Introduction
Glioma is one of the most lethal types of cancer in
the world and is a major cause of morbidity. Current treatment of
glioma predominantly focuses on standard procedure options,
including surgical intervention, radiotherapy, chemotherapy or a
combination of these methods; however, there has been no
significant improvement in prognosis (1). Glioma has proved to be resistant to
chemotherapy (2), yet the
molecular basis underlying this chemoresistance has not been fully
understood. Novel treatment strategies for reducing chemoresistance
are therefore required.
Osteopontin (OPN) is a secreted, integrin-binding
phosphoglycoprotein that is overexpressed in several primary
tumors. OPN has been shown to have numerous functions, including
roles in cell migration and survival and manipulation of tumor
phenotype (3). It has been
reported that OPN expression is elevated in glioma tissues
(4–6), which is consistent with the results
of our previous study (7);
however, less definitive evidence has been reported to explain the
chemoresistance of glioma cells.
The nuclear factor (NF)-κB family contains p65/RelA,
RelB, c-Rel, NF-κB1/p50 and NF-κB/p52, which dimerize and are held
in the cytoplasm by specific proteins. NF-κB may be activated by
the canonical or non-canonical pathway (8). The canonical pathway depends on the
NF-κB essential modulator, I-kappa-B kinase (IKK)β activation and
nuclear localization of RelA/p50 dimers; whereas the non-canonical
pathway depends on IKKα activation, probably by the upstream
NF-κB-inducing kinase nuclear localization of p52/RelB dimers
(9). Activation of NF-κB has been
reported to be associated with numerous types of cancer. It has
been demonstrated to influence cancer initiation, promotion, and
progression of lung (10,11), breast (12) and pancreatic cancer (13,14).
In glioma, NF-κB was shown to have higher expression levels in
glioblastoma (GBM) tissue, as compared with non-GBM tissue
(15). McFarland et al
(16) reported that NF-κB-induced
interleukin-6 ensured STAT3 activation and tumor aggressiveness in
glioblastoma. Bonavia et al (17) demonstrated that NF-κB was
implicated in the promotion of epidermal growth factor variant III,
in glioma angiogenesis and growth.
In the present study, it was found that treatment
with cisplatin (DDP) and temozolomide (TMZ) could induce
overexpression of OPN in human neuronal glioma astrocytoma (U251)
cells. Specific downregulation of OPN using RNA interference (RNAi)
enhanced the sensitivity of glioma U251 cells to DDP and TMZ via
targeting the nuclear factor κ-light-chain-enhancer of activated B
cells (NF-κB)/B cell lymphoma 2 (Bcl-2) pathway. This study has
confirmed that OPN plays an important role in chemoresistance.
Additionally, this is the first study, to the best of our
knowledge, indicating that downregulation of OPN by siRNA can
sensitize glioma cells to chemotherapy by suppressing the
NF-κB/Bcl-2 signaling pathway.
Materials and methods
Reagents
Cell culture media and supplements were purchased
from Gibco Life Technologies (Grand Island, NY, USA). Antibodies
against GAPDH and Bcl-2 were purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). The electrophoretic
mobility shift assay (EMSA) kit was obtained from Beyotime
Institute of Biotechnology (Shanghai, China). TMZ and DDP were
purchased from Sigma (St. Louis, MO, USA). The terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labeling
(TUNEL) assay kit was purchased from Beyotime Institute of
Biotechnology (Haimen, China).
Cell culture and treatment
The U251 human glioma cell line was purchased from
the Chinese Academy of Sciences Cell Bank (Shanghai, China). U251
cells were cultured in Dulbecco’s Modified Eagle Medium
supplemented with 10% fetal bovine serum and maintained in a 37°C,
5% CO2 incubator and routinely passaged at two- to
three-day intervals. U251 cells (1×106) were seeded in a
100-mm petri dish with 10 ml growth medium and were treated with
TMZ (100 μM) and DPP (100 μM) for 24 h on the third
day as indicated.
