Introduction
Atherosclerosis is a chronic inflammatory disease of
the artery wall, which involves multiple cell types of different
origins, and complex intracellular interactions and signaling
pathways (1–3). Understanding the function of genes
involved in atherosclerosis has been given much attention by
scientists and clinicians over recent years (4). Connexins are members of a family of
proteins encoded by at least 20 different mammalian genes, and are
expressed in a wide variety of tissues (5–7).
Connexins form transmembrane channels known as gap junctions, which
connect neighboring cells and allow passive diffusion of small
molecules (8). Connexin 37 (Cx37)
is a junction protein that has a central function in initiating the
inflammatory response. In a previous study by our group (9), it was found that the C allele of the
Cx37 gene is associated with susceptibility to coronary heart
disease, particularly in the male population. Boerma et al
(10) identified a link between a
single nucleotide polymorphism (SNP) in the human Cx37 gene and a
thickening of the carotid artery in the Swedish male population, in
which the C allele was over-represented in individuals with
atherosclerotic plaques. The C allele of this SNP has also been
associated with coronary heart disease in individuals from Taiwan,
northern China and Switzerland (11–13).
Subsequently, two studies performed on Japanese and Caucasian
populations revealed that T-SNP is a risk factor for acute
myocardial infarction (AMI), particularly in high-risk male
individuals (14,15). Seifi et al (16) suggested that the polymorphism in
the Cx37 gene has a significant effect on the manifestation of AMI
disease in Iranian individuals. However, Cx37 deletion in
apolipoprotein E-deficient mice was shown to increase
susceptibility to atherosclerosis (17).
Gene knockout studies have identified that Cx37
forms gap junction channels between endothelial cells. Two
polymorphic Cx37 variants (Cx37-S319 and Cx37-P319) have been
identified with a possible link to atherosclerosis (18). Although these results are
encouraging, the relative contribution and synergistic effects of
Cx37 on the formation of atherosclerotic plaques remain elusive.
The present study therefore hypothesized that Cx37 may affect
atherosclerotic plaque formation.
Schecter et al (19) delivered small interfering RNAs
(siRNAs) with lentiviruses, which have proven to be effective in
silencing target genes by means of RNA interference (RNAi). A
previous study showed that atherosclerosis in a pig model was most
similar to the condition in humans (20). In the present study, a lentiviral
vector was constructed to knockdown Cx37 and elucidate its role in
effecting atherosclerotic plaques in pigs. This vector was observed
by intravascular ultrasound (IVUS).
Materials and methods
Cell culture
HEK 293 cells were obtained from the Cell Bank of
the Chinese Academy of Science (Beijing, China). The cells were
cultured using previously reported methods (21), and maintained at 37°C, with 5%
CO2 in complete RPMI medium (Gibco-BRL, Carlsbad, CA,
USA). When the cell fusion rate reached 90%, the cells were divided
at a ratio of 1:4 and frozen in the logarithmic growth phase. Cell
recovery took place when the cells did not grow efficiently.
Preparation of lentiviral vectors and
target screening for RNAi
Three different sequences (sites A, B and C) of the
pig Cx37 gene were selected from GenBank as a target for RNAi. The
targeted sequence of mm-cx37-si-1 was as follows:
5′-GGUUAACGGUGCUCUUCAU-′3 location: 209; Targeted sequence of
mm-cx37-si-2: 5′-CCA AGGACCUACAUGUAGA-′3 location: 488; Targeted
sequence of mm-cx37-si-3: 5′-CAGACCCUUACCCUGAACA-′3 location: 841.
