Introduction
Parkinson’s disease (PD) is one of the most common
neurodegenerative diseases that affects >1% of the
>60-year-old population, worldwide (1). An important pathophysiological
mechanism of PD is associated with the progressive loss of
nigrostriatal dopaminergic neurons (2). With an increasing number of elderly
patients requiring surgical procedures, more patients with PD will
receive general anesthesia (3).
Sevoflurane is an inhalational general anesthetic,
with numerous beneficial properties, including low pungency, low
blood/gas partition coefficient, rapid inhalation induction and
recovery; sevoflurane, has therefore been widely used in clinical
anesthesia (4). As the use of
sevoflurane in general anesthesia has increased, there have been
increasing concerns regarding the safety of inhalational
anesthetics. Numerous studies have shown that persistent learning
deficits and social behavior dysfunction of animals may occur,
following exposure to general anesthesia (5,6). The
clinical concentration of sevoflurane has previously been shown to
inhibit neurotransmission in a dose-dependent manner, as determined
by a hippocampal slice study (7,8).
Mason et al (9)
demonstrated that inhalational anesthetics may change the
concentration of dopamine in the brain by impairing the
synaptosomes, which mediate dopaminergic transmission. Such
findings indicate that exposure to sevoflurane may affect
dopaminergic neuronal function, and influence the disease. However,
the effects of sevoflurane on patients with PD remains unknown.
The Drosophila melanogaster is an ideal model
organism that is often used in the study of neuroscience complex
biological function and disease research. Drosophila have an
intact neural circuit and a specific anatomical structure that is
beneficial for investigations into the mechanisms of
neuropharmacology, neuropathology and biochemistry (10).
The leucine-rich repeat kinase 2 (LRRK2) gene, which
is expressed in all examined tissues, spans a 144 kb genomic
region, with 51 exons encoding 2,527 amino acids. A previous study
treated mutations in the LRRK2 gene as a mature model of PD. LRRK2
mutations cause late-onset autosomal dominant PD with diverse
pathologies, including the formation of Lewy bodies, nigral
degeneration, and neurofibrillary tau-positive tangles (11). Liu et al (12) successfully generated a
LRRK2-associated Drosophila model of PD, in order to verify
that overexpression of LRRK2, which is one type of PD-associated
LRRK2 genetic mutation, led to retinal degeneration, selective loss
of dopamine (DA) neurons, and decreased climbing activity. DA is
the only catecholaminergic neurotransmitter present in the central
nervous system of Drosophila melanogaster, and it has an
important role in the progression of PD (12).
Numerous studies have demonstrated that the loss of
DA is a hallmark of PD pathology (14–16).
In Drosophila, as well as vertebrates, tyrosine hydroxylase
(TH) catalyzes the rate-limiting step in DA biosynthesis, and the
Drosophila TH gene has been shown to be specifically
expressed in all dopaminergic cells (17). The present study crossed TH-Gal4
Drosophila with upstream activation sequence-wild type LRRK2
(UAS-WT) Drosophila, in order to generate transgenic
Drosophila overexpressing LRRK2 specifically in dopaminergic
cells.
In Drosophila, mushroom bodies are critical
for associative learning and memory. Olfactory sensory neurons
receive stimulation, and emit signals to the projection neurons
(PNs), which are located in the antennal lobe. Then, PNs convey the
olfactory signals to Kenyon cells, the principal cells of the
mushroom body (18). As a paired
neuropil structure in the central brain, the mushroom bodies are
critical for associative learning and memory of
Drosophila.
The PNs are cholinergic, and through the olfactory
learning and memory circuit, nicotinic acetylcholine receptors are
crucial factors for driving the majority of spontaneous excitation.
Thus, patch clamp recordings of the miniature excitatory post
synaptic currents (mEPSCs) of PNs can be used to evaluate
cholinergic transmission in the Drosophila antennal lobe
(D). Impairment of synaptic plasticity has previously been
implicated in PD; therefore, the anomalous electrophysiological
changes of PNs are associated with the synaptic transmission
deficits that characterize neurodegenerative disease (20). The present study evaluated the
synaptic functions of the brain using a patch clamp, that recorded
electrophysiological signals of the PN from the antennal lobe.
Materials and methods
Drosophila strains
W1118 controls
W1118 Drosophila were used as controls for
comparisons with the experimental mutants used in the present
study. Both W1118 and experimental Drosophila were reared on
standard cornmeal agar medium, supplemented with dry yeast, and
maintained at 24°C in an atmosphere containing 60% relative
humidity.
Mutants
The cDNA encoding wild-type LRRK2 was obtained from
pcDNA3.1 (+) with BamHI/XhoI double digests, and
cloned into the pUAST vector at the BglII/XhoI site
(provided by Dr. Xicui Sun, Laboratory of Neurology, The First
Affiliated Hospital of Sun Yat-sen University, Guangzhou, China).
The plasmids were microinjected into W1118 Drosophila
embryos (Genetic Services, Inc., Cambridge, MA, USA), in order to
obtain UAS-WT LRRK2 Drosophila.
Cross
TH-Wild Type LRRK2 (TH-WT) transgenic
Drosophila were generated using the GAL4/UAS system, as
previously described (21), to
overexpress LRRK2 protein, specifically in the dopaminergic cells
of the brain.
Gender
In order to reduce the sampling bias, all
Drosophila used in the present study were male.
Statement of Animal Care and Use
Committee Approval
Animal handling and all experimental procedures used
in the present study were approved by the Animal Care and Use
Committee of Sun Yat-sen University (Guangzhou, China), and were
conducted in accordance with the National Institutes of Health
(NIH) Guide for the Care and Use of Laboratory Animals (NIH,
Bethesda, MA, USA).
Western blot analysis
The heads of the adult Drosophila were
collected and homogenized in lysis buffer (50 mM Tris-HCl, pH 7.5/1
mM EDTA/0.5 M NaCl/1% Triton X-100/1 mM DTT with protease
inhibitors) at 4°C for 15 min. After centrifuging at 12,000 × g for
10 min at 4°C, the supernatants were loaded onto SDS-PAGE gels,
separated by electophoresis and then transferred to polyvinylidene
fluoride membranes (0.45 mm, EMD Millipore, Billerica, MA, USA).