Lentiviral vector construction and
infection
A lentivirus-based vector for small interfering RNA
(siRNA) sequences targeting human OPN was constructed with
technical support from Shanghai GeneChem (Shanghai, China). A short
hairpin RNA (shRNA) template was designed and cloned into a
lentivirus vector containing a U6 promoter upstream of the cloning
restriction sites (AgeI and EcoRII). The
oligonucleotides encoding the shRNA were annealed and inserted
between the AgeI and EcoRII sites of the plasmid. The
OPN siRNA vector (pGC-LV) and packaging vectors (pHelper 1.0 and
pHelper 2.0) (Invitrogen Life Technologies, Carlsbad, CA, USA) were
cotransfected into 293FT cells cells (Cyagen Biosciences Inc.,
Santa Clara, CA, USA) using Lipofectamine® 2000
(Invitrogen, Grand Island, NY, USA). The culture supernatants were
collected, concentrated and stored at −70°C. U251 cells were
infected with lentiviral vectors at a multiplicity of infection of
five in the presence of polybrene (10 μg/ml).
Western blotting
To determine the level of Bcl-2 expression, total
protein was isolated in lysis buffer (137 mM NaCl, 0.1 mM sodium
orthovanadate, 0.1% Triton X-100, 15 mM ethylene glycol
tetra-acetic acid, 15 mM MgCl2, 25 mM
3-morpholinopropane-1-sulfonic acid, 20 μM leupeptin and 100 μM
phenylmethylsulfonyl fluoride, adjusted to pH 7.2). Equal amounts
of protein (30 μg) were loaded into the sample wells and separated
on a 12% SDS-PAGE gel. The electrophoresed proteins were
transferred to a polyvinylidene fluoride Immobilon-P membrane
(Millipore, Watford, UK) and subjected to immunoblot analysis with
anti-Bcl-2 antibody. GAPDH was used as an internal control.
Quantitative polymerase chain reaction
(qPCR)
Total RNA was purified from U251 glioma cells using
TRIzol® reagent (Invitrogen Life Technologies) according
to the manufacturer’s instructions. In order to validate the
differential relative expression of OPN, TaqMan-based qPCR was
performed using a SYBR PrimeScript RT-PCR kit (Takara Bio, Inc.,
Shiga, Japan) on the ABI Prism 7300 HT Sequence Detection system
(Applied Biosystems Life Technologies, Foster City, CA, USA),
according to the manufacturer’s instructions. Primers used were:
5′-CTG TGC CAT ACC AGT TAA-3′ (forward) and 5′-GAT GTC AGG TCT GCG
AAA-3′ (reverse), designed by Primer Premier 5.0 (Premier Biosoft,
Palo Alto, CA, USA). The amplification of β-actin with primers
5′-AAG ACC TGT ACG CCA ACA CAG T-3′ (forward) and 5′-AGA AGC ATT
TGC GGT GGA CGA T-3′ (reverse) was taken as an internal control. In
order to determine the relative amounts of the products, the
comparative Ct (threshold cycle) method was used according to the
instructions supplied by Applied Biosystems Life Technologies.
EMSA
The procedure for the EMSA was followed as
previously described (18).
Briefly, nuclear proteins were isolated from U251 cells in each
group. A total of 20 μg nuclear extract was preincubated for
10 min in binding buffer (1 μg
poly(deoxyinosinic-deoxycytidylic) acid, 10 mM Tris-HCl pH 7.5, 1
mM EDTA, 50 mM NaCl, 5% glycerol, 1 mM dithiothreitol and 1
μg/μl bovine serum albumin) on ice and consecutively reacted
with 1×105 dpm of γ-32P- (Amersham
Biosciences, Sunnyvale, CA, USA) labeled probe containing the NF-κB
binding site (5′-AGT TGA GGG GAC TTT CCC AGG C-3′) for 30 min at
room temperature. The nuclear extracts were electrophoresed on a 6%
polyacrylamide gel.
TUNEL
Groups of U251 cells were fixed with 4%
paraformaldehyde and washed in phosphate-buffered saline. The TUNEL
detection reagent was prepared according to the manufacturer’s
instructions (Beyotime Institute of Biotechnology). The U251 cells
were incubated with the TUNEL detection reagent at 37°C for 60 min
and then examined using a fluorescence microscope (Axio Imager A1;
Carl Zeiss AG, Oberkochen, Germany). DAPI staining was used as a
control in the corresponding microscope field.
Statistical analysis
Experimental procedures were repeated three times
and analyzed using SPSS 11.0 (SPSS, Inc., Chicago, IL, USA).