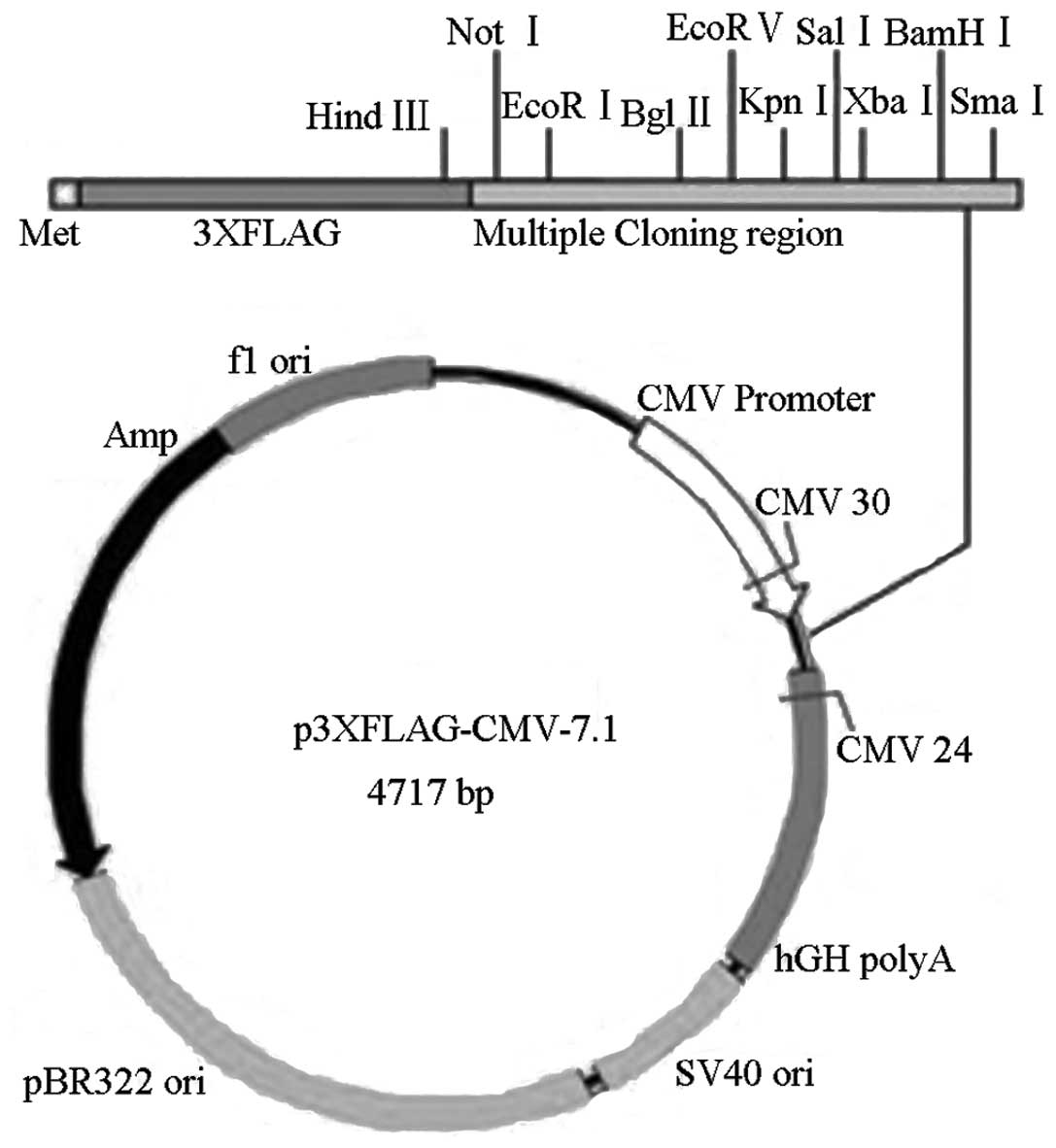
A FLAG-tagged expression construct for the full length Cx37
sequence was additionally constructed and an empty p3xFlag vector
was used as a negative control. The vector construct is shown
diagramatically in Fig. 1.
All cells were divided into six groups. The
experimental groups were comprised of i) overexpression of empty
p3xflag plasmid (0.4 μg), ii) p3xflag-Cx37 expression plasmid (0.4
μg), and iii-v) p3xflag-Cx37 expression plasmid (0.4 μg) with one
of the three different interference fragments. The respective
interference fragments were used at a concentration of 50 nM and
the transfection time was 72 h. Constructs were transfected using
Lipofectamine™ 2000 (Invitrogen Life Technologies, Carlsbad, CA,
USA). The wild-type Cx37 interference construct, the negative
control and p3xflag-Cx37 were cotransfected with RNAi plasmids and
mixed at a molar ratio of 5:1. The best interference fragment was
selected based on the results from the western blotting. The
preparation of lentiviral vectors expressing Cx37 involved the use
of BLOCK-It Lentiviral Pol II miR RNAi Expression system with green
fluorescence protein (GFP; Catalog No. K493800; Invitrogen). A
scrambled siRNA sequence (named mock-siRNA) with no known homology
to mammal genes served as control.
Cells were collected 72 h after transfection and
washed three times with cold phosphate-buffered saline (PBS). A
total of 0.2 ml radioimmunoprecipitation assay buffer containing a
protease inhibitor mixture was added, and the cells were incubated
on ice for 30 min. Finally, 1.5 ml cells were collected using a
cell scraper and transferred to a centrifuge tube on ice.
Ultrasonic lysis of the cells was performed for 30 sec at 4°C. The
supernatant was centrifuged at 22,500 × g for 30 min, and then
transferred to a new microcentrifuge tube and stored at −20°C.
Third, protein concentrations were determined using
a bicinchoninic acid (BCA) assay (Pierce Biotechnology, Rockford,
IL, USA). Solutions A and B from the BCA kit were mixed at a 50:1
ratio and incubated at room temperature for 30 min. Then, 200 μl of
the combined solution were added to each well of a 96-well plate,
as well as 10 μl of a concentration range of bovine serum albumin
at a 1:10 dilution. A blank using 10 μl double-distilled water was
used to normalize to zero. The plate was incubated at 37°C for 30
min in the microplate reader (SPECTROstar Omega; BMG Labtech GMBH,
Ortenberg, Germany) to determine the 570 nm absorbance value. A
standard curve was generated and the concentration of total protein
sample was calculated.