The membranes were blocked with TBST (pH 7.4, 10 mM TrisHCl/150 mM
NaCl/0.1% Tween 20) containing 5% fat-free milk for 2h, then probed
by incubation with a monoclonal mouse ANTI-FLAG® M2
antibody at a 1:1,000 dilution (F3165; Sigma-Aldrich, St Louis, MO,
USA), overnight at 4°C. The membranes were then incubated with a
polyclonal horseradish peroxidase (HRP)-conjugated goat-anti-mouse
immunoglobulin G secondary antibody (A3682; Sigma-Aldrich), at a
1:10,000 dilution. Chemiluminescent HRP substrate (EMD Millipore)
was used to detect the HRP, and the blots were visualized by
exposure to Kodak MR film (Kodak, Rochester, NY, USA). The
membranes were then stripped and reprobed with mouse anti-β-actin
antibody (sc-1616-R Santa Cruz) 1:4,000 dilution. β-actin was used
as a loading control. All western blot tests were performed three
times. All results have been quantified and analyzed with Image J
software version 1.46r (National Institutes of Health Bethesda, MA,
USA).
Sevoflurane exposure
All of the experimental groups were exposed to
sevoflurane three days prior to eclosion. The sevoflurane groups
(1, 2, or 3% sevoflurane exposure for 5 h) were placed in a special
anesthesia glass box, produced by the laboratory of Anatomy and
Neurology, (Zhongshan School of Medicine, Sun Yat-sen University).
The box measured 200 mm × 200 mm × 100 mm with a hole set in the
middle of the upper face for the delivery of gaseous anaesthetics
via an anesthesia machine. Air was used as a carrier and the
airflow was controlled at 2 l/min. The levels of sevoflurane,
O2 and CO2 in the chamber were monitored
using a gas monitor (GE Healthcare, Chalfont, UK), which displayed
the instantaneous gas concentration on an LCD monitor. The
temperature in the experimental room was controlled at 24°C with
60% humidity. The control groups received air without sevoflurane.
Twenty-four hours after 5 h anesthetic exposure, the anesthesia
groups were subjected to subsequent experimental procedures.
Electrophysiology
The brains were obtained from the Drosophila
two days prior to eclosion, 24 hours after sevoflurane or air
exposure. The entire brain, including the optic lobes, was removed
from the head and prepared for recordings in a standard external
solution containing 20 units/ml papain, with 1 mM L-cysteine, as
previously described (22,23). The standard external solution
contained (in mM): 101 NaCl, 1 CaCl2, 4
MgCl2, 3 KCl, 5 glucose, 1.25
NaH2PO4, and 20.7 NaHCO3, pH 7.2,
Osm 250. The dissected brains were then mounted in an RC-26
perfusion chamber (Warner Instruments, Hamden, CT, USA), and the
recording solution was bubbled through with 95% O2 and
5% CO2 (2 ml/min) during the experiment, with the
anterior brain facing up. Pipettes were targeted to PNs in the
dorsal neuron cluster of the antennal lobe.
Whole-cell recordings were performed with pipettes
(10–15 MΩ) filled with an internal solution containing the
following (in mM): 102 K-gluconate, 0.085 CaCl2, 1.7
MgCl2, 17 NaCl, 0.94 EGTA, and 8.5 HEPES with pH 7.2 and
235 mOsm. TTX was used to block sodium channels and PTX was used to
block GABA receptors as previously described (24).
The pipette solution for calcium and sodium currents (in mM)
consisted of: 102 D-gluconic acid, 102 CsOH, 0.085
CaCl2, 1.7 MgCl2, 17 NaCl, 0.94 EGTA and 8.5
HEPES, and pH 7.2, 235 Osm.
Voltage-clamp recordings were performed using
borosilicate glass electrodes (B150-86-10; Sutter Instrument Co.,
Novato, CA, USA) as previously described (24). Gigaohm seals were achieved prior to
recording the on-cell configuration, followed by the whole-cell
configuration, whilst in the voltage-clamp mode. The recordings
were made at room temperature, and only a single PN was examined in
each brain.
All electrophysiological recordings were carried out
using a BX51WI upright microscope (Olympus, Center Valley, PA,
USA). The signals were acquired using an EPC10 amplifier (HEKA
Elektronik, Lambrecht/Pfalz, Germany) and were filtered at 5 kHz,
using a built-in filter digitized at 5 kHz. Data analysis was
performed using the MiniAnalysis 6.0 program (Synaptosoft, Inc.,
Fort Lee, NJ, USA).
Climbing ability
All pupae were incubated in test tubes. After being
anesthetized, a number of the pupae were selected for
electrophysiological experiments, and the remainder were incubated
in the tube until eclosion. Following eclosion, the flies were
exposed to sevoflurane or air, and collected in order to perform
climbing assays, on days 5, 10, 15, 20, 25, 30, 35 and 40. A total
of 10 flies were placed in empty glass vials (10.5 cm × 2.5 cm). A
horizontal red line was drawn 8 cm above the bottom of the vial.
The Drosophila were allowed to accommodate to the vials for
10 min at room temperature, after which both the control and
experimental groups were assayed randomly, in a series of 10
repetitive trials for each. Before each trial, the vials were
gently tapped, in order for the Drosophila to remain at the
bottom of the vial. The number of Drosophila above the red
line of the vial was counted after 10 sec of climbing, and the same
batch of Drosophila was used to repeat the trials 10 times.
The values obtained were then averaged, and a group mean and
standard error were calculated. The mean values for the various
groups were statistically compared, using an unpaired student’s
t-test. All behavioral studies were performed at 24°C, under
standard lighting conditions.
Statistical analyses
The data are presented as the means ± standard
deviation. Comparisons between the groups were performed using a
one way analysis of variance, followed by a Bonferroni-Dunn post
hoc test or independent sample tests. All of the
electrophysiological data were analyzed using the MiniAnalysis 6.0
program (Synaptosoft Inc.). A P<0.05 was considered to indicate
a statistically significant difference.
Results
Generation of LRRK2 transgenic
Drosophila
Overexpression LRRK2 mutations are the most common
cause of familial PD, contributing to ~39% of all cases in certain
populations (25). To create a
model for the LRRK2-linked disease, transgenic Drosophila
carrying a full-length WT LRRK2 gene were generated. The WT group
was obtained by microinjecting a vector that contained UAS-LRRK2
into W1118 embryos. A Gal4/UAS bipartite system was used to
ectopically express the transgenes. This system took advantage of
the yeast GAL4 transcription factor, by binding specifically to the
UAS. Therefore, UAS-linked transgenes were expressed in specific
cells, under the control of a given promoter (promoter-GAL4). TH is
an enzyme that catalyzes the rate-limiting step in DA biosynthesis,
and is specific to dopaminergic cells (26). Therefore, TH-Gal4 was used as a
promoter, in order to generate flies which specifically
overexpressed the LRRK2 protein on dopaminergic cells (TH-WT). A
western blot analysis was conducted to confirm the overexpression
of LRRK2 in the TH-WT group. A strong band was observed at >250
kDa in the blots from the TH-WT group, that was not present in the
control W1118 or WT groups (Fig.
1). These results indicate that only TH-WT Drosophila
expressed the LRRK2 transgenes.