One-way analysis of variance was used to determine statistically
significant differences at a threshold of P<0.05.
Results
TMZ and DDP induce OPN overexpression and
NF-κB activation
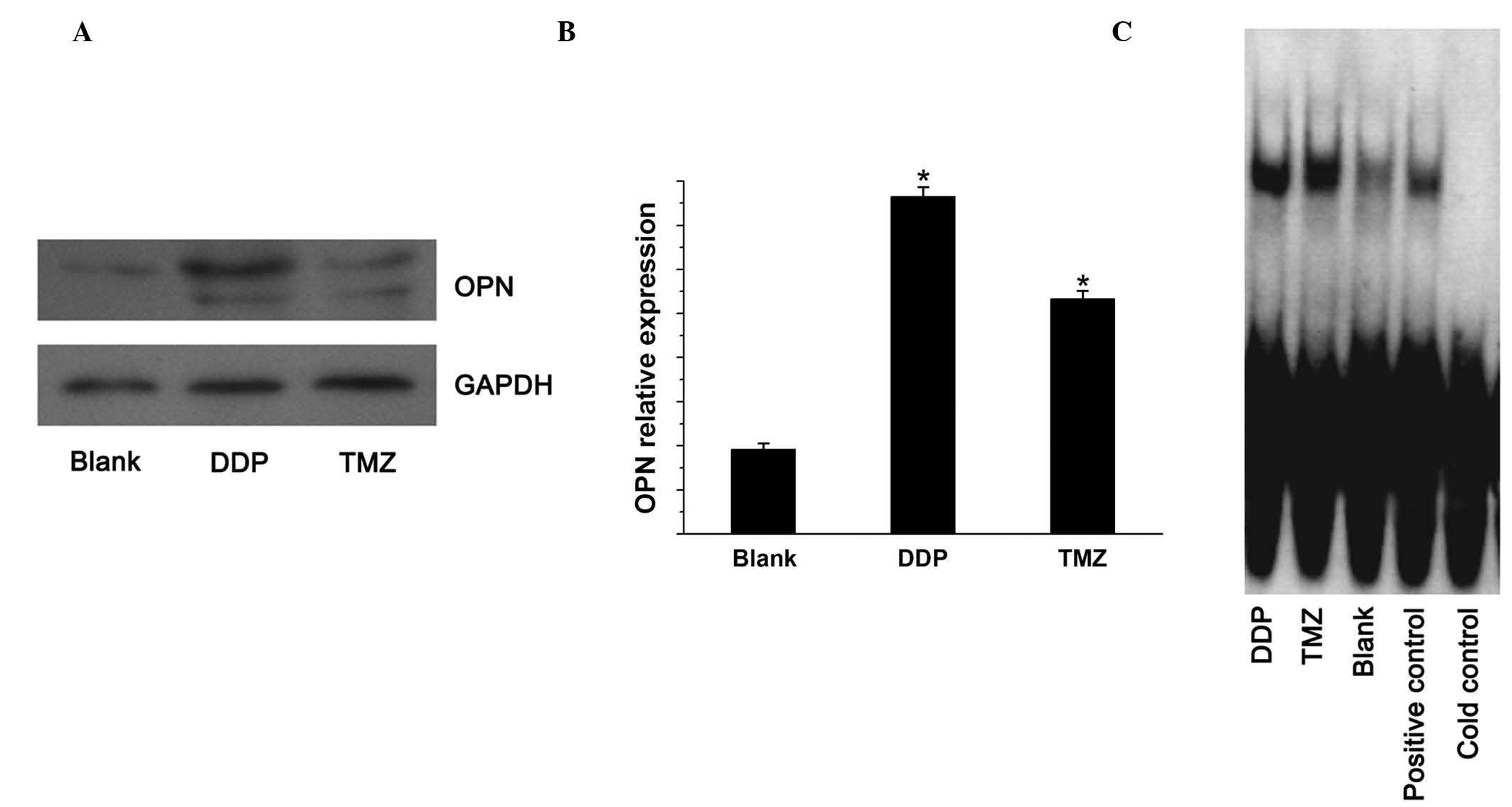
To examine the protein and mRNA expression of OPN in
U251 cells treated with TMZ and DDP, western blotting was performed
using specific antibodies for OPN and GAPDH (used as an internal
control), and qPCR was conducted using specific primers for OPN and
β-actin (used as an internal control), respectively. The levels of
OPN protein and mRNA in U251 cells treated with TMZ and DDP were
significantly higher than those in the negative controls (Fig. 1A and B). EMSA was subsequently used
to measure the state of NF-κB activation. TMZ and DDP were shown to
induce significant NF-κB activation (P<0.05 vs the blank control
group; Fig. 1C).