Western blot analysis
The total cellular protein of aortic plaque tissue
of pigs was extracted. 10% SDS-PAGE of the sample was performed.
The protein was transferred to the collodion membrane, followed by
blocking. The membrane was incubated with primary antibody
(anti-Cx37 antibody, 1:200, Abcam, Cambridge, MA, USA) at 4°C
overnight. Following washing for three times, the membrane was
incubated with secondary antibody (1:2,000) for 1 h, followed by
washing for three times. Protein expression was quantified using an
using enhanced chemiluminescence detection kit (Beijing Kang
Century Biotech Co., Ltd., Beijing, China).
Animal maintenance and set-up of
experimental groups
Sixty male Wuzhishan small pigs (four weeks old)
were obtained from Wuzhishan Pig Breeding Farm (Hainan, China). The
pigs were provided with a normal diet and water. After one week,
the pigs received a high-fat diet (5% lard, 1% sugar, 3%
cholesterol and 0.2% propylthiouracil) and fed three times daily.
All pigs were consistently provided with a high-fat diet for 10
months. This study was carried out in strict accordance with the
recommendations of the Guide for the Care and Use of Laboratory
Animals of the National Institute of Health. The animal use
protocol was reviewed and approved by the Institutional Animal Care
and Use Committee of the Affiliated Hospital of Nanjing Medical
University Wuxi People’s Hospital (Wuxi, China). The pigs were
divided into three groups; (1) Cx37 siRNA-treated group with an
infusion of 10 pl Cx37 lentiviral suspension, (2) Mock, untreated
group, and (3) Saline-treated group. These 3 groups received the
corresponding treatment for the last 2 months of 10 months,
respectively. The mock siRNA and saline treatment groups did not
receive any viral infusion. After two months of treatment,
abdominal aortic angiography and IVUS were performed in all pigs.
The plaque of aortic atherosclerosis in pigs was observed under
microscope (DM750; Leica Microsystems GmbH, Wetzlar, Germany) and
analyzed according to reported method (22). The expression of Cx37 mRNA was
detected by semi-quantiative PCR according to a reported method
(9). The expression of Cx37
protein was determined by western blot analysis.
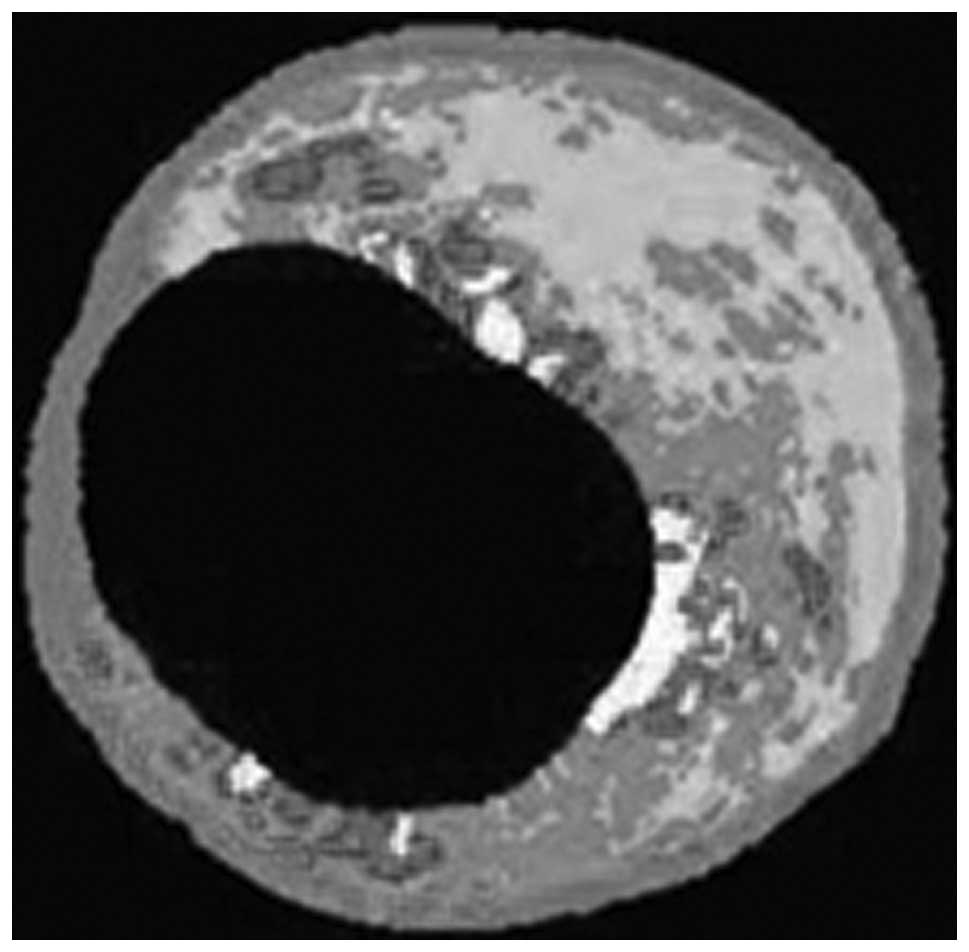
Abdominal aortic angiography and IVUS
analysis
After eight months of the experimental protocol,
abdominal aortic angiography and IVUS were performed on all pigs
under anesthesia by intravenous injection of ketamine
hydrochloride. All IVUS images were acquired using a 20 MHz Volcano
Eagle Eye™ IVUS catheter (Volcano Therapeutics, Inc., Rancho
Cordova, CA, USA). Once the abdominal aorta lesion had been
identified, the IVUS catheter was inserted distal to the lesion and
automatically pulled back to assess the severity and length of the
lesion (rate of 0.5 mm/sec). The location of the IVUS catheter was
determined using continuous fluoroscopy throughout the time of
pullback, as well as by recording anatomical landmarks observed
during IVUS imaging. To create adequate images, an average of two
pullbacks per artery was performed. Subsequently, the best play
loop was selected based on imaging resolution and quality.
