenchymal Stem
Adipogenic Differentiation Medium kit (Cyagen Biosciences,
Guangzhou, China). Induction medium was used until the cells
reached 100% confluence or post-confluence. Three days after
confluence, the medium was changed to maintenance medium; 24 h
later it was changed back to induction medium. Following completing
three cycles of induction and maintenance, the cells were incubated
for a further seven days in the adipogenic maintenance medium. The
non-induced control cells were fed only with adipogenic maintenance
medium. Adipogenic differentiation was confirmed by the formation
of neutral lipid-vacuoles stainable with Oil Red O (Sigma).
Non-induced cells were used as a control.
In vitro osteogenic differentiation
Cells were treated with osteogenic medium
(Osteogenic Differentiation kit; Cyagen Biosciences) for two weeks
and the induction medium was changed every three days. Osteogenesis
was assessed by von Kossa staining (Cyagen Biosciences).
Non-induced cells were used as a control.
Total RNA was isolated from hepatic differentiated
AT-MSCs (Trizol; Invitrogen, Carlsbad, CA, USA); 2 μg total RNA was
used for reverse transcription (RevertAid First Strand cDNA
Synthesis Kit; Fermentas, Burlington, Ontario, Canada). The cDNA
was amplified using LaTaq (Takara BIO, Otsu, Shiga, Japan).
Primers, synthetized by Sangon Biotech (Shanghai, China), were used
to correspond with human gene sequences (Genbank database,
www.ncbi.nlm.nih.gov/genbank): ALB
sense, 5′-CCCCAAGTGTCAACTCCAA-3′ and antisense,
5′-AAAGCAGGTCTCCTTATCGT-3′; AFP sense, 5′-GGCTGACATTATTATCGGACAC-3′
and antisense, 5′-GTTCCTCTGTTATTTGTGGC-3′; and β-actin sense,
5′-TGAAGGTCGGAGTCAACGGATTTGGT-3′ and antisense, 5′-CAT
GTGGGCCATGAGGTCCACCAC-3′ were used as an internal control for PCR.
Amplification reactions were performed using an Eppendorf
thermocycler (Mastercycler Nexus Gradient, Eppendorf, Germany) at
94°C for 30 seconds, 55°C for 30 seconds and 72°C for 60 seconds
for 35 cycles. The PCR products were then separated by
electrophoresis on 1.5% agarose gels (Sigma). The PCR sequencing
product was confirmed by automatic sequencing (ABI 3730XL; Applied
Biosystems, Foster City, CA, USA).
Periodic acid-Schiff (PAS) staining (Beyotime) was
used for the detection of glycogen storage in hepatogenic
differentiated cells. Cells were fixed with 10% formaldehyde
(Beyotime) oxidized in 1% periodic acid (Beyotime) for 10 minutes
and rinsed twice with water. Subsequently, cells were treated with
Schiff’s reagent (Beyotime) for 10 minutes and then rinsed with
water.
Uptake of low-density lipoprotein
The uptake of lipoprotein was detected with the
Dil-Ac-LDL staining kit (Biomedical Technologies, Stoughton, MA,
USA).
Animal model and cell
transplantation
Animal experiments were conducted with permission
from the Ethical Committee for Animal Experimentation of Xiamen
University (Xiamen China) and according to P.R. China legislation.
Immune-deficient BALB/c nude mice were obtained from the National
Rodent Laboratory Animal Resources, (Shanghai, China); the animals
were kept in animal house of Xiamen University according to
internationally accepted principles. Animals were housed under
standard laboratory conditions of light (12-h light/dark cycle) at
25±2°C, with a humidity of 55±5%, rats were fed a standard mice
pellet diet and tap water ad libitum. All the mice were
male. Mice were administered a single abdominal injection of olive
oil containing 10% carbon tetrachloride (CCl4; Xiamen
Chemical Company) at a dose of 100 μl/20 g body weight (bw). Cells
for transplantation or control solutions were injected into the
tail vein: Group A control mice were injected with 100 μl 10%
CCl4/20 g bw only (n=9), group B received 100 μl PBS
(n=9) and group C received 100 μl PBS containing AT-MSCs
(5×105 cells) (n=9). Following this procedure, three
mice from each group were sacrificed on days one, three and seven.
Blood samples were collected and whole livers were removed, fixed
and prepared for further analysis. The serum concentrations of
ammonia, alanine aminotransferase (ALT), aspartate aminotransferase
(AST) and direct bilirubin (DBIL) were detected by histological
analysis of liver tissue at days one, three and seven. To evaluate
engraftment of AT-MSCs in the liver, two additional groups of mice
were used: Group I mice were administered 100 μl/20 g bw of 10%
CCl4, followed by AT-MSCs (5×105 cells) after
24 h by injection into the tail vein (n=3); group II mice were
injected with AT-MSCs (5×105 cells) only (n=3). One
month later, these mice were sacrificed, and the livers were
removed and fixed for further study. Histological analysis of liver
tissues was conducted by serial tissue sectioning and staining with
hematoxylin and eosin (H&E; Beyotime) or immunohistochemical
examination for human-specific ALB expression, as described
above.
Two-way mixed lymphocyte reaction (MLR)
assay
The two-way MLR assay was used in order to detect
whether lymphocyte aggregation was inhibited by AT-MSCs. The MLR
was performed in 96-well microtiter plates using RPMI 1640
(Gibco-BRL) medium supplemented with 10% FBS. Purified T cells
derived from two different donors were plated at 2×105
cells per donor per well. Peripheral blood mononuclear cells
(PBMCs) from two different donors were used as the ‘responder
cells’ for the MLR. PBMCs were prepared by centrifugation of
leukapheresed peripheral blood cells (Innovadyne, Santa Rosa, CA,
USA). The derived lymphocytes were tested using flow cytometry with
antibodies for CD3 and CD19 (BD Biosciences). T cells were mixed in
complete culture medium at 2×105 cells per donor per
well in 96-well microtiter plates. Adipose tissue-derived cells
were added to the MLR at 1, 250, 2,500, 5,000 or 10,000 cells per
well. Stimulator cells were irradiated with 5,000 rads of
γ-radiation with a cesium irradiator (XH BRI-1000; Baxter,
Shanghai, China) prior to being added to the culture wells at the
various concentrations. Control cultures consisted of T cells from
two donors plated in medium alone (no stimulator cells). Triplicate
cultures were set up for each condition. Cultures were pulsed with
bromodeoxyuridine (BRDU; Millipore) on day 5 for 10 h. The optical
density (OD) of the plates was then read with a spectrophotometer
microplate reader (Biorad) set at a wavelength of 450 nm. All the
above steps were performed at RT. The percentage of suppression was
calculated using the following formula: Percentage
suppression=(1-[(Test cell + MLR OD value) ÷ MLR OD value])
×100%.
Statistical analysis
Results are expressed as mean ± standard deviation.
Statistical analyses were performed using least significant
difference (LSD) t-test after one-way analysis of variance (ANOVA)
or Student’s t-test. P<0.05 was considered to indicate a
statistically significant difference between values.
Results
Characterization of AT-MSCs
Morphology and ultramicrostructure of
AT-MSCs
AT-MSCs were cultivated from the mononuclear cell
fraction of adipose tissue samples obtained from healthy donors.