OPN siRNA enhances apoptosis induced by
TMZ and DDP in U251 cells
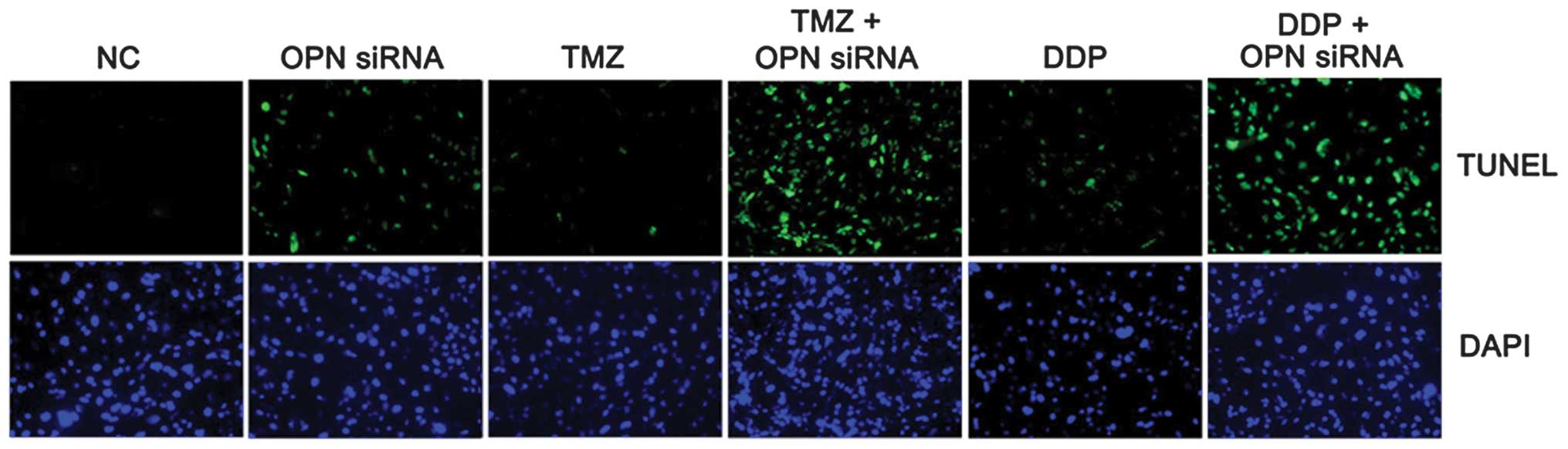
It was next assessed whether OPN contributed to the
resistance of U251 cells to chemotherapy. Cells were infected with
either OPN or control siRNA and then incubated with TMZ and DDP. It
was found that U251 cells exhibited increased apoptosis when OPN
was specifically knocked down by lentivirus-mediated OPN siRNA
(Fig. 2). These findings suggested
that OPN contributed to the resistance of glioblastoma cells to TMZ
and DDP.
OPN siRNA enhances the sensitivity of
glioma U251 cells to TMZ and DDP by targeting the NF-κB/Bcl-2
pathway
Constitutive NF-κB activation was evaluated by EMSA.
TMZ and DDP induced significant activation of NF-κB as well as
Bcl-2 expression in U251 cells. In addition, lentivirus-mediated
OPN siRNA was shown to block NF-κB activation and Bcl-2 expression
induced by TMZ and DDP (P<0.05; Fig. 3).
Discussion
It is well known that glioma cells are resistant to
chemotherapeutic drugs (19).
However, the molecular mechanisms of chemoresistance in glioma
cells are not fully understood. OPN is a secreted non-collagenous
phosphoglycoprotein that has been shown to be commonly
overexpressed in several types of tumor (20). It has been reported that OPN
inhibits apoptosis of adherent endothelial cells deprived of growth
factors (21) and prevents
curcumin-induced apoptosis (22).