Continuous electrocardiography monitoring was performed during the
procedure to gate the IVUS frames for analysis. IVUS-virtual
histology (VH) data were recorded on the imaging system hard drive
and then extracted and archived for analysis. Analysis was based on
border contour calculation from greyscale. Tissue maps provided by
the software (dark green for fibrous tissue, light green for
fibrofatty tissue, red for necrotic core and white for dense
calcium) were used to analyze each independent frame. Once the
total length of each lesion had been determined, a 20 mm vascular
segment containing the vascular lesion was selected for analysis.
This segment was then divided into equal 2.0-mm subsections,
generating a total of 10 series of cross-sections per vascular
segment. Then, the abdominal cavity was opened. An incision was
made on the left paracolic sulci and side peritoneum, exposing the
descending colon and its mesentery to the right, and exposing the
abdominal aorta. Wire positioning was upstream to block blood flow.
The aortic plaques were injected with Cx37 siRNA virus suspension
and mock-siRNA virus suspension or saline and then the sutured
vascular and abdominal areas were injected using a needle at ~30°
angle.
Serum lipid level measurements
Detection of serum lipid was conducted by analysis
of orbital venous sinus blood. The plasma levels of total
cholesterol (TC), plasma triglyceride (TG), low- and high-density
lipoprotein cholesterol (LDL-C and HDL-C, respectively) were
measured by the oxidase method (Center Laboratory of Wuxi People’s
Hospital, Wuxi, China) (23).
Statistical analysis
Data are expressed as the mean ± standard deviation.
Continuous variables between two groups were compared using
independent t-tests and χ2 tests. Multiple groups were
compared by analysis of variance. All tests were conducted using
SPSS 17.0 software for Windows (SPSS, Inc., Chicago, IL, USA).
Proportions were compared using Fisher’s exact test with expected
frequency of <5. χ2 testing was applied in all other
cases. P<0.05 was considered to indicate a statistically
significant difference.
Results
Body weight and serum lipid profile
No significant differences were identified in body
weight among the Cx37 siRNA group and untreated groups, e.g. the
mock and saline subgroups. This indicated that the virus was safe
to transfect with regard to the overall health status of the
animals. Likewise, serum cholesterol and triglyceride levels in the
treatment group did not differ significantly from those in the
untreated groups (Table I). This
finding suggested that the therapeutic effects of gene transfer
were independent of serum lipid levels.
 | Table IChange in body weight and serum lipid
profiles. |
Table I
Change in body weight and serum lipid
profiles.