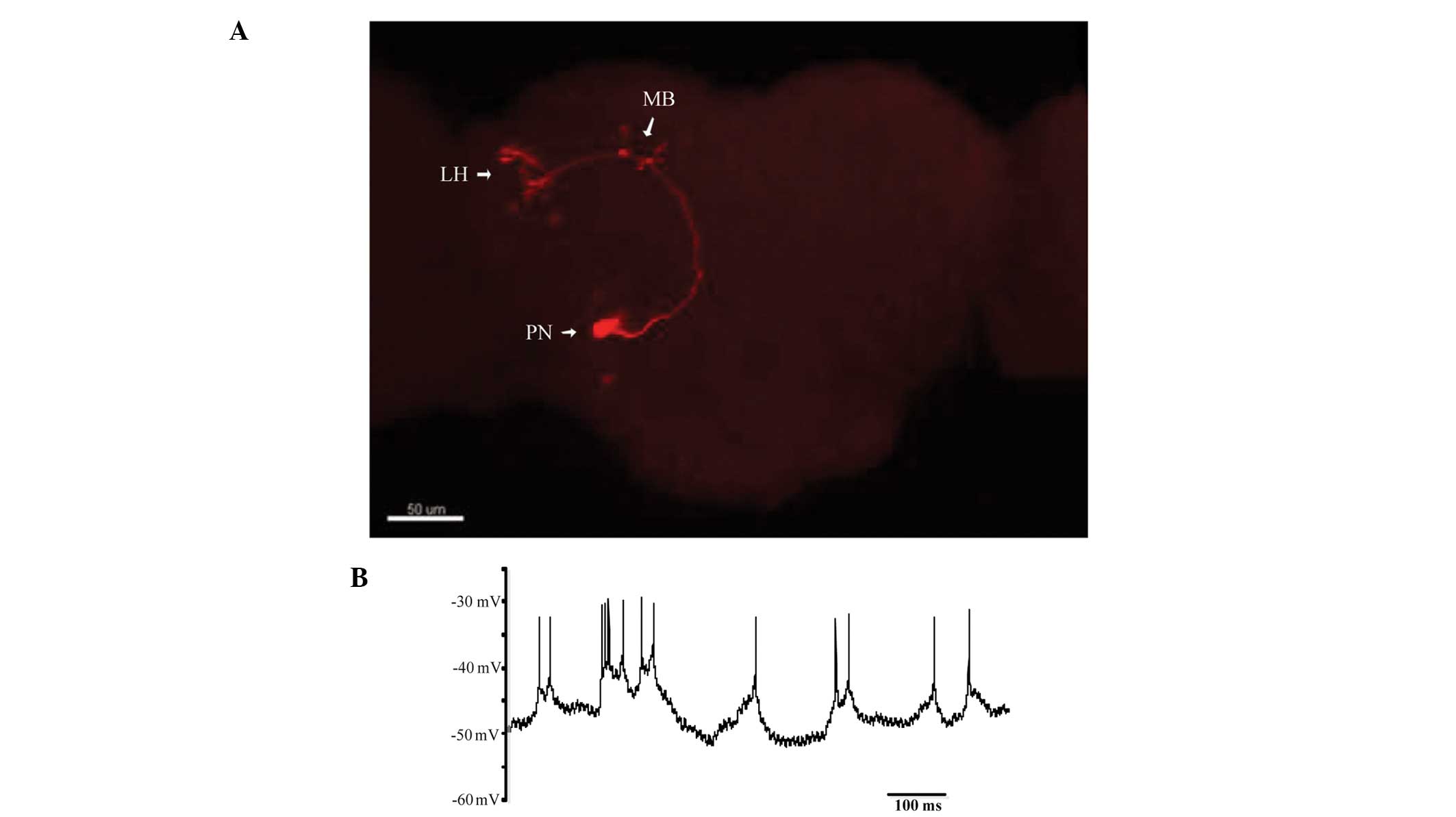
Confocal images
Confocal images of the Drosophila brain
(Fig. 2A) showed the detailed
morphology of the olfactory PN, as labeled with biocytin. In the
olfactory learning and memory system of the Drosophila, the
olfactory receptor neurons expressed the same odorant receptor that
projected their axons to the same glomerulus in the antennal lobe.
PNs sent olfactory information through their axons, to the mushroom
body and lateral horn. In the absence of stimuli, PNs continued to
receive a continuous and spontaneous barrage of excitatory
postsynaptic potentials (EPSPs), and spikes recorded in the PN
somata were <10 mV (Fig. 2B). A
nicotinic acetylcholine receptor antagonist blocked the spontaneous
EPSPs in the PNs, and the spikes were blocked by TTX. The
morphology and identity of a recorded neuron was confirmed by
injecting with 0.4% biocytin in the recording pipette for at least
30 min in the whole cell configurations. After electrophysiological
recording, the brain was fixed in phosphate buffered 4%
formaldehyde at 4°C for 10 h and then washed in 1% PBS three times,
blocked and incubated in blocking buffer (0.1 MPBS, 0.1% Triton
X-100, 1% BSA) containing streptavidin-CY3 (Molecular Devices) for
3 h at room temperature, followed by three washes at 5 min
intervals in PBS. A confocal microscope (LSM 710, Zeiss, Jena
Germany) with a ×40 objective was used to acquire photos of
dendritic arborization of the visual projection neurons.
Electrophysiological recordings
A patch clamp was used to record the
electrophysiological effects of sevoflurane on the frequency and
amplitude of mEPSCs of PNs, in the isolated Drosophila pupa
brain of the various genotypes, 24 h following exposure to
sevoflurane or air. Cholinergic mEPSCs were obtained in the
presence of 1 μmol TTX and 50 μmol pertussis toxin. The standard
characteristics of spontaneous postsynaptic currents and mEPSCs are
compared in Fig. 3A.