In our previous study (7), it was
found that OPN siRNA induced apoptosis of glioma cells. In the
present study, it was found that TMZ and DDP could induce OPN
overexpression. The aim of this study was to determine whether OPN
was involved in chemoresistance in glioma cells and to identify
novel targeted strategies to enhance chemosensitivity through
specific OPN inhibition. The dysfunction of apoptosis represents a
critical step in tumorigenesis. Cancer cells commonly show
resistance to radiotherapy and chemotherapy, and the overexpression
of various anti-apoptotic proteins in cancer cells contributes to
chemoresistance (23). In the
present study, it was observed that OPN was significantly
upregulated in DDP- and TMZ-treated U251 cells. Additionally,
lentivirus-mediated OPN siRNA greatly enhanced DDP and TMZ-induced
apoptosis in U251 cells. This suggests that DDP and TMZ treatments,
in combination with lentivirus-mediated OPN siRNA, may provide a
novel approach to sensitizing glioma cells to chemotherapy.
The transcription factor NF-κB serves as a principal
mediator of resistance to chemotherapy in several types of tumor.
Constitutive activation of NF-κB facilitates cancer cell survival
and reduces sensitivity towards chemotherapeutic drugs (24). It has been reported that NF-κB
activation in human colon cancers diminishes the level of apoptosis
induced by chemotherapy, whilst inhibition of NF-κB has been shown
to enhance the sensitivity to anti-neoplastic-induced apoptosis
in vitro and in vivo (25–27).
Bcl-2 overexpression has been shown to inhibit apoptosis in
vitro in response to several chemotherapeutic agents (28), and resistance to chemotherapy in
glioblastoma has been linked to the expression of genes of the
Bcl-2 family (29,30). Targeting Bcl-2 may provide a novel
therapeutic approach to overcome chemoresistance, as proposed for
small-cell lung cancer (31). In
the present study, U251 cells were examined by EMSA to determine
changes in DNA binding of NF-κB in response to TMZ and DDP. It was
found that treatment with TMZ and DDP could induce NF-κB
activation. In addition, TMZ and DDP treatment increased Bcl-2
expression. It has previously been shown that NF-κB activates Bcl-2
expression in t(14;18) lymphoma cells (32). Furthermore, the NF-κB/Bcl-2
signaling pathway has been suggested to function in chemoresistance
(33). In the present study, TMZ
and DDP, in combination strategies with specific inhibition of OPN
by siRNA, led to inhibition of NF-κB activation and Bcl-2
expression, which resulted in enhanced chemosensitivity. These data
demonstrated that chemotherapy induced NF-κB activity in glioma
cells, and specific inhibition of OPN blocked this activation of
NF-κB and led to enhanced tumoricidal responses. The identification
of OPN targets in chemoresistance may lead to novel target-directed
strategies in the treatment of glioma cells to improve therapeutic
responses.
In conclusion, the present study demonstrates for
the first time, to the best of our knowledge, that treatment with
DDP and TMZ can induce increased OPN expression in U251 cells,
which results in activation of NF-κB and an increase in Bcl-2
expression. Specific downregulation of OPN with RNAi enhanced the
sensitivity of glioma U251 cells to DDP and TMZ. These results
demonstrate that lentiviral-mediated OPN siRNA can sensitize U251
cells to chemotherapy by targeting the NF-κB/Bcl-2 pathway.
Acknowledgements
This study was supported financially by grants from
the National High Technology Research and Development Program 863
(2012AA02A508), the National Natural Science Foundation of China
(91229121 and 81272792), the Jiangsu Province’s Key Discipline of
Medicine (XK201117), the Key Foundation of Jiangsu Province’s
Science and Technology Commission (BL2012028), the Provincial
Initiative Program for Excellency Disciplines, Jiangsu Province,
the Jiangsu Province’s Medical Major Talent Program (RC2011051),
the Open Project of the Jiangsu Province’s Key Discipline of
Medicine (KF201231) and Nanjing Medical University Technological
Development Program (2010NJM075).