| | | Untreated groups |
|---|
| | |
|
|---|
| Parameters | Time-point | Cx37 siRNA group
(n=20) | Mock-siRNA
(n=20) | Saline (n=20) |
|---|
| Weight (kg) | Baseline | 21.51±10.34
(n=20) | 22.24±7.38
(n=20) | 23.78±8.99
(n=20) |
| 8 months | 30.37±8.36a (n=18) | 29.45±6.89a (n=19) | 28.12±7.65a (n=17) |
| 10 months | 34.89±4.16a (n=15) | 34.54±3.87a (n=17) | 31.96±4.84a (n=15) |
| Total cholesterol
(mmol/l) | Baseline | 1.97±0.51 (n=20) | 2.01±0.46 (n=20) | 1.98±0.65 (n=20) |
| 8 months | 11.23±1.29a (n=18) | 12.26±1.32a (n=19) | 11.78±1.09a (n=17) |
| 10 months | 14.67±2.23a (n=15) | 15.31±2.01a (n=17) | 14.56±2.65a (n=15) |
| LDL-C (mmol/l) | Baseline | 1.01±0.27 (n=20) | 0.98±0.34 (n=20) | 1.02±0.36 (n=20) |
| 8 months | 8.24±1.23a (n=18) | 9.12±1.29a (n=19) | 8.65±1.31a (n=17) |
| 10 months | 10.23±2.21a (n=15) | 9.98±2.65a (n=17) | 10.31±2.07a (n=15) |
| HDL-C (mmol/l) | Baseline | 0.65±0.28
(n=20) | 0.64±0.31
(n=20) | 0.71±0.29
(n=20) |
| 8 months | 2.31±0.25a (n=18) | 2.41±0.22a (n=19) | 2.35±0.37a (n=17) |
| 10 months | 2.65±0.31a (n=15) | 2.60±0.32a (n=17) | 2.59±0.29a (n=15) |
| Triglyceride
(mmol/l) | Baseline | 0.34±0.12
(n=20) | 0.35±0.10
(n=20) | 0.37±0.12
(n=20) |
| 8 months | 0.33±0.10a (n=18) | 0.34±0.11a (n=19) | 0.38±0.13a (n=17) |
| 10 months | 0.41±0.12a (n=15) | 0.39±0.13a (n=17) | 0.42±0.11a (n=15) |
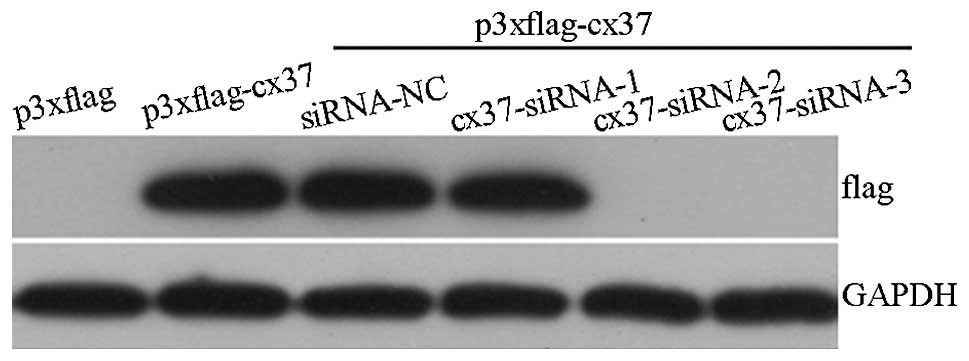
Gene silencing in vitro
The most effective targeting sites for siRNA in the
Cx37 sequence were screened by western blot analysis (Fig. 2). HEK293 cells were transfected
with lentivirus-based vectors expressing three different Cx37
siRNAs. Gene silencing analysis identified that the Cx37 lentivirus
sequences 2 and 3 were the most effective vectors in blocking Cx37
expression. Consequently, Cx37-site 3 lentiviruses were selected
for further in vivo studies.
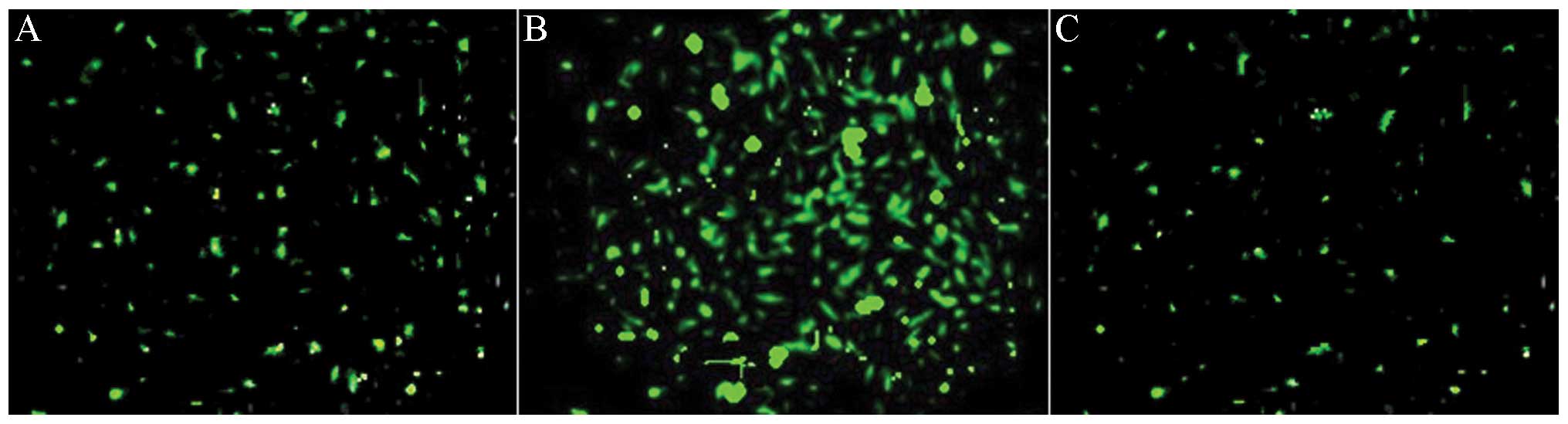
Efficient transfection of lentivirus
Previous studies have shown that local virus
delivery to precollar-induced abdominal aortic atherosclerosis of
pigs has resulted in efficient transfection to abdominal aortic
atherosclerosis (24). GFP
expression provides an efficient and convenient method by which to
check lentiviral transfection efficiency. Therefore, GFP was
analyzed in the abdominal aortic atherosclerosis 1 week after
transfection (Fig. 3A). siRNA
transfection (indicated by strong green fluorescence) was observed
most significantly after 2 weeks after transfection (Fig. 3B). When the study was terminated,
GFP remained weakly visible, two months after transfection
(Fig. 3C). These results
demonstrated the efficiency of the in vivo transfection of
siRNA lentiviruses in the abdominal aortic atherosclerosis. Local
transfection of the virus did not affect the normal functioning of
the animals and did not result in weight change (34.89+4.16 kg in
transfected pigs vs. 34.54+3.87 kg in mock-siRNA and 31.96±4.84 kg
in saline).