Cells were selected based on plastic adherence to ensure the
removal of any contaminating hematopoietic cells, AT-MSCs expanded
easily in vitro and exhibited a fibroblast-like morphology
(Fig. 2A). The expression of
mesenchymal stem cell markers, detected by immunofluorescence, was
high in cultured AT-MSCs. The majority of cultured AT-MSCs
expressed vimentin, and >90% highly expressed CD90 and CD105
(Fig. 2B). Following subsequent
passages, differentiated cells displayed homogeneous morphologies
and high rates of proliferation. Examination of AT-MSCs by electron
microscopy displayed the presence of numerous surface microvilli.
However, it also revealed a limited presence of organelles,
including Golgi bodies, rough endoplasmic reticula, mitochondria;
by contrast, the differentiated cells showed significant presence
of organelles, including plate-like bodies (Fig. 2C).
 | Figure 2AT-MSC morphology. (A) AT-MSCs showed
a fibroblast-like morphology, forming a CFU-F upon confluence. (B)
Cells were stained for 1) vimentin and CD90 (FITC, green), 2) CD105
(Dylight, red), and 3) nuclei stained with DAPI. (C)
Ultramicrostructure of AT-MSCs: Organelles had a naïve profile. (D)
CCK-8 detection of growth kinetics; AT-MSCs of P3–5 had similar
characteristics. (E) Cell cycle analysis showed that most cells
were in dormant phase. CFU-F, colony forming unit fibroblast; FITC,
fluorescein isothiocyanate; AT-MSCs, adipose tissue-derived
mesenchymal stem cells; P, passage; OD, optical density. |
Cell cycle and growth patterns
AT-MSCs at P3–P5, showed a dynamic growth pattern,
with duplication time of 3.00±0.28 days. In direct proliferation
experiments, AT-MSCs of different passages (P3–P5) showed similar
biological characteristics (Fig.
2D) and a stage of rapid cell proliferation approximately five
days following cell culture (Fig.
2E). The patterns of proliferation as well as the cell cycle
profiles demonstrated that these AT-MSCs displayed classical stem
cell features.
Phenotypic characterization of AT-MSC
populations
Cell surface markers of AT-MSCs at P3–P5 were
analyzed by flow cytometry. The average expression of the following
markers from cells of all donors (n=6) were: CD11b (2±0.4%), HLA-DR
(3.4±0.8%), PDL-1 (1.4±0.4%), CD29 (96±1.3%), CD34 (5.5±5.2%), CD45
(2.6±0.7%), CD73 (97±2.6%), CD90 (97.5±2%), CD105 (96.7±1.7%),
CD271 (2.3±1.2%) (Fig. 3A). These
results confirmed that the AT-MSCs expressed characteristic stem
cell-associated surface markers CD29, CD73, CD90, CD105, while
lacking expression of CD34, CD45, HLA-DR and PDL-1 (Fig. 3A). The hematopoietic lineage
markers CD34, CD45 and other markers CD90, CD105 and CD73 were