References
|
1
|
Field KM, Drummond KJ, Yilmaz M, Tacey M,
Compston D, Gibbs P and Rosenthal MA: Clinical trial participation
and outcome for patients with glioblastoma: multivariate analysis
from a comprehensive dataset. J Clin Neurosci. 20:783–789. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Koshkin PA, Chistiakov DA and Chekhonin
VP: Role of microRNAs in mechanisms of glioblastoma resistance to
radio- and chemotherapy. Biochemistry (Mosc). 78:325–334. 2013.
View Article : Google Scholar
|
|
3
|
Shevde LA, Das S, Clark DW and Samant RS:
Osteopontin: an effector and an effect of tumor metastasis. Curr
Mol Med. 10:71–81. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Matusan-Ilijas K, Behrem S, Jonjic N,
Zarkovic K and Lucin K: Osteopontin expression correlates with
angiogenesis and survival in malignant astrocytoma. Pathol Oncol
Res. 14:293–298. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Toy H, Yavas O, Eren O, Genc M and Yavas
C: Correlation between osteopontin protein expression and
histological grade of astrocytomas. Pathol Oncol Res. 15:203–207.
2009. View Article : Google Scholar
|
|
6
|
Atai NA, Bansal M, Lo C, Bosman J,
Tigchelaar W, Bosch KS, Jonker A, De Witt Hamer PC, Troost D,
McCulloch CA, Everts V, Van Noorden CJ and Sodek J: Osteopontin is
up-regulated and associated with neutrophil and macrophage
infiltration in glioblastoma. Immunology. 132:39–48. 2011.
View Article : Google Scholar :
|
|
7
|
Yan W, Qian C, Zhao P, Zhang J, Shi L,
Qian J, Liu N, Fu Z, Kang C, Pu P and You Y: Expression pattern of
osteopontin splice variants and its functions on cell apoptosis and
invasion in glioma cells. Neuro Oncol. 12:765–775. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rothwarf DM, Zandi E, Natoli G and Karin
M: IKK-gamma is an essential regulatory subunit of IkappaB kinase
complex. Nature. 395:297–300. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu R, Tan J, Lin Y, Jia R, Yang W, Liang
C, Geng Y and Qiao W: HIV-1 Vpr activates both canonical and
noncanonical NF-κB pathway by enhancing the phosphorylation of
IKKα/β. Virology. 439:47–56. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li Z, Guo Y, Jiang H, Zhang T, Jin C,
Young CY and Yuan H: Differential regulation of MMPs by E2F1, Sp1
and NF-kappa B controls the small cell lung cancer invasive
phenotype. BMC Cancer. 14:2762014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li F, Huang L, Su XL, Gu QH and Hu CP:
Inhibition of nuclear factor-κB activity enhanced chemosensitivity
to cisplatin in human lung adeno-carcinoma A549 cells under
chemical hypoxia conditions. Chin Med J (Engl). 126:3276–3782.
2013.
|
|
12
|
Fuke Y, Hishinuma M, Namikawa M, Oishi Y
and Matsuzaki T: Wasabi-derived 6-(methylsulfinyl)hexyl
isothiocyanate induces apoptosis in human breast cancer by possible
involvement of the NF-κB pathways. Nutr Cancer. 66:879–887. 2014.