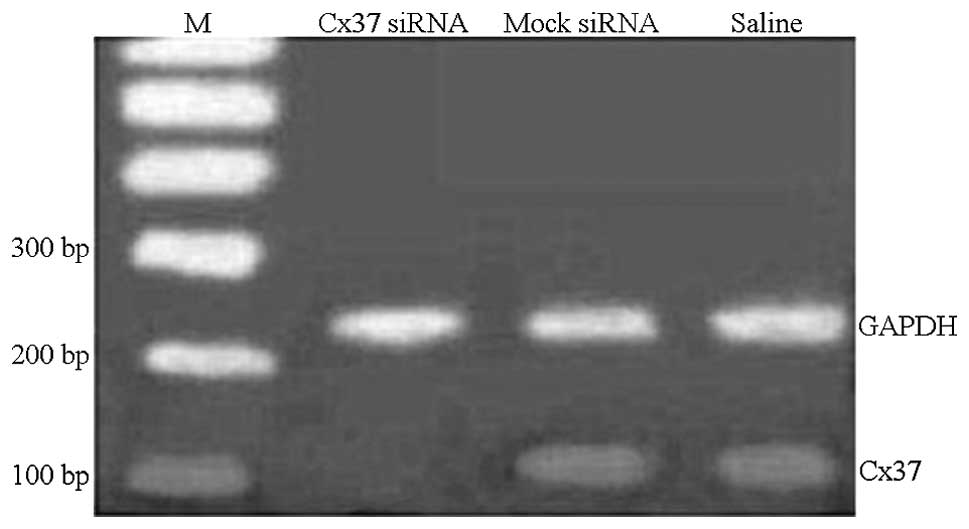
Gene silencing in vivo
To evaluate the efficacy of lentivirus-mediated gene
silencing in vivo on abdominal aortic atherosclerosis of
pigs, Cx37 mRNA and protein expression levels were analyzed by qPCR
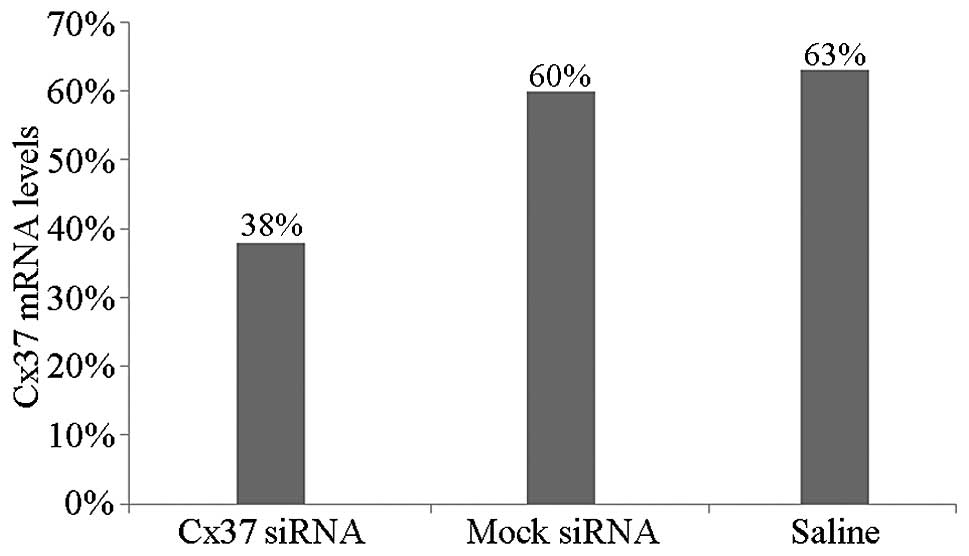
and western blot analyses. Cx37 mRNA levels in the Cx37 siRNA group
were decreased to 38% as compared with those in the mock-siRNA
group, which were decreased to 60%, and to 63% in the saline group
(P<0.05) (Figs. 4 and 5). The mock group showed no significant
change in Cx37 mRNA expression as compared with the control group.