observed by flow cytometry in subsequent cultures of AT-MSCs. These
markers were considered the minimum criteria for MSCs. Expression
of the MSC markers was found to differ among passages. Of note,
AT-MSCs of passage 0, AT-MSCs that were separated from human
adipose tissue without cell culture, expressed higher CD34 and CD45
and lower CD73, CD90 and CD105. With increasing time of AT-MSCs in
culture, hematopoietic lineage markers (CD34, CD45) were decreased,
while expression of CD73, CD90 and CD105 intensified (Fig. 3B). Therefore, SVF in P0 expressed
significantly different marker profiles from that of AT-MSCs at
P1–P3 (P<0.05, one-way ANOVA and P<0.05, LSD-t-test).
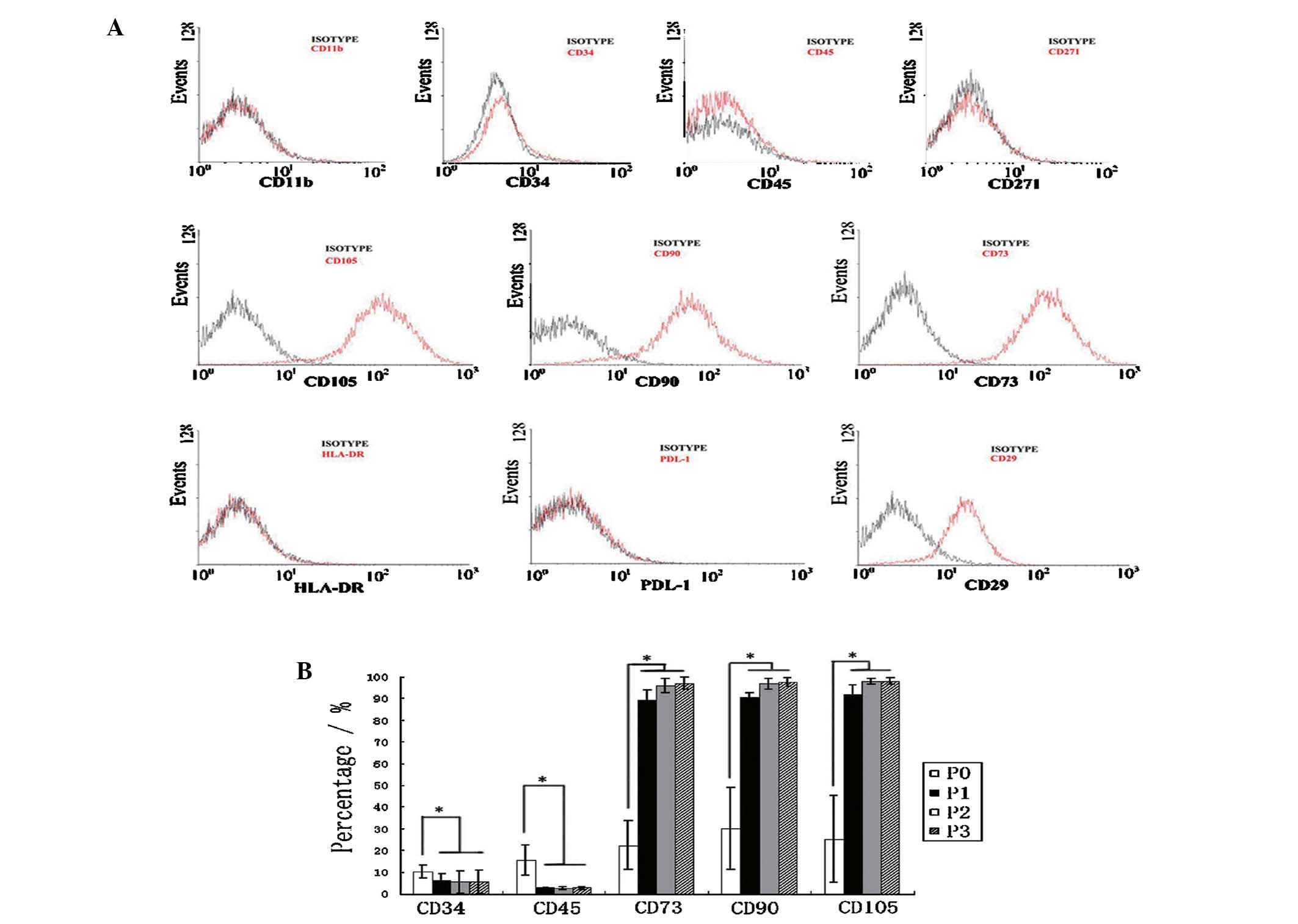 | Figure 3AT-MSCs express a unique set of CD
markers. (A) Flow cytometric analysis of the expression of multiple
CD antigens (red lines, representative histograms; black lines,
respective isotype controls). (B) Changes in expression of CD
markers at different passages. Values represent mean ± standard
error of the mean (n=6). Data were analyzed by LSD-t-test following
a one way analysis of varience. The expression of all markers shown
at P0 differed significantly from that shown at P1/P2/P3
(P<0.05, LSD-t-test); with no difference in CD marker levels at
P1, P2, P3 (P>0.05, LSD-t test). AT-MSCs, adipose tissue-derived
mesenchymal stem cells; P, passage; HLA-DR, human leukocyte
antigen-DR; PDL-1, programmed death-ligand 1; LSD, least
significant difference. |
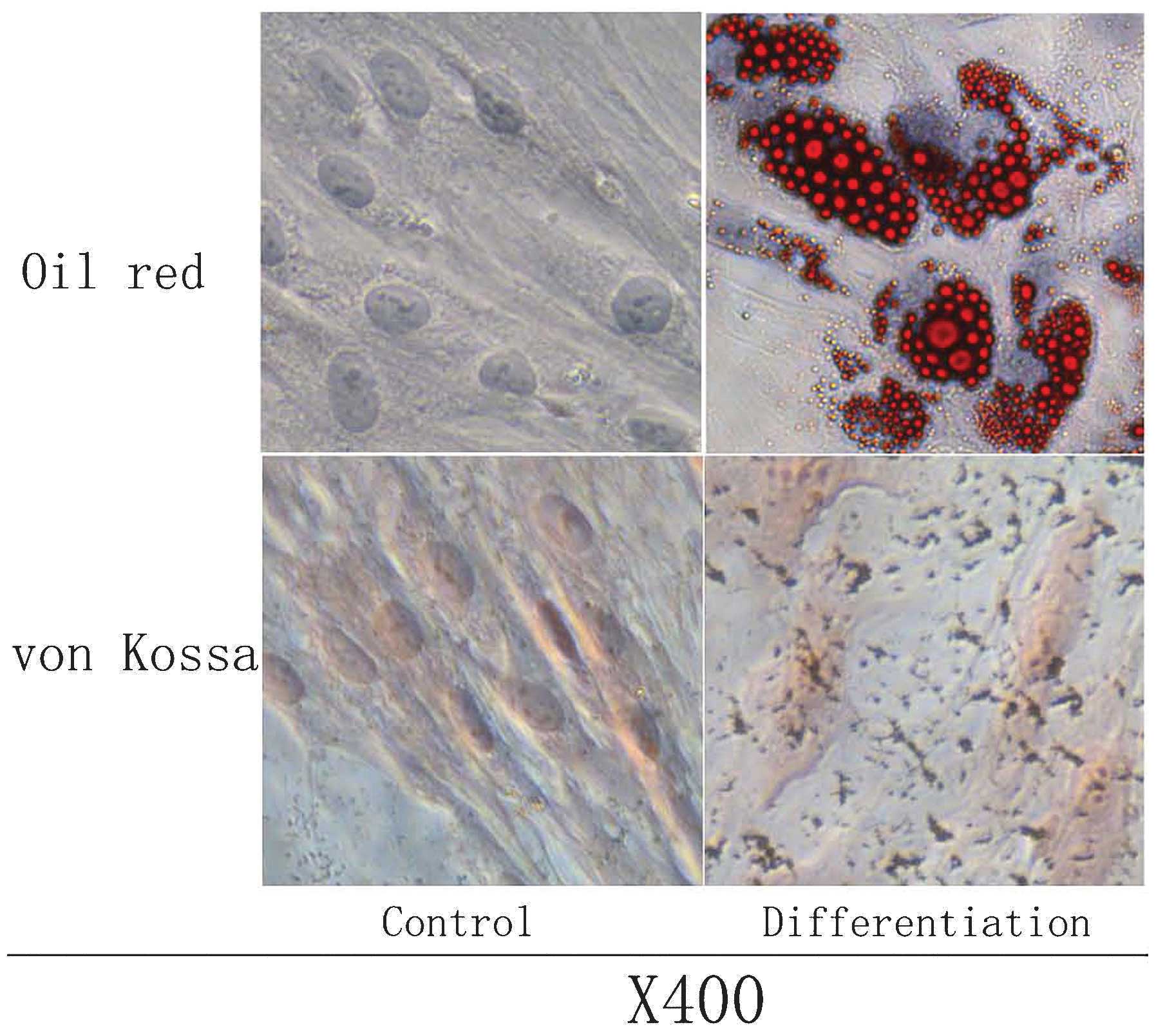
Multi-differentiation capacity of
AT-MSCs
The osteogenic and adipogenic potential of AT-MSCs
was evaluated at P3–P5. The adipogenic potential was assessed by
induction of post-confluent AT-MSCs. Vacuoles appeared after three
days of induction, and a consistent cell vacuolation was evident in
the cytoplasm. Vacuoles stained strongly for fatty acids with Oil O
Red. The lipid vacuoles were identified as bright red inclusions
within cells, while the nuclei were stained dark blue with DAPI.
Evidence for osteogenic differentiation was observed as
morphological changes, which appeared during the first week of
subculture. At the end of the 21-day induction period, calcium
crystals were clearly visible in culture, and cell differentiation
was confirmed by von Kossa staining for calcium. Extracellular
calcium mineralization following osteogenic differentiation was
visualized as black stains, and the nuclei stained with Neutral Red
appeared as pink (Fig. 4).
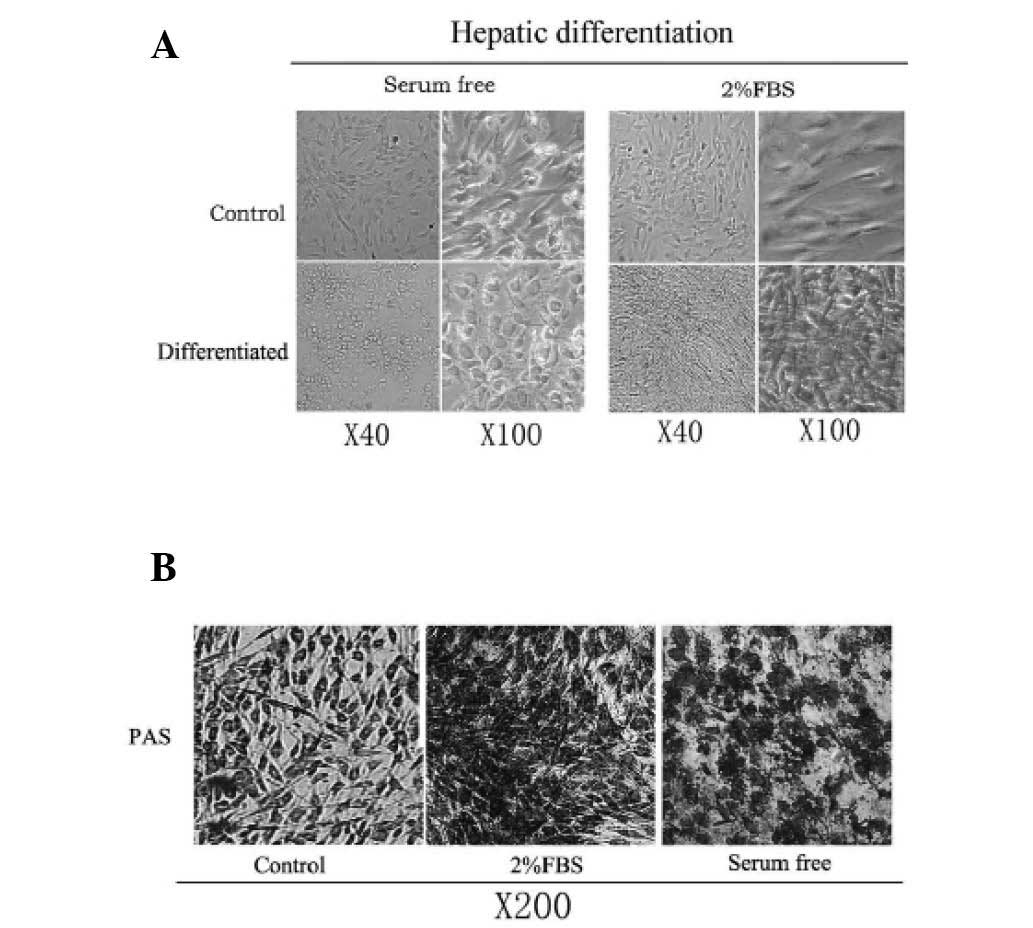
Hepatic differentiation of AT-MSCs in
vivo
Morphological changes in cultured
AT-MSCs
Protocols shown in Fig.