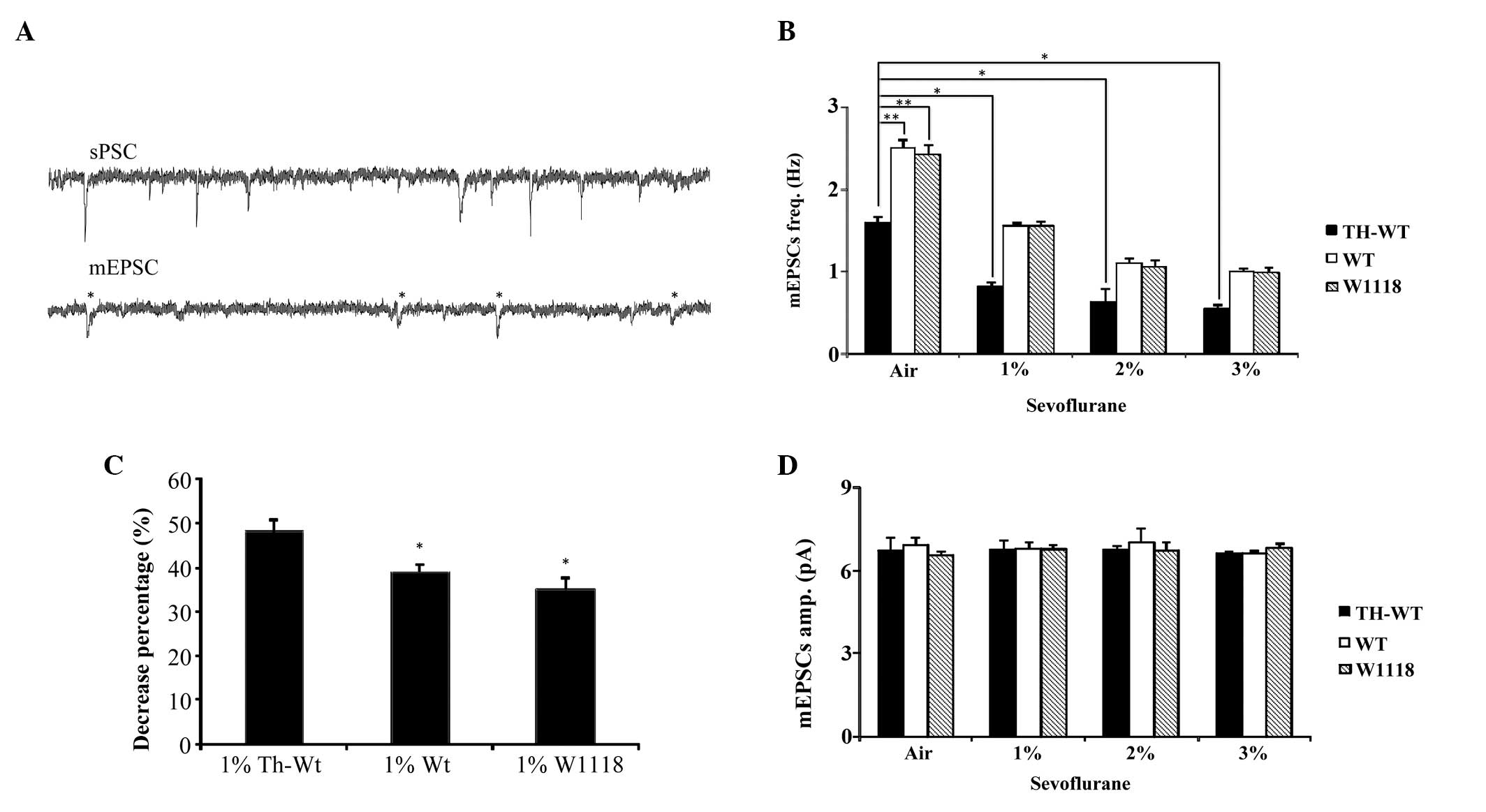 | Figure 3Miniature excitatory synaptic
currents (mEPSCs) obtained from projection neurons (PNs) of the
Drosophila brain of the three different genotypes, treated
with various concentrations of sevoflurane. (A) Representative
spontaneous postsynaptic currents (sPSCs) and mEPSCs were recorded
from PNs of the wild type (WT) Drosophila brains. The mEPSCs
wree obtained in the presence of tetrodotoxin and pertussis toxin,
*standard mEPSC. (B) The mean frequency of mEPSCs in the
tyrosine hydroxylase (TH)-WT, WT and W1118 Drosophila, under
normal conditions and treated with sevoflurane at various
concentrations. A Bonferroni post hoc test was used to
determine significance. **P<0.01;
*p<0.05. (C) The percentage decrease of the frequency
of mEPSCs, following treatment with 1%
sevoflurane.*P<0.05. (D) The mean amplitude of mEPSCs
in TH-WT, WT and W1118 Drosophila under normal conditions
and following treatment with sevoflurane at various concentrations.
TH-WT, tyrosine hydroxylase-wild type Drosophila; WT,
wild-type Drosophila; W118, control Drosophila; Hz,
hertz; freq, frequency; amp, amplification. |
The frequency of mEPSCs was compared between the
three Drosophila genotypes, following exposure to air. The
frequency of mEPSCs was markedly lower in the TH-WT group
(1.60±0.05 Hz), as compared with the WT (2.51±0.07 Hz) and W1118
(2.41±0.10 Hz) groups. The frequency of mEPSCs in the WT group was
not significantly different, as compared with the W1118 group
(Fig. 3B). These results
demonstrate that without the presence of the TH-Gal4 promoter the
mutant UAS-LRRK2 gene had no effect on the frequency of mEPSCs in
the PNs of the Drosophila. The significant decrease in the
frequency of mEPSCs of PNs in the TH-WT group may be attributed to
the successful overexpression of the LRRK2 protein in dopaminergic
cells.
The effects of sevoflurane on normal and transgenic
Drosophila is currently unknown. Therefore, in the present
study W118, WT and TH-WT Drosophila were exposed to various
concentrations of sevoflurane for 5h. In response to all three
concentrations of sevoflurane, the frequency of mEPSCs of PNs in
the normal W1118 Drosophila was significantly decreased
(1.54±0.05 Hz in 1%, 1.05±0.06 hz in 2%, and 0.97±0.05 Hz in 3%
sevoflurane), as compared with the control W1118 group, which was
exposed to air (2.41±0.10 Hz; Fig.
3B) These results indicate that sevoflurane may decrease the
frequency of mEPSCs of PNs in the normal W1118
Drosophila.
The frequency of mEPSCs in the transgenic
Drosophila were also significantly decreased, following
exposure to sevoflurane. The frequency of mEPSCs in the WT group
(1.55±0.04 Hz in 1%, 1.11±0.04 Hz in 2%, and 1.00±0.10 Hz in 3%
sevoflurane) was much lower, as compared with the WT group that was
exposed to air (2.51±0.07 Hz). In the TH-WT group, the decreased
frequency of mEPSCs was more evident (0.82±0.04 Hz in 1%, 0.63±0.16
Hz in 2%, and 0.55±0.04 Hz in 3% sevoflurane), as compared with the
group exposed to air (1.60±0.05 Hz; Fig. 3B). These results suggest that
sevoflurane may decrease the frequency of mEPSCs, not only in
normal W1118 Drosophila, but also in transgenic WT and TH-WT
Drosophila.
