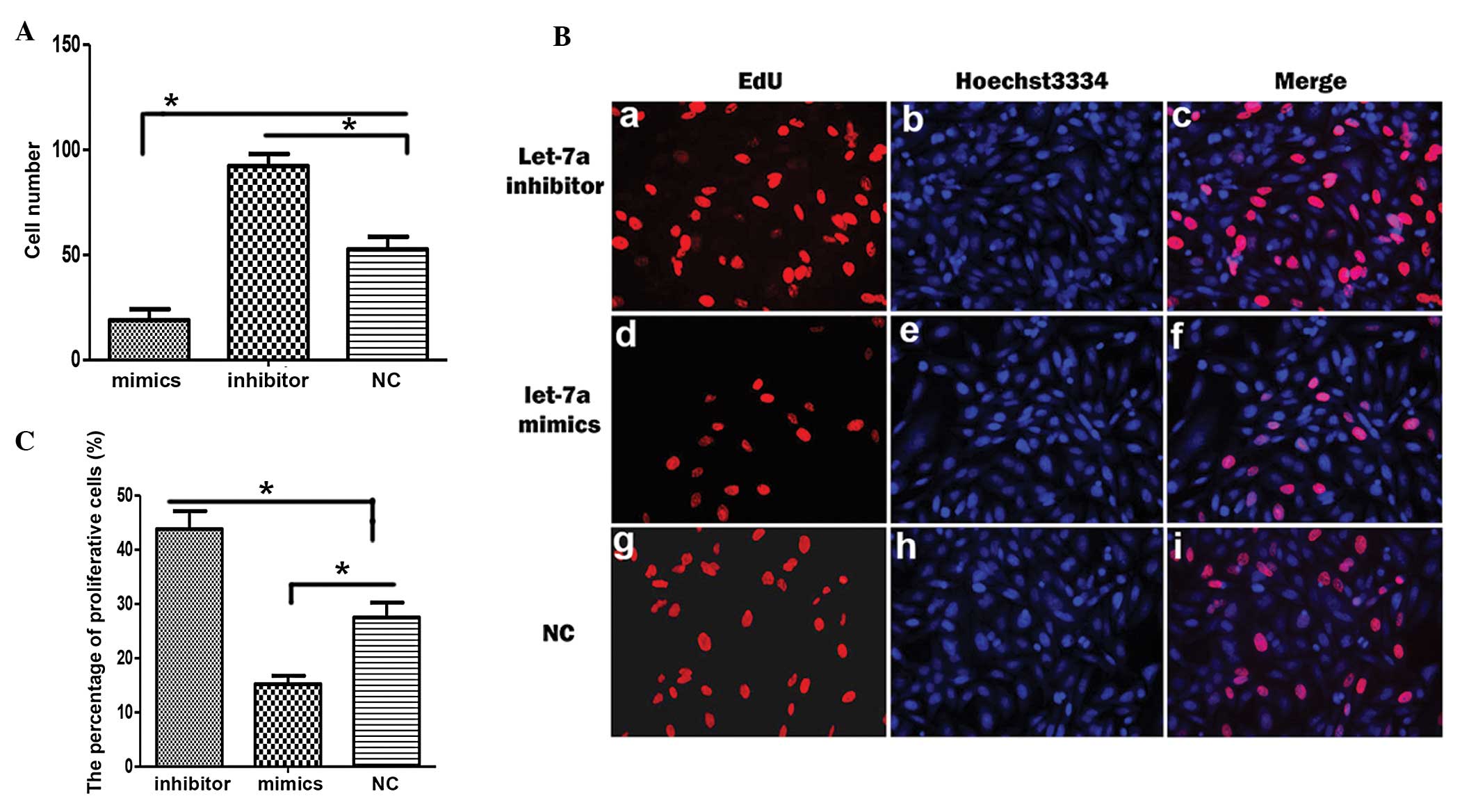
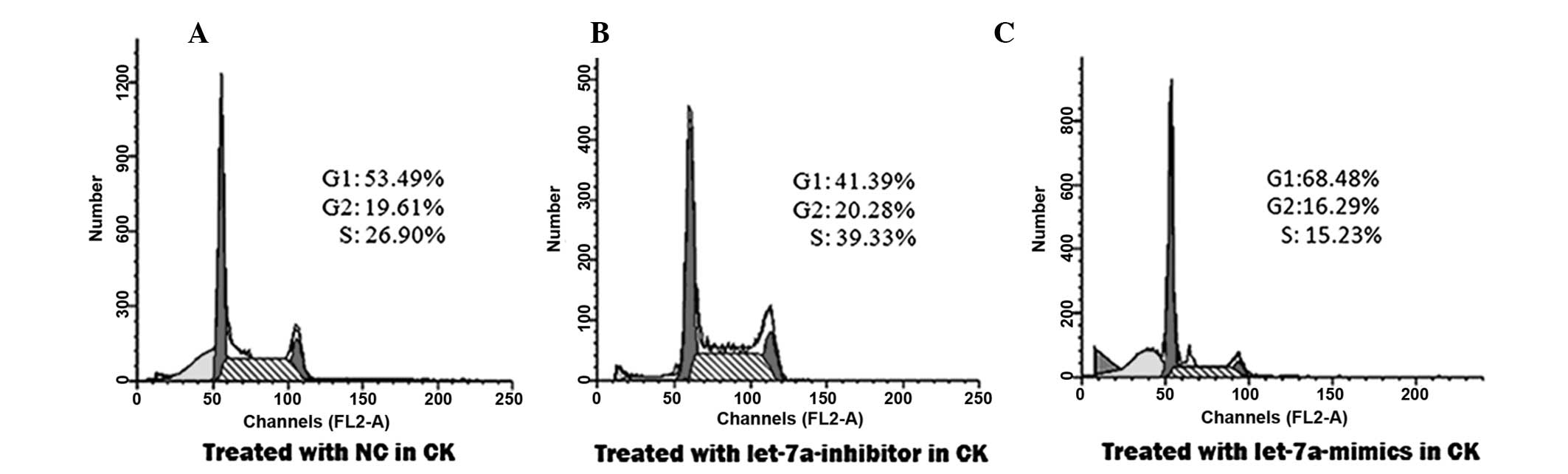
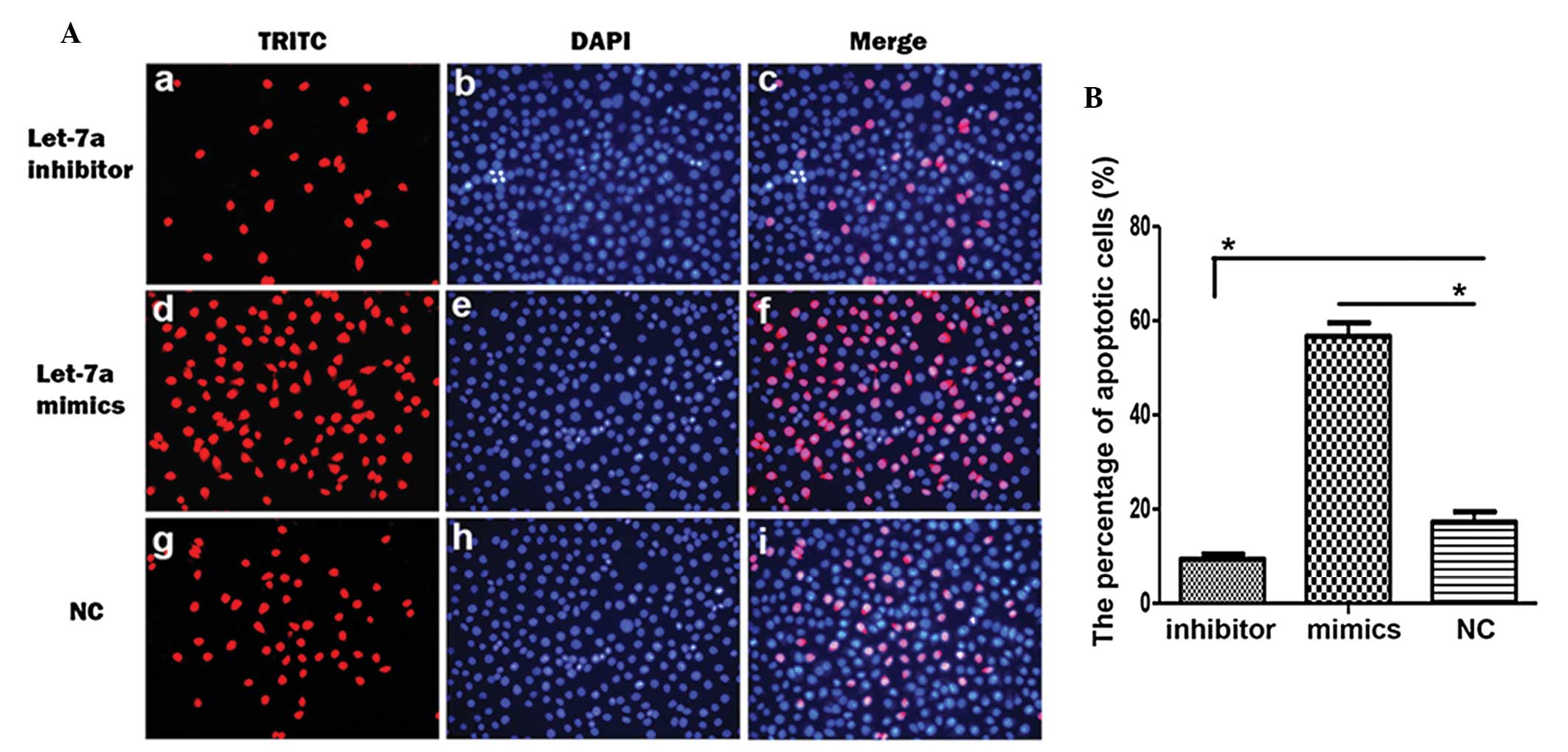
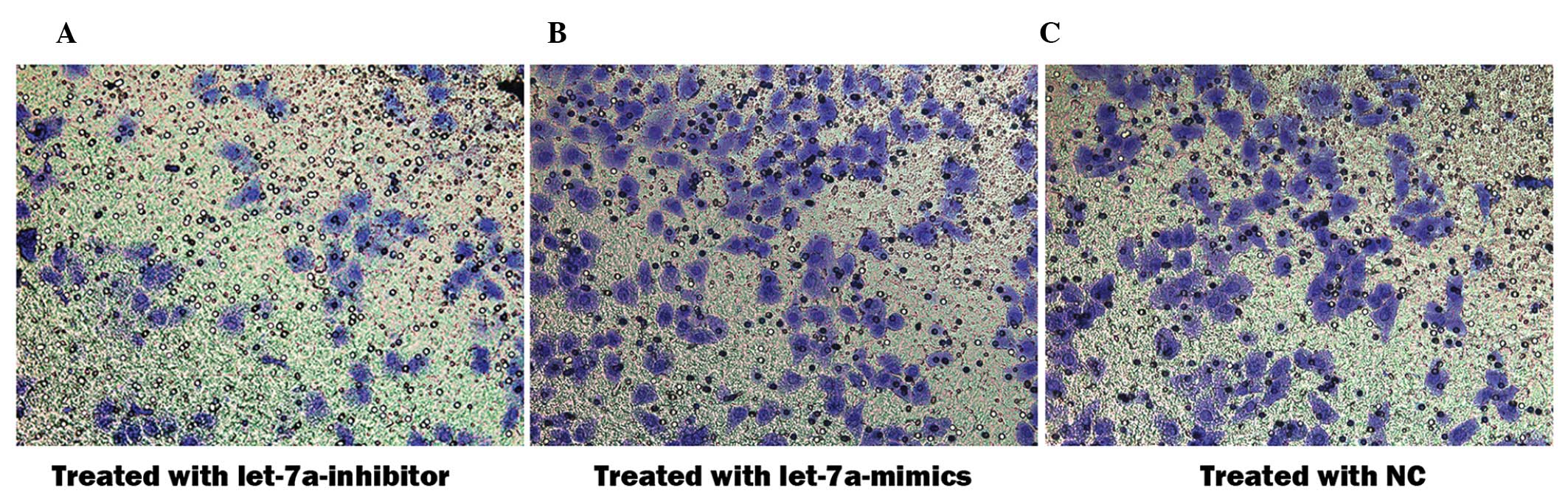
|
1
|
Louw L: Acquired cholesteatoma: summary of
the cascade of molecular events. J Laryngol Otol. 127:542–549.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Preciado DA: Biology of cholesteatoma:
special considerations in pediatric patients. Int J Pediatr
Otorhinolaryngol. 76:319–321. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Alves AL, Pereira CS, de Carvalho MF,
Fregnani JH and Ribeiro FQ: EGFR expression in acquired middle ear
cholesteatoma in children and adults. Eur J Pediatr. 171:307–310.
2012. View Article : Google Scholar
|
|
4
|
Barbara M, Raffa S, Murè C, et al:
Keratinocyte growth factor receptor (KGF-R) in cholesteatoma
tissue. Acta Otolaryngol. 128:360–364. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jin BJ, Min HJ, Jeong JH, Park CW and Lee
SH: Expression of EGFR and microvessel density in middle ear
cholesteatoma. Clin Exp Otorhinolaryngol. 4:67–71. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yamamoto-Fukuda T, Takahashi H and Koji T:
Expression of keratinocyte growth factor (KGF) and its receptor in
a middle-ear cavity problem. Int J Pediatr Otorhinolaryngol.