1A and B were used to detect whether FBS could influence the
hepatic induction procedure, in particular the morphological
changes of the cells. Differentiation medium with or without 2% FBS
was added to confluent AT-MSCs. During the initiation step of
hepatic differentiation, the cells treated with serum-free media
gradually lost their fibroblastic morphology and developed a
broader, flatter shape; these cells subsequently developed a
polygonal shape during differentiation (Fig. 5A). The contraction of the cytoplasm
progressed further during maturation, and during differentiation
the majority of treated cells became dense and round with clear or
double nuclei. By contrast, hepatic differentiation medium with 2%
FBS showed no significant morphological changes (Fig. 5A). The induced cells became more
dense and elongated, both in the presence and absence of TSA.
AT-MSCs were analyzed for glycogen-storage ability using PAS
staining. As shown in (Fig. 5B),
the serum-free medium-induced hepatocyte-like cells were strongly
positive for PAS staining. However, cells with 2% FBS in the
differentiation medium showed relatively weaker staining, and
undifferentiated AT-MSCs were weakly positive for PAS staining. It
was concluded that the serum-free induction medium was important
for the differentiation of AT-MSCs into hepatocyte-like cells, as
this dramatically changed the morphology of AT-MSCs from
fibroblastic to epithelial.
Function of hepatocyte-like cells derived
from AT-MSCs
Assays were performed to investigate the functional
competence of AT-MSCs-derived hepatocyte-like cells without serum.
At day 14, hepatocyte-like cells expressed both ALB and AFP, as
detected by immunostaining using anti-human specific antibodies
(Fig. 6A). ALB is a
hepatocyte-specific marker of mature functional hepatocytes, while
AFP is a marker for immature hepatocytes. Hepatocyte-like cells
also showed an ability to store glycogen (Fig. 5B). Furthermore, following 2 weeks
of induction, LDL uptake was observed in the hepatocyte-like cells,
but did not occur in untreated cells (Fig. 6C). LDL is a lipoprotein that
carries cholesterol in hepatocytes. During maturation (28 days),
the majority of induced hepatocyte-like cells became competent for
LDL uptake, a phenotype that was enhanced by addition of TSA to the
induction medium (Fig. 6C).
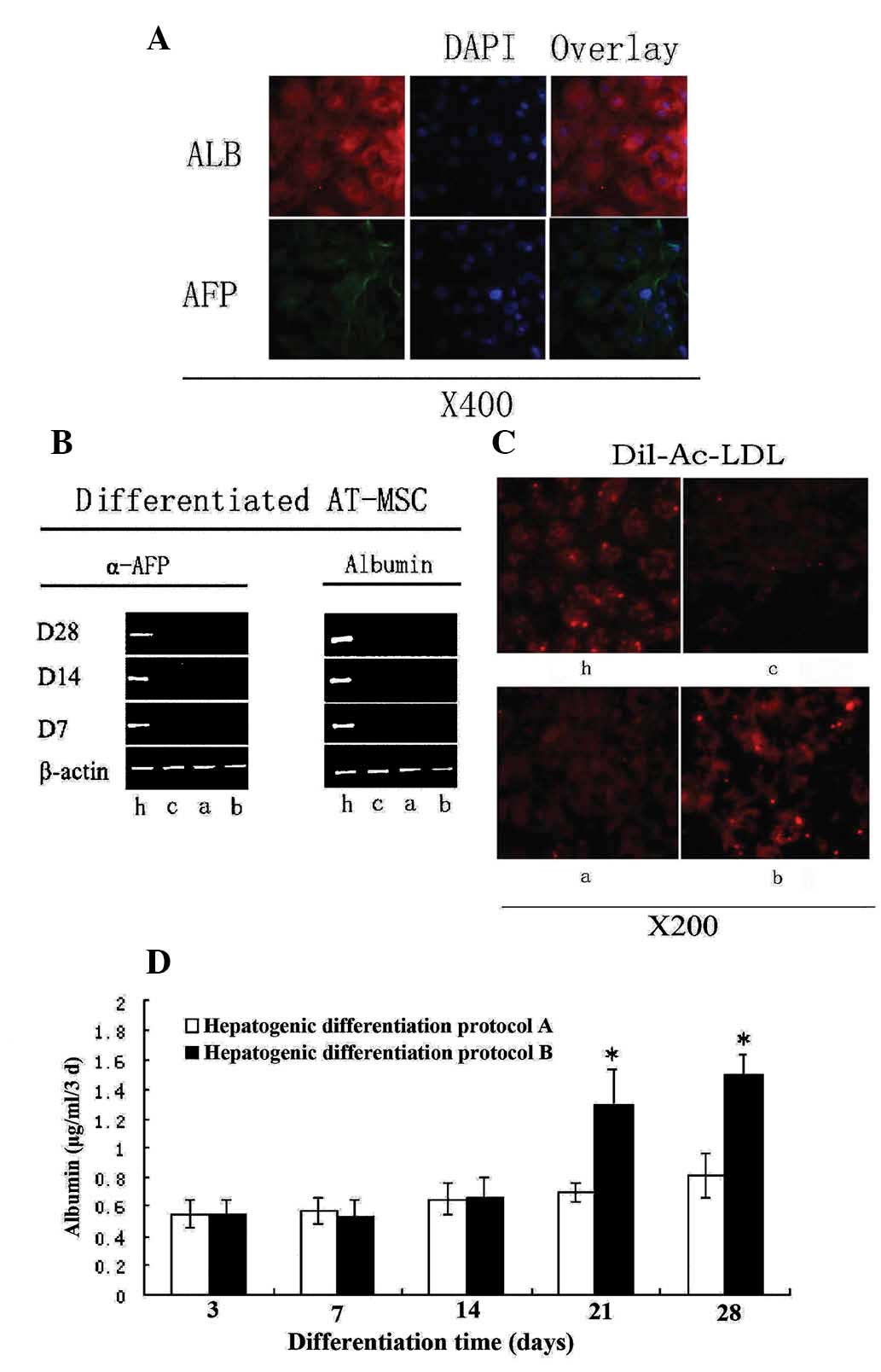 | Figure 6Differentiation potential of AT-MSCs
exposed to hepatogenic differentiation medium. (A) ALB expression
at day 14 and AFP expression at day 7. (B) Reverse
transcription-polymerase chain reaction showed expression levels of
ALB and AFP (using β-actin as a control). (C) AT-MSCs-derived
hepatocyte-like cells were analyzed for LDL-uptake at day 28. (D)
ALB levels secreted by AT-MSCs-derived hepatocyte-like cells. Data
are presented as mean ± standard deviation and analyzed by
Student’s t-test (n=3), *P<0.05 protocol A vs.