In order to further investigate the various effects
of sevoflurane on the three Drosophila groups, the
percentage decrease of the frequency of mEPSCs in the flies,
following exposure to sevoflurane, was determined. A concentration
of 1% sevoflurane was considered to be effective, since all three
groups presented significant decreases in the frequency of mEPSCs
of PNs following exposure to 1% sevoflurane. In addition, there
were no statistical differences between 1% sevoflurane and the
other concentrations used. The percentage decrease was compared
between the groups exposed to air and 1% sevoflurane. The decreased
frequency of mESPCs in the TH-WT flies (48.32%±3.08%) was
significantly higher, as compared with the WT (39.17%±1.42%) and
W1118 groups (35.10%±2.66%). There was no statistical difference
between the WT and W1118 groups (Fig.
3C).
Furthermore, there was no significant difference
between the amplitude of mEPSCs among the different genotypes, and
the groups treated with different concentrations of sevoflurane
(Fig. 3D). These findings indicate
that sevoflurane could affect normal W1118 flies by decreasing the
frequency of mEPSCs of PNs; however, this effect was more severe in
the TH-WT flies with PD.
Locomotor activity
PD is a movement disorder; therefore, the present
study investigated how LRRK2 overexpression affected locomotor
ability of the transgenic Drosophila, with or without
anesthesia. A climbing assay, which has been previously used in
transgenic Drosophila models of PD, was performed to assess
locomotor activity. After a single exposure to sevoflurane or air,
the Drosophila were collected in order to perform the
reduplicative climbing assays, at various time points. The climbing
ability of the W1118 and WT groups remained essentially unchanged
up to 30 days, but significantly decreased following this time
point (Fig. 4A). This finding is
concordant with the results of a previous report, by Feany and
Bender (27). The transgenic TH-WT
group presented equivalent climbing ability to the W1118 and WT
groups within 15 days. However, between days 20 and 40, the
climbing abilities of the transgenic TH-WT group gradually
decreased, as compared with the W1118 and WT groups (P<0.05,
Fig. 4A). These results indicate
that overexpression of LRRK2 in dopaminergic cells reduced the
locomotor abilities of the TH-WT flies. This reduction was detected
in the climbing assay 20 days following eclosion. However, there
was no statistically significant difference in the climbing
abilities of the WT, as compared with the W1118 Drosophila,
from day 1 to 40 after eclosion. These data suggest there were no
observable effects on locomotor ability during this time course,
following LRRK2 cDNA microinjection into the W1118
Drosophila embryos, other than overexpression of LRRK2
protein.
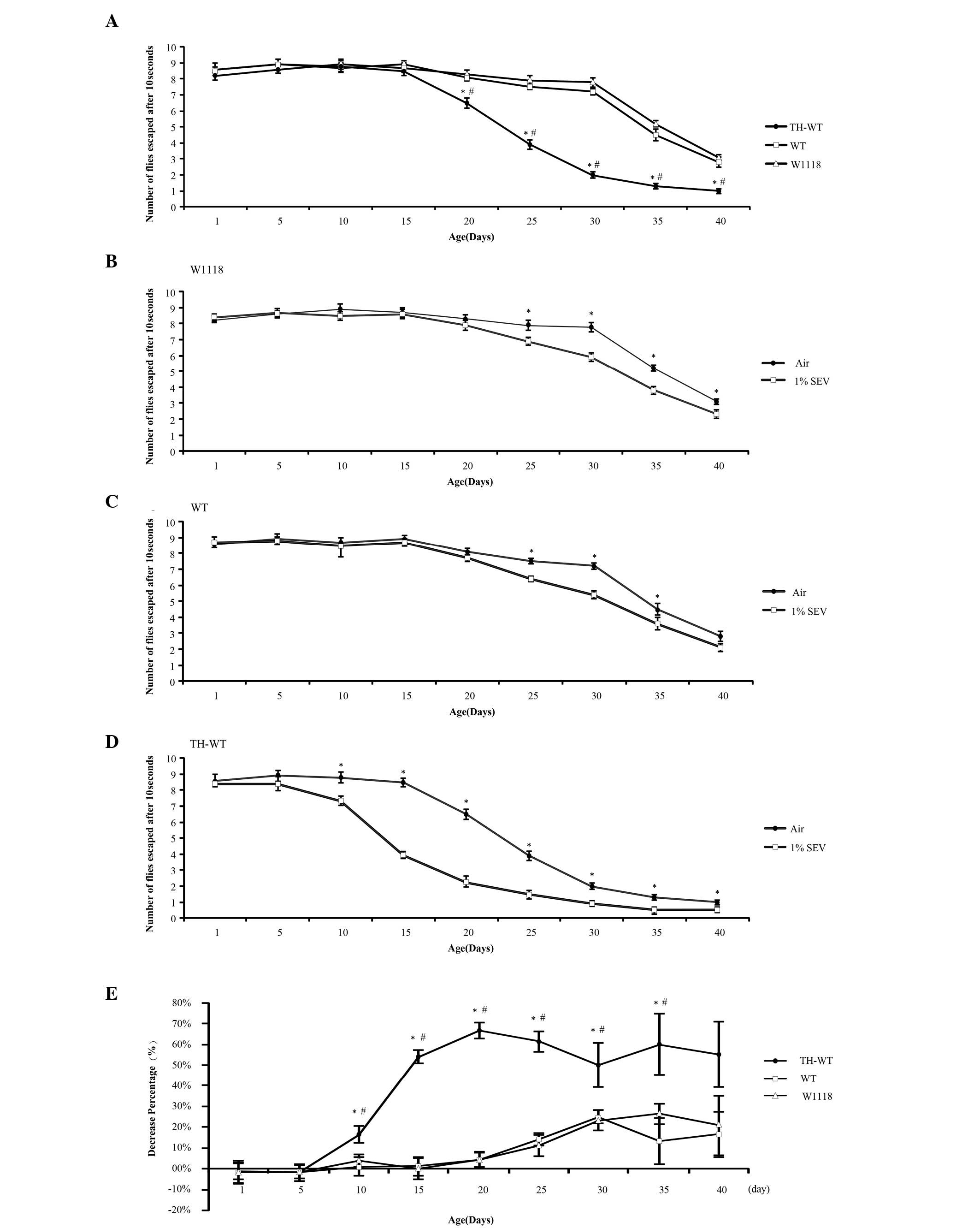 | Figure 4Sevoflurane exposure to the three
genotypes of Drosophila, resulted in various alterations to
locomotor activity. Each data point represents a group of 10
Drosophila, which underwent 10 replicated assays, to
calculate the means and error bars. (A) Climbing ability of the
transgenic and control Drosophila, from day 1–40, without
sevoflurane exposure. *P<0.05, tyrosine
hydroxylase-wild type (TH-WT) vs WT Drosophila;
#P<0.05, TH-WT vs W1118 Drosophila. (B)
Climbing ability of the W1118 Drosophila following exposure
to air and 1% sevoflurane; *P<0.05. (C) Climbing
ability of the WT Drosophila following exposure to air and
1% sevoflurane; *P<0.05. (D) Climbing ability of the
TH-WT Drosophila following exposure to air and 1%
sevoflurane; *P<0.05. (E) Percentage decrease of the
three types of Drosophila, following exposure to 1%
sevoflurane. *P<0.05, TH-WT vs WT Drosophila;
#P<0.05, TH-WT vs W1118 Drosophila. All of the
data were analyzed by a one way analysis of variance, followed by a
Bonferoni’s post hoc test. TH-WT, tyrosine hydroxylase-wild
type Drosophila; WT, wild-type Drosophila; W118,
control Drosophila. |
An electrophysiological recording was conducted to
compare the effects of the three different concentrations of
sevoflurane. A concentration of 1% sevoflurane was considered to be
effective. In the climbing assay experiment, 1% sevoflurane was
regarded as the effective concentration, for comparing the results
with the group exposed to air. In the normal W1118
Drosophila there were no differences in the locomotor
abilities, between the experimental and control groups, until 25
days after eclosion (Fig. 4B).