76:76–81. 2012. View Article : Google Scholar
|
|
7
|
Kuczkowski J, Bakowska A, Pawelczyk T,
Narozny W and Mikaszewski B: Cell cycle inhibitory protein p27 in
human middle ear cholesteatoma. ORL J Otorhinolaryngol Relat Spec.
68:296–301. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu W, Ren H, Ren J, et al: The role of
EGFR/PI3K/Akt/cyclinD1 signaling pathway in acquired middle ear
cholesteatoma. Mediators Inflamm. 2013:6512072013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sakamoto T, Kondo K, Yamasoba T, et al:
Overexpression of ErbB-2 protein in human middle ear
cholesteatomas. Laryngoscope. 114:1988–1991. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen X and Qin Z: Post-transcriptional
regulation by microrna-21 and let-7a microRNA in paediatric
cholesteatoma. J Int Med Res. 39:2110–2118. 2011. View Article : Google Scholar
|
|
11
|
Friedland DR, Eernisse R, Erbe C, Gupta N
and Cioffi JA: Cholesteatoma growth and proliferation:
posttranscriptional regulation by microRNA-21. Otol Neurotol.
30:998–1005. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Djuranovic S, Nahvi A and Green R: A
parsimonious model for gene regulation by miRNAs. Science.
331:550–553. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bartel DP: MicroRNAs: genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Akimoto R, Pawankar R, Yagi T and Baba S:
Acquired and congenital cholesteatoma: determination of tumor
necrosis factor-alpha, intercellular adhesion molecule-1,
interleukin-1-alpha and lymphocyte functional antigen-1 in the
inflammatory process. ORL J Otorhinolaryngol Relat Spec.
62:257–265. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bujía J, Kim C, Boyle D, et al:
Quantitative analysis of interleukin-1-alpha gene expression in
middle ear cholesteatoma. Laryngoscope. 106:217–220. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bujia J, Kim C, Ostos P, et al:
Interleukin 1 (IL-1) and IL-1-receptor antagonist (IL-1-RA) in
middle ear cholesteatoma: an analysis of protein production and
biological activity. Eur Arch Otorhinolaryngol. 253:252–255. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kato A, Ohashi Y, Masamoto T, et al:
Interleukin-6 and tumour necrosis factor alpha synthesized by
cholesteatoma cells affect mucociliary function in the eustachian
tube. Acta Otolaryngol. 38(Suppl 5): 90–97. 1998.
|
|
18
|
Mehta D, Daudia A, Birchall JP and
Banerjee AR: The localization of matrix metalloproteinases-8 and
-13 in cholesteatoma, deep-meatal and post-auricular skin: a
comparative analysis. Acta Otolaryngol. 127:138–142. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Thornton JE and Gregory RI: How does Lin28
let-7 control development and disease? Trends Cell Biol.
22:474–482. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wong TS, Man OY, Tsang CM, et al: MicroRNA
let-7 suppresses nasopharyngeal carcinoma cells proliferation
through downregulating c-Myc expression. J Cancer Res Clin Oncol.
137:415–422. 2011. View Article : Google Scholar :
|
|
21
|
Childs G, Fazzari M, Kung G, et al:
Low-level expression of microRNAs let-7d and miR-205 are prognostic
markers of head and neck squamous cell carcinoma. Am J Pathol.
174:736–745. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mayr C, Hemann MT and Bartel DP:
Disrupting the pairing between let-7 and Hmga2 enhances oncogenic
transformation. Science. 315:1576–1579. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Johnson SM, Grosshans H, Shingara J, et
al: RAS is regulated by the let-7 microRNA family. Cell.
120:635–647. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Park SM, Shell S, Radjabi AR, et al: Let-7
prevents early cancer progression by suppressing expression of the
embryonic gene HMGA2. Cell Cycle. 6:2585–2590. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Frankel LB, Christoffersen NR, Jacobsen A,
et al: Programmed cell death 4 (PDCD4) is an important functional
target of the microRNA miR-21 in breast cancer cells. J Biol Chem.
283:1026–1033. 2008. View Article : Google Scholar
|
|
26
|
Zhao H, Dupont J, Yakar S, Karas M and
LeRoith D: PTEN inhibits cell proliferation and induces apoptosis
by downregulating cell surface IGF-IR expression in prostate cancer
cells. Oncogene. 23:786–794. 2004. View Article : Google Scholar : PubMed/NCBI
|