protocol B. h, HepG2; c, control; a, hepatogenic differentiation
protocol A; b, hepatogenic differentiation protocol B; AT-MSC,
adipose tissue mesenchymal stem cells; ALB, albumin; AFP,
α-fetoprotein; LDL, low density lipoprotein; Dil-Ac-LDL,
dioctadecyl-tetramethyl-indocarbocyanine perchlorate acetylated
LDL. |
TSA can boost the functions of
hepatocyte-like cells derived from AT-MSCs
In an attempt to enhance the in vitro
differentiation of AT-MSCs, TSA, a selective and reversible histone
deacetylase inhibitor (16), was
added to the culture media following exposure of cells to
hepatogenic factors over 14 days of incubation. Treatment of
undifferentiated AT-MSCs with increased concentrations of TSA
resulted in wide-spread cell death and cell detachment (16); therefore, 1.5 μM TSA was used in
experiments for the present study. Total RNA was isolated at 7, 14
and 28 days post-differentiation of AT-MSCs into hepatic lineage,
and the expression of several hepatic genes was examined by RT-PCR.
Undifferentiated cells were used as negative controls and HepG2 was
used as the positive control. The expression pattern of
differentiated AT-MSCs from protocol B (Fig. 1B) was used to compare the levels of
the ALB and AFP genes relative to human β-actin at different
time-points by RT-PCR. ALB expression was significantly enhanced by
induction of differentiation. As AT-MSCs differentiated into
hepatocyte-like cells, matured and became more functional, AFP
expression gradually diminished, more notably when TSA was added
from day 14 onwards (Fig. 6C).
In order to further assess the effect of TSA on
differentiated cellular function, the capacity of LDL uptake of
hepatocyte-like cells from protocol B was evaluated (Fig. 1B). TSA enhanced the LDL uptake
capacity dramatically. When different cell types were co-cultured
with LDL for 8 h, the signal indicating LDL uptake in the
hepatocyte-like cells in the TSA induction medium was considerably
brighter in comparison with cells in the basic induction medium.
The negative control cells (undifferentiated AT-MSCs) were
considerably darker than the induced cells treated according to
protocol A or B (Fig. 6C).
TSA, when added exclusively to AT-MSCs at 100%
confluence and without pre-treatment with hepatogenic medium, was
not effective in stimulating mesenchymal-to-hepatic transition.
However, AT-MSCs treated with TSA from day 14 onwards exhibited
significantly upregulated ALB secretion rates (P<0.05, Student’s
t-test) when compared with cells in basic differentiation cultures
(Fig. 6D).
Transplantation of AT-MSCs into
CCl4-injured nude mice results in the improvement of
liver function
Transaminase activity and direct bilirubin levels
were measured at selected time-points to examine liver function
following a single injection of CCl4. Transaminase
activity peaked one day post-CCl4 injection, and
pathological examination of H&E-stained sections revealed large
areas of inflammation and hepatocyte denaturation (Fig. 7A). In comparison with normal liver
tissue, both transaminase activity and direct bilirubin levels
returned to normal seven days following the single injection of
CCl4. Transaminase activity corresponded with H&E
staining of liver pathology, while the serum ALT, DBIL and AST
levels in the CCl4/PBS groups were significantly higher
than those in the CCl4/AT-MSCs groups (Fig. 7B–D) (P<0.05, Student’s t-test).
The therapeutic effects of AT-MSCs transplanted into mice with
CCl4-induced acute liver injury were demonstrated by the
decrease in serum ALT, AST activity and DBIL levels. Although
differences in pathologies between CCl4/PBS and
CCl4/AT-MSCs groups were not found, the results of the
present study showed that transplantation of AT-MSCs had a
beneficial effect on liver function in vivo.
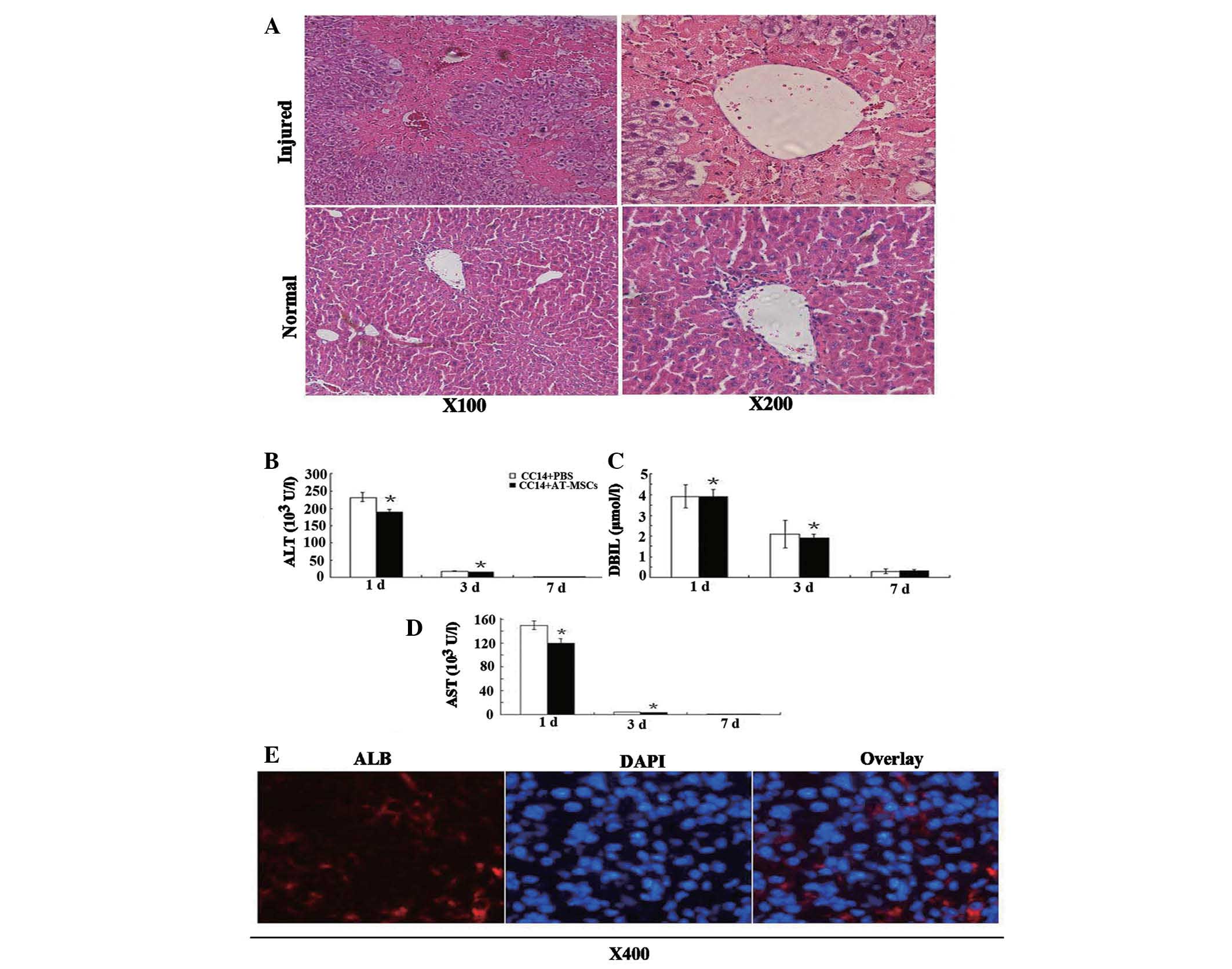 | Figure 7Therapeutic effect of AT-MSC
administration. (A) One day following a single injection of 10%
CCl4 (100 μl/20 g body weight) into the tail vein of
nude mice, H&E staining showed pathological changes in liver
cells, i.e., heavily damaged central veins of the liver and the
morphological characteristics for hepatocytes vanished; (B–D) ALT,
AST and DBIL levels decreased dramatically following AT-MSCs
administration. Data are presented as mean ± standard deviation;
analyzed by Student’s t-test, n=3, *P<0.05 control
vs. AT-MSC-treated group; (E) Human ALB detection in the livers of
nude mice one month following injection of 5×105 AT-MSCs
into CCl4-injured mice. AT-MSC, adipose tissue
mesenchymal stem cells; H&E, hematoxylin and eosin; ALT,
alanine aminotransferase; AST, aspartate aminotransferase; DBIL,
direct bilirubin; ALB, albumin. |
AT-MSCs reside in CCl4 injured
livers and express liver-specific markers
Liver sections were examined by histochemical
immunofluorescence with human ALB-specific antibodies (Fig. 7E), which demonstrated the
incorporation of AT-MSCs into injured livers. Human ALB-positive
cells were found in liver sections following injection of
undifferentiated AT-MSCs; however, these cells did not exhibit the
typical hepatocyte morphology (Fig.