View Article : Google Scholar
|
|
13
|
Wang Y, Zhou Y, Jia G, Han B, Liu J, Teng
Y, Lv J, Song Z, Li Y, Ji L, Pan S, Jiang H and Sun B: Shikonin
suppresses tumor growth and synergizes with gemcitabine in a
pancreatic cancer xenograft model: Involvement of NF-κB signaling
pathway. Biochem Pharmacol. 88:322–333. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Du QH, Xu YB, Zhang MY, Yun P and He CY:
Propofol induces apoptosis and increases gemcitabine sensitivity in
pancreatic cancer cells in vitro by inhibition of nuclear factor-κB
activity. World J Gastroenterol. 19:5485–5492. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang H, Wang H, Zhang W, Huang HJ, Liao WS
and Fuller GN: Analysis of the activation status of Akt, NFkappaB,
and Stat3 in human diffuse gliomas. Lab Invest. 84:941–951. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
McFarland BC, Hong SW, Rajbhandari R,
Twitty GB Jr, Gray GK, Yu H, Benveniste EN and Nozell SE:
NF-κB-induced IL-6 ensures STAT3 activation and tumor
aggressiveness in glioblastoma. PLoS One. 8:e787282013. View Article : Google Scholar
|
|
17
|
Bonavia R, Inda MM, Vandenberg S, Cheng
SY, Nagane M, Hadwiger P, Tan P, Sah DW, Cavenee WK and Furnari FB:
EGFRvIII promotes glioma angiogenesis and growth through the NF-κB,
interleukin-8 pathway. Oncogene. 31:4054–4066. 2012. View Article : Google Scholar
|
|
18
|
Yin BL, Hao H, Wang YY, Jiang YJ and Xue
S: Downregulating osteopontin reduces angiotensin II-induced
inflammatory activation in vascular smooth muscle cells. Inflamm
Res. 58:67–73. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dumitru CA, Weller M and Gulbins E:
Ceramide metabolism determines glioma cell resistance to
chemotherapy. J Cell Physiol. 221:688–695. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Courter D, Cao H, Kwok S, Kong C, Banh A,
Kuo P, Bouley DM, Vice C, Brustugun OT, Denko NC, Koong AC, Giaccia
A and Le QT: The RGD domain of human osteopontin promotes tumor
growth and metastasis through activation of survival pathways. PLoS
One. 5:e96332010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Khan SA, Lopez-Chua CA, Zhang J, Fisher
LW, Sørensen ES and Denhardt DT: Soluble osteopontin inhibits
apoptosis of adherent endothelial cells deprived of growth factors.
J Cell Biochem. 85:728–736. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Song G, Ming Y, Mao Y, Bao S and Ouyang G:
Osteopontin prevents curcumin-induced apoptosis and promotes
survival through Akt activation via alpha v beta 3 integrins in
human gastric cancer cells. Exp Biol Med (Maywood). 233:1537–1545.
2008. View Article : Google Scholar
|
|
23
|
Malaguarnera L: Implications of apoptosis
regulators in tumorigenesis. Cancer Metastasis Rev. 23:367–387.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Arlt A and Schäfer H: NFkappaB-dependent
chemoresistance in solid tumors. Int J Clin Pharmacol Ther.
40:336–347. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Thomas RP, Farrow BJ, Kim S, May MJ,
Hellmich MR and Evers BM: Selective targeting of the nuclear
factor-kappaB pathway enhances tumor necrosis factor-related
apoptosis-inducing ligand-mediated pancreatic cancer cell death.
Surgery. 132:127–134. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dhandapani KM, Mahesh VB and Brann DW:
Curcumin suppresses growth and chemoresistance of human
glioblastoma cells via AP-1 and NFkappaB transcription factors. J
Neurochem. 102:522–538. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jani TS, DeVecchio J, Mazumdar T, Agyeman
A and Houghton JA: Inhibition of NF-kappaB signaling by quinacrine
is cytotoxic to human colon carcinoma cell lines and is synergistic
in combination with tumor necrosis factor-related
apoptosis-inducing ligand (TRAIL) or oxaliplatin. J Biol Chem.
285:19162–19172. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hannun YA: Apoptosis and the dilemma of
cancer chemotherapy. Blood. 89:1845–1853. 1997.PubMed/NCBI
|
|
29
|
Guensberg P, Wacheck V, Lucas T, Monia B,
Pehamberger H, Eichler HG and Jansen B: Bcl-xL antisense
oligonucleotides chemosensitize human glioblastoma cells.
Chemotherapy. 48:189–195. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gielen PR, Aftab Q, Ma N, Chen VC, Hong X,
Lozinsky S, Naus CC and Sin WC: Connexin43 confers Temozolomide
resistance in human glioma cells by modulating the mitochondrial
apoptosis pathway. Neuropharmacology. 75:539–548. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fennell DA: Bcl-2 as a target for
overcoming chemoresistance in small-cell lung cancer. Clin Lung
Cancer. 4:307–313. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Heckman CA, Mehew JW and Boxer LM:
NF-kappaB activates Bcl-2 expression in t(14;18) lymphoma cells.
Oncogene. 21:3898–3908. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fahy BN, Schlieman MG, Mortenson MM,
Virudachalam S and Bold RJ: Targeting Bcl-2 overexpression in
various human malignancies through NF-kappaB inhibition by the
proteasome inhibitor bortezomib. Cancer Chemother Pharmacol.
56:46–54. 2005. View Article : Google Scholar : PubMed/NCBI
|