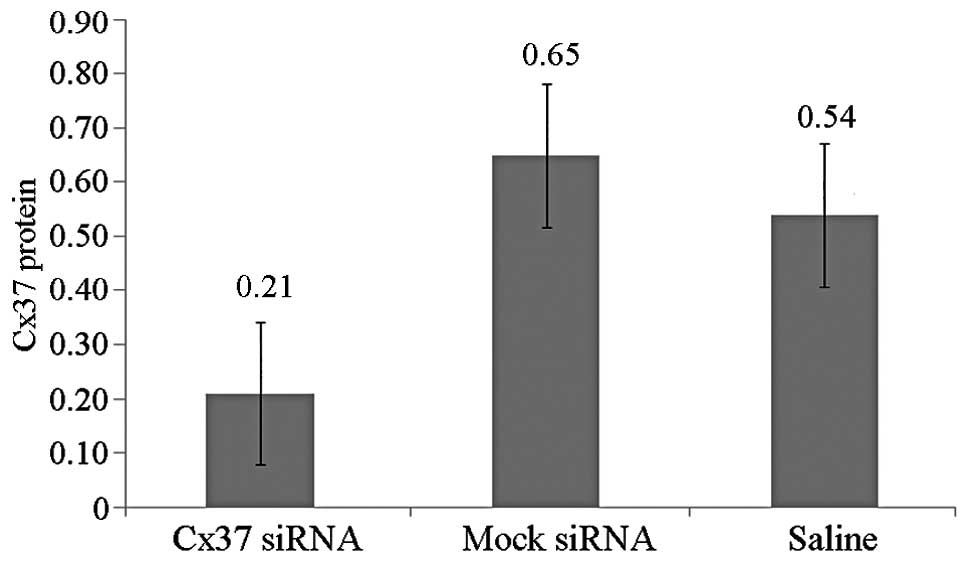
Western blot analysis indicated that Cx37 protein was lowest in the
Cx37-RNAi group as compared with the mock and saline-treated groups
(0.21±0.07 vs. 0.65±0.06 vs. 0.54±0.07) (Fig. 6). Therefore, the local application
of siRNA-lentivirus efficiently silenced the target mRNA.
Effects of Cx37 siRNA on atherosclerosis
plaque
The percentage of plaque necrosis following 10
months (after injection Cx37 siRNA) decreased in the Cx37 siRNA
groups as compared with that following eight months (prior to
injection of Cx37 siRNA) (5.26±2.11 vs. 7.83±1.03%, P<0.05). In
the mock-siRNA and saline groups, no differences in percentages of
plaque necrosis following eight months were observed (P=0.074 and
0.061, respectively). In the Cx37 siRNA group, plaque volumes at 10
months (following RNAi) decreased as compared with those at eight
months (prior to RNAi) (21.03±6.24 vs. 31.23±10.23, P<0.01). By
contrast, plaque volumes increased between 8 and 10 months
(38.54±13.56 vs. 32.12±11.21 mm3, 37.36±14.21 vs.
30.21±12.02 mm3, P=0.031 and P=0.027, respectively) in
the mock siRNA and saline groups. Changes in the percentages of
plaque necrosis and plaque volumes in groups are shown in Table II and Figs. 7 and 8.
 | Table IIChanges in the percentage of plaque
necrosis and volumes between the three treatment groups. |
Table II
Changes in the percentage of plaque
necrosis and volumes between the three treatment groups.
| Variable | Cx37 siRNA | Mock siRNA | Saline |
|---|
| Necrotic (%) |
| 8 months | 7.83±1.03 | 7.63±1.34 | 7.78±1.28 |
| 10 months | 5.26±2.11 | 8.79±3.36 | 9.01±3.02 |
| Plaque volume
(mm3) |
| 8 months | 31.23±10.23 | 32.12±11.21 | 30.21±12.02 |
| 10 months | 21.03±6.24 | 38.54±13.56 | 37.36±14.21 |
Discussion
The frequency of the C allele at base pair 1019 of
the Cx37 gene in patients with coronary heart disease was observed
to be significantly higher as compared with that of healthy
controls (17). The present in
vivo study demonstrated that Cx37 gene interference in the pig
resulted in a reduced volume but improved the stability of the
atherosclerosis plaque. To the best of our knowledge, the present
study was the first to report the effect of Cx37 RNAi in
atherosclerotic plaques.
Atherosclerosis is a chronic inflammatory disease of
the arterial wall (25,26), which has been associated with
numerous genetic factors (27).
Previous studies have demonstrated that Cx37 gene polymorphism is a
risk factor of coronary heart disease. Cx37 and associated genes
may therefore be suitable therapeutic targets in atherosclerosis,
since they have been shown to be important in recognizing highly
evolutionarily conserved molecular motifs in pathogens. Previous
studies demonstrated that Cx37 has a significant role in
atherosclerosis (9,17). Therefore, in the present study,
Cx37 genes were selected as therapeutic targets for atherosclerotic
plaques.