7E). This indicated that AT-MSCs may have potential clinical
application as treatment for acute liver injury.
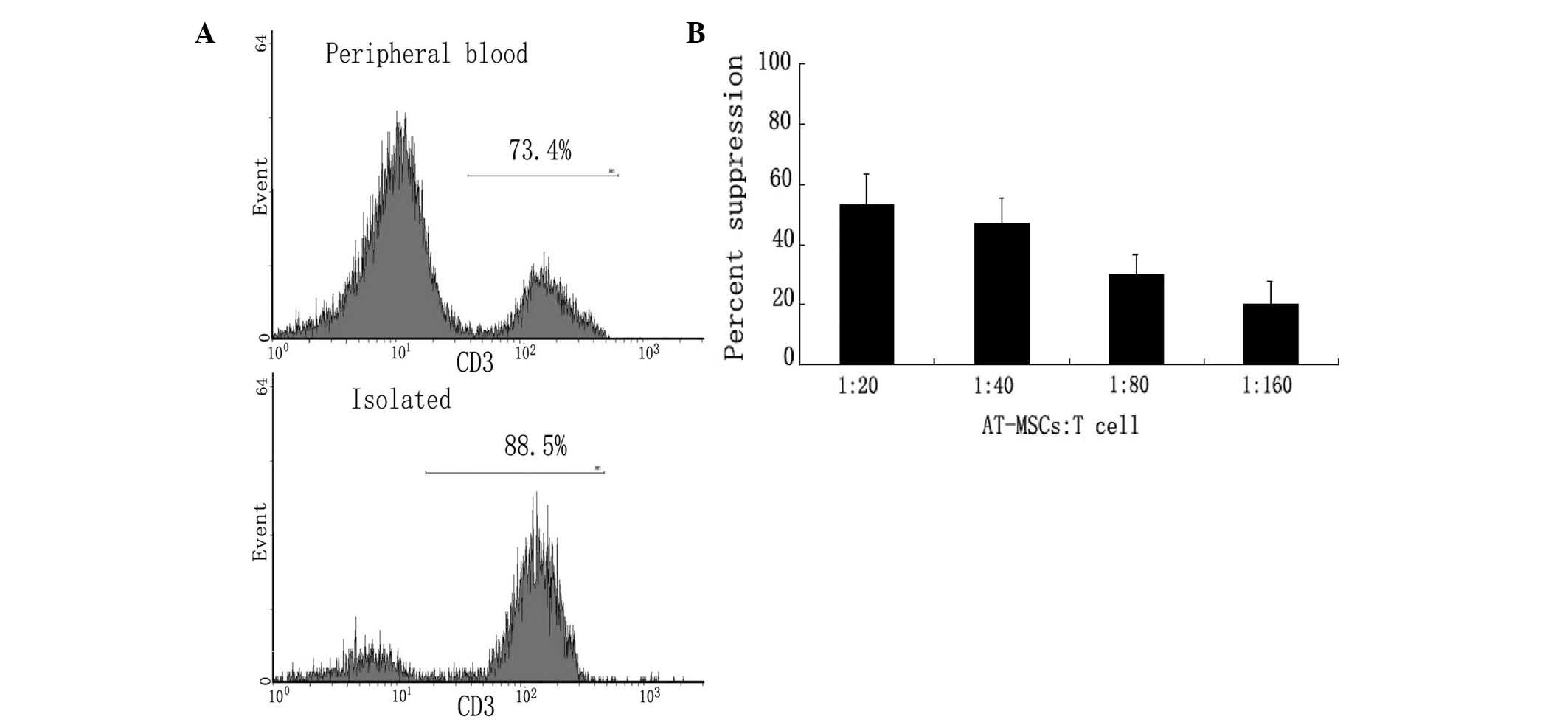
Low immunogenicity of the AT-MSCs
The potential clinical use of AT-MSCs is attractive
due to their low immunogenicity. Both activated and inactivated
AT-MSCs lacked expression of co-stimulatory molecules, including
CD40, CD54, CD80, CD86, or HLA-DR and HLA-ABC (data not shown). The
inability of AT-MSCs to stimulate a T-cell response may be due to
an inherently low immunogenicity status, or active
immunosuppressive mechanisms mediated by the AT-MSCs. MLR assays
with allogeneic T cells were performed to determine whether the
fat-derived cells were immunosuppressive. The ratio of T cells to
total lymphocytes increased from 73.4% in peripheral blood to 88.5%
following isolation (Fig. 8A).
AT-MSCs suppressed MLR cultures in a dose-dependent manner
(Fig. 8B); T-cell aggregation was
decreased compared with that of with that of controls (data not
shown). In addition, AT-MSCs significantly suppressed T-cell
proliferation in MLR. These data indicated that allogeneic AT-MSCs
may be a potential source of cells for tissue repair or
replacement. The present study also has important implications with
respect to the ready availability of adult stem cells for clinical
applications, and to the practical and commercial aspects of their
manufacture and quality assurance. AT-MSCs are not only inherently
non-immunogenic and have the ability to suppress proliferation of
alloantigen- or mitogen-stimulated T cells, but they can also
suppress immunoglobulin production by mitogen-stimulated B cells
(27). The low immunogenicity and
immunosuppressive characteristics show that human adipose tissue
may be an ideal source for cell transplantation (27–29).