However, following the exposure to 1% sevoflurane, the WT flies had
significantly decreased locomotor ability, from day 25–35, as
compared with the control group (Fig.
4C). This decrease was even more evident in the transgenic
TH-WT group, in which a significant difference was observed between
days 10 and 40 (Fig. 4D). In
conclusion, sevoflurane not only decreased the locomotor abilities
of the normal W1118 and WT Drosophila, but also deteriorated
the climbing capacities of the transgenic TH-WT
Drosophila.
In order to determine the difference in the effects
of sevoflurane on the W1118 and transgenic Drosophila, the
percentage decrease of the climbing abilities was compared between
the W1118, WT and TH-WT groups, with or without exposure to 1%
sevoflurane. Notably, from day 10–40, the percentage decrease of
the climbing abilities of the TH-WT group was significantly lower,
as compared with the WT and W1118 groups. The percentage decrease
of the WT Drosophila remained similar to that of the W1118,
except at day 35. These comparisons indicate that sevoflurane led
to a more severe deterioration of locomotor ability in the TH-WT,
as compared with the WT and W1118 flies (Fig. 4E).
Discussion
Genetic mutations of LRRK2 are considered to be the
most common known genetic cause of familial PD, with a similar
clinical progression and neurochemical genotype to typical
late-onset disease. In the present study, in order to acquire a
Drosophila model with typical PD characteristics, TH-Gal4
was used as a promoter to specifically induce LRRK2 overexpression
in DA neurons. Previous studies have successfully established a
Drosophila model of PD using the Gal4/UAS system, to
generate transgenic Drosophila overexpressing wild-type
LRRK2 (12). Based on the various
types of Gal4 promoter, researchers may specifically overexpress
LRRK2 in any cell type, depending on their requirements. DA neurons
have important roles in the pathogenesis of PD, and the
overexpression of LRRK2 in DA neurons can lead to severe DA lesions
and apoptosis (13). A previous
study demonstrated that overexpression of LRRK2 in all neurons,
under the control of the pan neuronal promoter elav-GAL4, lead to a
less severe genotype in flies, as compared with those specifically
overexpressing LRRK2 in DA neurons. However, the protein expression
levels were higher in the head homogenates of the elav-GAL4 LRRK2
Drosophila. LRRK2 triggers the loss of anti-TH
immunostaining; however, there is no significant loss in anti-elav
or anti-5-HT immunostaining (28–30).
These findings may explain why the protein expression levels of
LRRK2 in DA neurons are reduced by elav-GAL4, indicating that
LRRK2-induced toxicity is preferentially localized to DA neurons in
the brain, which is concordant with human PD. TH catalyzes the
rate-limiting step in DA biosynthesis, therefore the
Drosophila TH gene is specifically expressed in all
dopaminergic cells (17).
As previously reported, mEPSCs of PNs in the
Drosophila central nervous system are associated with
synaptic stability and plasticity (31). Therefore, mEPSCs may have a
critical role in the functional and structural aspects of the
synapses of PNs (32). Talantova
et al (33) previously
demonstrated that a decreased frequency of mEPSCs may cause early
synaptic injury, due to concurrent extrasynaptic
N-methyl-D-aspartare receptor-mediated nitric oxide production, tau
phosphorylation, and caspase-3 activation. In the present study,
the frequency of mEPSCs in the PNs of transgenic TH-WT
Drosophila brains was significantly decreased, following
exposure to air, as compared with the W1118 and WT groups. These
results suggest that the synaptic transmission of Drosophila
with PD was lower, as compared with the normal Drosophila.
The frequency of mEPSCs of PNs in the transgenic TH-WT
Drosophila may be declined due to disorder of the
dopamine-cholinergic system.
In addition, mEPSCs are thought to be involved in
synaptic plasticity in the Drosophila central nervous system
(32–34). mEPSCs are evoked by single vesicle
release, which is triggered by release of presynaptic calcium ions
(35). Alterations to the
frequency and amplitude of mEPSCs are ascribed to presynaptic and
postsynaptic action (36). The
amplitude of mEPSCs reflects the response of the postsynaptic
receptor to a single vesicle, while the frequency is partially due
to changes to the presynaptic calcium channel (35). In the present study, following
exposure to various concentrations of sevoflurane, the frequency of
mEPSCs of PNs in the normal W1118 flies was significantly
decreased. These results indicate that sevoflurane may affect
normal W1118 Drosophila by reducing the presynaptic calcium
channels of PNs. Conversely, the amplitude of mEPSCs remained the
same. Therefore, the effects of sevoflurane on postsynaptic action
are not as evident as they are on presynaptic calcium ions.
Furthermore, the WT and TH-WT groups exhibited a similar decrease
in the frequency of mEPSCs of PNs, as compared with the W1118
group. However, the differences of the negative effects between the
three concentrations of sevoflurane on the frequency of mEPSCs were
not as significant as initially predicted. Therefore, 1%
sevoflurane was considered to be an effective concentration and was
used for all further experiments.
Percentage decreases were calculated by comparing
the frequency of mEPSCs in the Drosophila exposed to 1%
sevoflurane, with the Drosophila exposed to air. The
percentage decrease of TH-WT group (48.32%±3.08%) was significantly
higher, as compared with the WT (39.17%±1.42%) and W1118 groups
(35.10%±2.66%), and there was no statistical difference between the
WT and W1118 groups. These results indicate that sevoflurane may
cause more severe effects on Drosophila with PD. The
possible mechanism of anesthetic-induced PD related mEPSC
impairment may be due to the potential effectss of the anesthetics,
on synaptic morphology and function. It may be hypothesized that
sevoflurane may regulate synaptic developmental processes and
modulate aberrant synaptic formation or ectopic neuron
distribution, leading to impairment of synaptic plasticity and
maturation, which have already been damaged by the progression of
PD (37). In the present study,
sevoflurane markedly reduced the frequency of mEPSCs of
Drosophila with PD, and this may be due to the basic
impairment of synaptic plasticity implicated in PD.