Discussion
AT-MSCs have great potential for clinical
applications in regenerative medicine. Transplantation of AT-MSCs
may provide an easier, more efficient and safer method for the
treatment of patients with liver disease than whole organ
transplantation (17). Adipose
tissue can be obtained in large quantities with minimally invasive
procedures and AT-MSCs can be easily isolated and cultured in
vitro (17–19). AT-MSCs have a broader
differentiation potential than previously anticipated and can
differentiate into all mesodermal lineage cells, including
osteocytes, adipocytes and chondrocytes (20,21).
AT-MSCs have characteristics similar to those of BMSCs; AT-MSCs
have the ability to differentiate into osteogenic and adipogenic
cells in vitro; however they do not express hematopoietic
cell antigens. The mechanism of MSC differentiation into
hepatocyte-like cells in vitro has not been determined
extensively: Baertschiger et al (22) reported that co-culturing with Huh-7
cells was sufficient to induce hepatic differentiation. Chien et
al (23) found that coating
culture plates with different proteins affected human
placenta-derived multi-potent cells, which when cultured in
differentiation medium on poly-L-lysine-coated plates, changed
morphology and became polygonal in shape. These morphological
changes were less obvious when cells were cultured on
fibronectin-coated plates.
TSA is a trigger for the further differentiation of
human MSCs towards endodermal lineages (24). High levels of TSA can induce cell
apoptosis and promote differentiation by inducing cell cycle arrest
during the G0/G1 and G1/S phases (25). Vinken et al (25) reported that TSA counteracted the
loss of liver-specific functions in primary rat hepatocytes
(26) and enhanced intercellular
communication through gap junctions. In the present study, TSA
significantly boosted the function of hepatocyte-like cells derived
from AT-MSCs, regardless of increases in the ALB mRNA and protein
levels, in addition to enhancing their glycogen storage
abilities.
In vivo, the transplanted cells were exposed
to a dynamic host microenvironment laden with soluble mediators and
immunoreactive cells. The present study hypothesized that at least
three ubiquitous microenvironmental factors may affect the
differentiation and function of the MSCs. Interleukin-1α (IL-1α) is
a key regulator of hematopoiesis and the inflammatory process
(30). Recent studies on mice have
shown that MSCs have an inherent ability to counteract the
deleterious inflammatory effects of IL-1α in injured tissues
(31) In addition, tumor necrosis
factor α, which has been shown to increase chemotaxis of MSCs,
would also be clinically important if the efficiency of
concentrating MSCs to the site of tissue injury was improved
(32). Furthermore, stromal
cell-derived factor-1α is a promoter of non-specific MSC migration
(33,34). Therefore, co-therapy with a
pharmacological agent, such as a cytokine receptor antagonist, may
negate the deleterious effects of the microenvironment and optimize
the therapeutic potential of MSCs (31,35).
Human BMSCs used for transplantation have been expanded without
significant loss of their differentiation capacities. Following
transplantation into unconditioned adult mice, BMSCs not only
migrated to the bone marrow but also into other tissues. Impairment
of the tissue was shown to cause increased BMSC implantation not
only in bone marrow and muscle but it was also shown to lead to
further engraftment in the brain, heart and liver (36).
Oyagi et al (37) reported that BMSCs reduced
hepatocyte apoptosis and promoted cell proliferation. In addition,
they showed that autologous MSC transplantation prolonged the
survival of dogs (38) and swine
(39) receiving living donor liver
transplantation. The present study clearly demonstrated that
AT-MSCs can ease mouse acute hepatic injury in vivo by
decreasing inflammation and apoptosis as well as increasing
proliferation and recovery (as evidenced by decreased ALT, AST and
DBIL levels). It was demonstrated that AT-MSCs are a novel source
of cells that may be used to treat hepatic injury and/or
dysfunction. The mechanisms by which hepatic differentiation occurs
in vivo and hepatic function is restored, however, are still
not fully elucidated. Oyagi et al (37) reported that BMSCs secreted HGF and
suppressed inflammation when transplanted into
CCl4-injured rats. HGF had the capacity to induce
hepatic differentiation and suppress hepatocyte death (40). Silva et al (41) reported that the transplantation of
MSCs reduced fibrosis through the secretion of cytokines, in
particular vascular endothelial growth factor. BMSCs have the
capacity to differentiate into hepatocyte-like cells in response to
growth factor stimulation (37).
Results of the present study showed that AT-MSCs can only reside in
CCl4-injured livers, suggesting that hepatic
differentiation of AT-MSCs may be induced by HGF and/or other
cytokines secreted by the CCl4-injured livers. It
remains elusive whether MSCs contribute to tissue repair by
differentiation into tissue-specific cell types, or whether they
produce trophic factors at the site of injury, which can stimulate
tissue repair (42,43). MSCs are responsive to their
environment, adapting function to local circumstances, and
immunosuppressive properties appear to be induced under
inflammatory conditions (43).
Modification of the culture medium could modulate the properties of
MSCs, for example, by enhancing immunosuppressive function and
reducing susceptibility for lysis by cytotoxic T cells (44).
In conclusion, ALB was detected in the livers of the
CCl4-injured mice one month post-transplantation. This
suggested that transplantation of the human AT-MSCs could relieve
the impairment of acute CCl4-injured livers in nude
mice. This therefore implied that adipose tissue was a source of
multipotent stem cells which had the potential to differentiate
into mature, transplantable hepatocyte-like cells in vivo
and in vitro. In addition, the present study determined that
TSA was essential to promoting differentiation of human MSC towards
functional hepatocyte-like cells. The relief of liver injury
following treatment with AT-MSCs suggested their potential as a
novel therapeutic method for liver disorders or injury. Human
AT-MSCs may become a useful source for hepatocyte regeneration and
may provide an alternative to liver transplantation.
Acknowledgements
This study was funded by the National Natural
Science Foundation of China (no. 30872484) and the Science
Foundation of Nanjing Medical University (no. 2012NJMU134).
References
|
1
|
van Poll D, Parekkadan B, Cho CH, et al:
Mesenchymal stem cell-derived molecules directly modulate
hepatocellular death and regeneration in vitro and in vivo.
Hepatology. 47:1634–1643. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kashofer K and Bonnet D: Gene therapy
progress and prospects: stem cell plasticity. Gene Ther.
12:1229–1234. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kern S, Eichler H, Stoeve J, Klüter H and
Bieback K: Comparative analysis of mesenchymal stem cells from bone
marrow, umbilical cord blood, or adipose tissue. Stem Cells.
24:1294–1301. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Devine SM, Cobbs C, Jennings M,
Bartholomew A and Hoffman R: Mesenchymal stem cells distribute to a
wide range of tissues following systemic infusion into nonhuman
primates. Blood. 101:2999–3001. 2003. View Article : Google Scholar
|
|
5
|
Tepliashin AS, Chupikova NI, Korzhikova
SV, et al: Comparative analysis of cell populations with a
phenotype similar to that of mesenchymal stem cells derived from
subcutaneous fat. Tsitologiia. 47:637–643. 2005.(in Russian).
|
|
6
|
Malhi H, Irani AN, Gagandeep S and Gupta
S: Isolation of human progenitor liver epithelial cells with
extensive replication capacity and differentiation into mature
hepatocytes. J Cell Sci. 115:2679–2688. 2002.PubMed/NCBI
|
|
7
|
Young HE, Steele TA, Bray RA, et al: Human
reserve pluripotent mesenchymal stem cells are present in the
connective tissues of skeletal muscle and dermis derived from
fetal, adult, and geriatric donors. Anat Rec. 264:51–62. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lowes KN, Croager EJ, Olynyk JK, Abraham
LJ and Yeoh GC: Oval cell-mediated liver regeneration: Role of
cytokines and growth factors. J Gastroenterol Hepatol. 18:4–12.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zuk PA, Zhu M, Ashjian P, et al: Human
adipose tissue is a source of multipotent stem cells. Mol Biol
Cell. 13:4279–4295. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lagasse E, Connors H, Al-Dhalimy M, et al:
Purified hematopoietic stem cells can differentiate into
hepatocytes in vivo. Nat Med. 6:1229–1234. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Alison MR, Poulsom R, Jeffery R, et al:
Hepatocytes from non-hepatic adult stem cells. Nature. 406:2572000.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Herzog EL, Chai L and Krause DS:
Plasticity of marrow-derived stem cells. Blood. 102:3483–3493.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vassilopoulos G, Wang PR and Russell DW:
Transplanted bone marrow regenerates liver by cell fusion. Nature.