Movement disorder is a common symptom of patients
with PD (38). As compared with
the immediate effects observed on mEPSCs, the effects of LRRK2
overexpression on locomotor behavior are chronic, which become more
significant as the Drosophila age (39). Furthermore, the life span of the
W1118 Drosophila is ~45.5 days following eclosion (40); however, it is even longer in the
transgenic LRRK2 Drosophila (25). As a result, the locomotor abilities
of the Drosophila were assessed between days 1 and 40
following eclosion. The climbing abilities of the W1118 and WT
groups remained unchanged before 30 days, but gradually declined
thereafter. This finding indicates that, without being activated by
TH-Gal4, the locomotor activities of both the WT and W1118
Drosophila were identical. A vector containing UAS-WT LRRK2
with a TH-Gal4 promoter resulted in the successful generation of
TH-WT Drosophila with an overexpression of the LRRK2
protein. During the climbing assay, the climbing abilities of the
TH-WT group were significantly decreased from day 20–40 following
eclosion, indicating that the overexpression of LRRK2 led to
locomotor impairment in the Drosophila after day 20.
Previous studies have demonstrated that such impairment is
associated with the loss of DA neurons in the brain (41,42).
Therefore, it may be speculated that locomotor impairment of the
Drosophila overexpressing LRRK2, specifically in the DA
neurons, may result from the loss of dopaminergic transmission.
In the present study, the climbing abilities of the
W1118 Drosophila following exposure to 1% sevoflurane were
significantly decreased from day 25–35, suggesting that sevoflurane
could deteriorate the locomotor ability of the W1118
Drosophila; however, such deterioration could only be
distinguished 25 days following eclosion. After exposure to
sevoflurane during pupa, the synaptosomes in the brains of the
Drosophila may deteriorate, resulting in deficits to
locomotor ability. Deterioration also occurred in the transgenic WT
group, that was quite similar to that of the W1118 group.
As Mason et al (9) previously suggested, inhalational
anesthetics may change the concentration of DA in the brain by
impairing the synaptosomes that mediate dopaminergic transmission;
therefore, it may be deduced that the negative effects of
sevoflurane could be found in patients with PD. In the present
study, sevoflurane appeared to cause markedly severe damage to the
locomotor ability of TH-WT Drosophila, whose climbing
ability significantly decreased from day 10. In order to verify the
differences in the effects of sevoflurane between the W1118 and
transgenic groups, the percentage decreases were compared between
the W1118, WT and TH-WT, which were calculated by comparing the
frequency of mEPSCs of the Drosophila exposed to 1%
sevoflurane, with the Drosophila exposed to air. Notably,
between days 10 and 40, the percentage decrease of the climbing
abilities of the TH-WT group was significantly lower, as compared
with the WT and W1118 groups. These comparisons indicate that
sevoflurane led to a more severe deterioration in the locomotor
ability of the TH-WT, as compared with the WT and W1118 groups.
Overexpression of LRRK2 in dopaminergic cells leads to
neurofibrillary tau-positive tangles, nigral degeneration, Lewy
bodies and specific loss of dopaminergic neurons in the aging
brain; which may be the cause of the deteriorating locomotor
abilities in the Drosophila overexpressing LRRK2, 15 days
after eclosion (43,44).
Sevoflurane increases brain extracellular concentrations of DA
during general anesthesia by impairing the transport synaptosomes
of DA. Such mechanisms may lead to further damage of the
dopaminergic system and locomotor dysfunction (9).
In conclusion, sevoflurane not only had negative
effects on normal W1118 Drosophila, but also severely
aggravated the prognosis of PD in a LRRK2-associated
Drosophila model, by means of synaptic cholinergic deficits
and impairment of locomotor abilities. The possibility of other
impairments in the prognosis of PD by sevoflurane, however, remain
uncertain. There are also doubts as to whether sevoflurane will
have the same effects on humans with PD. Therefore, further
investigations are required in order to fully understand the
effects of sevoflurane on patients with PD.
Acknowledgements
The authors of the present study would like to thank
Xicui Sun, Peisen Huang and Runcong Nie for instructions regarding
the successful creation of a cross-transgenic model. They would
also like to acknowledge the help of Youlan Li in proofreading the
manuscript and data analysis. This study was supported by a grant
from the Guangdong Science Foundations (grant no.
S2011010003739).
References
|
1
|
Nicholson G, Pereira AC and Hall GM:
Parkinson’s disease and anaesthesia. Br J Anaesth. 89:904–916.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Belin AC and Westerlund M: Parkinson’s
disease: a genetic perspective. FEBS J. 275:1377–1383. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
García-Pérez L, Linertová R, Lorenzo-Riera
A, Vázquez-Díaz JR, Duque-González B and Sarría-Santamera A: Risk
factors for hospital readmissions in elderly patients: a systematic
review. QJM. 104:639–651. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang X, Xue Z and Sun A: Subclinical
concentration of sevoflurane potentiates neuronal apoptosis in the
developing C57BL/6 mouse brain. Neurosci Lett. 447:109–114. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Culley DJ, Baxter MG, Yukhananov R and
Crosby G: Long-term impairment of acquisition of a spatial memory
task following isoflurane-nitrous oxide anesthesia in rats.
Anesthesiology. 100:309–314. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Satomoto M, Satoh Y, Terui K, et al:
Neonatal exposure to sevoflurane induces abnormal social behaviors
and deficits in fear conditioning in mice. Anesthesiology.
110:628–637. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Haseneder R, Kratzer S, von Meyer L, Eder
M, Kochs E and Rammes G: Isoflurane and sevoflurane
dose-dependently impair hippocampal long-term potentiation. Eur J
Pharmacol. 623:47–51. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ishizeki J, Nishikawa K, Kubo K, Saito S
and Goto F: Amnestic concentrations of sevoflurane inhibit synaptic
plasticity of hippocampal CA1 neurons through gamma-aminobutyric
acid-mediated mechanisms. Anesthesiology. 108:447–456. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mason LJ, Cojocaru TT and Cole DJ:
Surgical intervention and anesthetic management of the patient with
Parkinson’s disease. Int Anesth Clin. 34:133–150. 1996. View Article : Google Scholar
|
|
10
|
Ng M, Roorda RD, Lima SQ, Zemelman BV,
Morcillo P and Miesenböck G: Transmission of olfactory information
between three populations of neurons in the antennal lobe of the
fly. Neuron. 36:463–474. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zimprich A, Biskup S, Leitner P, et al:
Mutations in LRRK2 cause autosomal-dominant parkinsonism with
pleomorphic pathology. Neuron. 44:601–607. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu Z, Wang X, Yu Y, et al: A Drosophila
model for LRRK2-linked parkinsonism. Proc Natl Acad Sci USA.
105:2693–2698. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Choi DC, Chae YJ, Kabaria S, et al:
MicroRNA-7 Protects against 1-Methyl-4-Phenylpyridinium-Induced
Cell Death by Targeting RelA. J Neuroscience. 34:12725–12737. 2014.
View Article : Google Scholar
|
|
14
|
White KE, Humphrey DM and Hirth F: The
dopaminergic system in the aging brain of Drosophila. Front
Neurosci. 4:2052010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Braak H and Del Tredici K: Invited
Article: Nervous system pathology in sporadic Parkinson disease.
Neurology. 70:1916–1925. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Obeso JA, Rodriguez-Oroz MC, Goetz CG, et
al: Missing pieces in the Parkinson’s disease puzzle. Nat Med.
16:653–661. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Friggi-Grelin F, Coulom H, Meller M, Gomez
D, Hirsh J and Birman S: Targeted gene expression in Drosophila
dopaminergic cells using regulatory sequences from tyrosine
hydroxylase. J Neurobiol. 54:618–627. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ueno K, Naganos S, Hirano Y, Horiuchi J
and Saitoe M: Long-term enhancement of synaptic transmission
between antennal lobe and mushroom body in cultured Drosophila
brain. Physiology. 591:287–302. 2013. View Article : Google Scholar
|
|
19
|
D’Souza RD, Parsa PV and Vijayaraghavan S:
Nicotinic receptors modulate olfactory bulb external tufted cells
via an excitation-dependent inhibitory mechanism. J
Neurophysiology. 110:1544–1553. 2013. View Article : Google Scholar
|
|
20
|
Ran D, Cai S, Wu H and Gu H: Di
(2-ethylhexyl) phthalate modulates cholinergic mini-presynaptic
transmission of projection neurons in Drosophila antennal lobe.
Food Chem Toxicol. 50:3291–3297. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Brand AH and Perrimon N: Targeted gene
expression as a means of altering cell fates and generating
dominant phenotypes. Development. 118:401–15. 1993.PubMed/NCBI
|
|
22
|
Gu H and O’Dowd DK: Cholinergic synaptic
transmission in adult Drosophila Kenyon cells in situ. J Neurosci.
26:265–272. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gu H and O’Dowd DK: Whole cell recordings
from brain of adult Drosophila. J Vis Exp. (6): 2482007.
|
|
24
|
Yan Y, Yang Y, You J, et al: Permethrin
modulates cholinergic mini-synaptic currents by partially blocking
the calcium channel. Toxicol Lett. 201:258–263. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Venderova K, Kabbach G, Abdel-Messih E, et
al: Leucine-Rich Repeat Kinase 2 interacts with Parkin, DJ-1 and
PINK-1 in a Drosophila melanogaster model of Parkinson’s disease.
Hum Mol Genet. 18:4390–4404. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tai Y, Chen L, Huang E, et al: Protective
effect of alpha-synuclein knockdown on methamphetamine-induced
neurotoxicity in dopaminergic neurons. Neural regeneration
research. 9:951–958. 2014. View Article : Google Scholar : PubMed/NCBI
Feany MB and Bender WW: A Drosophila model
of Parkinson’s disease. Nature. 404:394–398. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dauer W and Przedborski S: Parkinson’s
disease: mechanisms and models. Neuron. 39:889–909. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Forno LS: Neuropathology of Parkinson’s
disease. J Neuropathol Exp Neurol. 55:259–272. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mouradian MM: Recent advances in the
genetics and pathogenesis of Parkinson disease. Neurology.
58:179–185. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yamasaki M, Hashimoto K and Kano M:
Miniature synaptic events elicited by presynaptic Ca2+
rise are selectively suppressed by cannabinoid receptor activation
in cerebellar Purkinje cells. J Neurosci. 26:86–95. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang J, Yang Y, Li H, Cao J and Xu L:
Amplitude/frequency of spontaneous mEPSC correlates to the degree
of long-term depression in the CA1 region of the hippocampal slice.
Brain Res. 1050:110–117. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Talantova M, Sanz-Blasco S, Zhang X, et
al: Aβ induces astrocytic glutamate release, extrasynaptic NMDA
receptor activation, and synaptic loss. Proc Natl Acad Sci USA.
110:E2518–E2527. 2013. View Article : Google Scholar
|
|
33
|
Yasuyama K, Meinertzhagen IA and Schürmann
FW: Synaptic organization of the mushroom body calyx in Drosophila
melanogaster. J Comp Neurol. 445:211–226. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Su H and O’Dowd DK: Fast synaptic currents
in Drosophila mushroom body Kenyon cells are mediated by
alpha-bungarotoxin-sensitive nicotinic acetylcholine receptors and
picrotoxin-sensitive GABA receptors. J Neurosci. 23:9246–9253.
2003.PubMed/NCBI
|
|
35
|
Simkus CR and Stricker C: The contribution
of intracellular calcium stores to mEPSCs recorded in layer II
neurones of rat barrel cortex. J Physiol. 545:521–535. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kato R, Tachibana K, Nishimoto N, et al:
Neonatal exposure to sevoflurane causes significant suppression of
hippocampal long-term potentiation in postgrowth rats. Anesth
Analg. 117:1429–1435. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shaltiel-Karyo R, Davidi D, Menuchin Y, et
al: A novel, sensitive assay for behavioral defects in Parkinson’s
disease model Drosophila. Parkinsons Dis. 2012:6975642012.
|
|
38
|
Kwon Y, Kim JW, Ho Y, et al: Analysis of
antagonistic co-contractions with motorized passive movement device
in patients with parkinson’s disease. Bio-medical materials and
engineering. 24:2291–2297. 2014.
|
|
39
|
Oxenkrug GF: The extended life span of
Drosophila melanogaster eye-color (white and vermilion) mutants
with impaired formation of kynurenine. J Neural Transm. 117:23–26.
2010. View Article : Google Scholar
|
|
40
|
Jenner P and Olanow CW: The pathogenesis
of cell death in Parkinson’s disease. Neurology. 66:S24–S36. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Reynolds NP, Soragni A, Rabe M, et al:
Mechanism of membrane interaction and disruption by α-synuclein. J
Am Chem Soc. 133:19366–19375. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Rajput A, Dickson DW, Robinson CA, et al:
Parkinsonism, Lrrk2 G2019S, and tau neuropathology. Neurology.
67:1506–1508. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ross OA, Toft M, Whittle AJ, et al: Lrrk2
and Lewy body disease. Ann Neurol. 59:388–393. 2006. View Article : Google Scholar : PubMed/NCBI
|