422:901–904. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang X, Willenbring H, Akkari Y, et al:
Cell fusion is the principal source of bone-marrow-derived
hepatocytes. Nature. 422:897–901. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wagner W, Wein F, Seckinger A, et al:
Comparative characteristics of mesenchymal stem cells from human
bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol.
33:1402–1416. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Snykers S, Vanhaecke T, De Becker A, et
al: Chromatin remodeling agent trichostatin A: a key-factor in the
hepatic differentiation of human mesenchymal stem cells derived of
adult bone marrow. BMC Dev Biol. 7:242007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yamamoto H, Quinn G, Asari A, et al:
Differentiation of embryonic stem cells into hepatocytes:
biological functions and therapeutic application. Hepatology.
37:983–993. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim JW, Kim SY, Park SY, et al:
Mesenchymal progenitor cells in the human umbilical cord. Ann
Hematol. 83:733–738. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Reyes M, Lund T, Lenvik T, et al:
Purification and ex vivo expansion of postnatal human marrow
mesodermal progenitor cells. Blood. 98:2615–2625. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Teratani T, Yamamoto H, Aoyagi K, et al:
Direct hepatic fate specification from mouse embryonic stem cells.
Hepatology. 41:836–846. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yamamoto Y, Teratani T, Yamamoto H, et al:
Recapitulation of in vivo gene expression during hepatic
differentiation from murine embryonic stem cells. Hepatology.
42:558–567. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Baertschiger RM, Serre-Beinier V, Morel P,
et al: Fibrogenic potential of human multipotent mesenchymal
stromal cells in injured liver. PLoS One. 4:e66572009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chien CC, Yen BL, Lee FK, et al: In vitro
differentiation of human placenta-derived multipotent cells into
hepatocyte-like cells. Stem Cells. 24:1759–1768. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Herold C, Ganslmayer M, Ocker M, et al:
The histone-deacetylase inhibitor Trichostatin A blocks
proliferation and triggers apoptotic programs in hepatoma cells. J
Hepatol. 36:233–240. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vinken M, Henkens T, Vanhaecke T, et al:
Trichostatin a enhances gap junctional intercellular communication
in primary cultures of adult rat hepatocytes. Toxicol Sci.
91:484–492. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Henkens T, Papeleu P, Elaut G, et al:
Trichostatin A, a critical factor in maintaining the functional
differentiation of primary cultured rat hepatocytes. Toxicol Appl
Pharmacol. 218:64–71. 2007. View Article : Google Scholar
|
|
27
|
Bochev I, Elmadjian G, Kyurkchiev D, et
al: Mesenchymal stem cells from human bone marrow or adipose tissue
differently modulate mitogen-stimulated B-cell immunoglobulin
production in vitro. Cell Biol Int. 32:384–393. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
McIntosh K, Zvonic S, Garrett S, et al:
The immunogenicity of human adipose-derived cells: temporal changes
in vitro. Stem Cells. 24:1246–1253. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Battiwalla M and Hematti P: Mesenchymal
stem cells in hematopoietic stem cell transplantation. Cytotherapy.
11:503–515. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dinarello CA: The interleukin-1 family: 10
years of discovery. FASEB J. 8:1314–1325. 1994.PubMed/NCBI
|
|
31
|
Ortiz LA, Dutreil M, Fattman C, et al:
Interleukin 1 receptor antagonist mediates the anti-inflammatory
and antifibrotic effect of mesenchymal stem cells during lung
injury. Proc Natl Acad Sci USA. 104:11002–11007. 2007. View Article : Google Scholar
|
|
32
|
Ponte AL, Marais E, Gallay N, et al: The
in vitro migration capacity of human bone marrow mesenchymal stem
cells: comparison of chemokine and growth factor chemotactic
activities. Stem Cells. 25:1737–1745. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Potian JA, Aviv H, Ponzio NM, Harrison JS
and Rameshwar P: Veto-like activity of mesenchymal stem cells:
functional discrimination between cellular responses to
alloantigens and recall antigens. J Immunol. 171:3426–3434. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tang J, Wang J, Yang J, et al: Mesenchymal
stem cells over-expressing SDF-1 promote angiogenesis and improve
heart function in experimental myocardial infarction in rats. Eur J
Cardiothorac Surg. 36:644–650. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Greco SJ and Rameshwar P:
Microenvironmental considerations in the application of human
mesenchymal stem cells in regenerative therapies. Biologics.
2:699–705. 2008.
|
|
36
|
Mouiseddine M, François S, Semont A, et
al: Human mesenchymal stem cells home specifically to
radiation-injured tissues in a non-obese diabetes/severe combined
immunodeficiency mouse model. Br J Radiol. 80(Spec 1): S49–55.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Oyagi S, Hirose M, Kojima M, et al:
Therapeutic effect of transplanting HGF-treated bone marrow
mesenchymal cells into CCl4-injured rats. J Hepatol.
44:742–748. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pan MX, Hou WL, Zhang QJ, et al: Infusion
of autologous mesenchymal stem cells prolongs the survival of dogs
receiving living donor liver transplantation. J South Med Univ. (in
Chinese).
|
|
39
|
Kuo YR, Goto S, Shih HS, et al:
Mesenchymal stem cells prolong composite tissue allotransplant
survival in a swine model. Transplantation. 87:1769–1777. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Matsuda Y, Matsumoto K, Ichida T and
Nakamura T: Hepatocyte growth factor suppresses the onset of liver
cirrhosis and abrogates lethal hepatic dysfunction in rats. J
Biochem. 118:643–649. 1995.PubMed/NCBI
|
|
41
|
Silva GV, Litovsky S, Assad JA, et al:
Mesenchymal stem cells differentiate into an endothelial phenotype,
enhance vascular density, and improve heart function in a canine
chronic ischemia model. Circulation. 111:150–156. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Phinney DG and Prockop DJ: Concise review:
mesenchymal stem/multipotent stromal cells: the state of
transdifferentiation and modes of tissue repair - current views.
Stem Cells. 25:2896–2902. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Krampera M, Cosmi L, Angeli R, et al: Role
for interferon-gamma in the immunomodulatory activity of human bone
marrow mesenchymal stem cells. Stem Cells. 24:386–398. 2006.
View Article : Google Scholar
|
|
44
|
Rasmusson I, Uhlin M, Le Blanc K and
Levitsky V: Mesenchymal stem cells fail to trigger effector
functions of cytotoxic T lymphocytes. J Leukoc Biol. 82:887–893.
2007. View Article : Google Scholar : PubMed/NCBI
|