MicroRNAs (miRNAs), a class of short RNAs, are
involved in numerous biological processes and the development of
human disease through specific posttranscriptional downregulation
of gene expression (28). RNAi is
an effective method for selectively silencing mRNA for a wide range
of proteins. This method has been applied in several diseases for
gene intervention. Although a number of delivery approaches are
available, significant challenges remain, including the success
rate, safety and off-target effects of the RNA in vivo. To
overcome these limitations, a lentivirus expression cassette was
used to increase transfection efficiency. Lentiviral
vector-delivered siRNAs have been previously used to successfully
silence gene function in primary mammalian cells, stem cells and
rabbits (29). In the present
study, lentivirus-siRNA was delivered site-specifically to the
targeted plaque site at high titers (i.e., by instilling the
lentivirus suspension into the pigs’ abdominal aorta artery). The
efficacy of this method was confirmed by the observation of GFP
fluorescence in the abdominal aortic plaque during the first and
second week following transfection. Stronger fluorescence was
observed two months following transfection. No adverse effects were
observed in the pigs used in the present study. In addition, no
significant differences in body weight among the Cx37 siRNA group,
mock siRNA group and saline group were found, which indicated the
safety of virus transfection in these animals. The absence of
significant differences in the serum lipid among the Cx37 siRNA,
mock siRNA and saline groups excluded the possibility that the
therapeutic effects in the Cx37 siRNA group were caused by
nonspecific stimulation. However, following the two-month Cx37
siRNA injection, the plaque necrosis and the plaque volume
percentages decreased. However, no differences in the mock-siRNA
and saline groups in percentages of plaque necrosis from injection
of mock siRNA and saline were observed. By contrast, plaque volume
increased in the mock siRNA and saline groups. Therefore,
lentiviral vectors expressing siRNA comprise a safe, efficient and
specific tool to study gene function and therapy.
The effects of interference of Cx37 genes on
advanced atherosclerotic lesions have not been investigated
previously. The present study demonstrated that Cx37 is critical in
the progression of atherosclerosis. The pigs treated with Cx37
siRNA consistently exhibited lipid levels similar to those of the
mock and saline subgroups, and no statistically significant
differences were observed prior to and following treatment in the
three groups. The percentage of plaque necrosis and plaque volume
in the Cx37 siRNA group post treatment were lower as compared with
those prior to treatment. Both values in the control and mock
subgroups were higher than those prior to treatment. Inhibited
metabolism of active macrophages in the plaques may explain the
attenuation of atherosclerosis induced by Cx37 gene
interference.
The mechanisms underlying the therapeutic effects of
Cx37 are currently not fully understood; however, they may be
associated with its recognition patterns. If an SNP in the Cx37
gene causes a cytosine-to-thymine replacement at position 1019
(C1019T), a nonconservative amino acid replacement of proline with
serine, occurs in the regulatory C-terminus of the Cx37 protein
(P319S). This amino acid replacement may lead to functional changes
of the protein and different responses to regulatory mechanisms,
such as phosphorylation. The creation of a new phosphorylation site
may result in a greater capacity for modulating the function of gap
junctions that are regulated by this protein. This may modify
endothelial cell function and lead to susceptibility to
cardiovascular diseases. Recent studies have demonstrated that Cx37
is expressed in endothelial cells, monocytes/macrophages and
platelets (1). The
electrophysiological characteristics determined in the present
study are similar to but distinct from previously characterized
connexins (30). However, the
effects of Cx37 siRNA on atherosclerosis plaques and the mechanisms
of Cx37 action remain to be identified.
In conclusion, lentivirus-mediated siRNA can be used
to efficiently knock down Cx37 genes in abdominal aortic plaques of
pigs fed with a high-fat diet.
References
|
1
|
Pfenniger A, Chanson M and Kwak BR:
Connexins in atherosclerosis. Biochim Biophys Acta. 1828:157–166.
2013. View Article : Google Scholar
|
|
2
|
Otsuka F, Yahagi K, Sakakura K and Virmani
R: Why is the mammary artery so special and what protects it from
atherosclerosis. Ann Cardiothorac Surg. 2:519–526. 2013.PubMed/NCBI
|
|
3
|
Steinberg D: In Celebration of the 100th
anniversary of the lipid hypothesis of atherosclerosis. J Lipid
Res. 54:2946–2949. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu Y, Liu Q, Xu Y, et al: Rutaecarpine
suppresses atherosclerosis in ApoE−/− mice through up-regulating
ABCA1 and SR-BI within RCT. J Lipid Res. 55:1634–1647. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Saez JC, Berthoud VM, Branes MC, Martinez
AD and Beyer EC: Plasma membrane channels formed by connexins:
their regulation and functions. Physiol Rev. 83:1359–1400.
2003.PubMed/NCBI
|
|
6
|
Fang JS, Angelov SN, Simon AM and Burt JM:
Cx37 deletion enhances vascular growth and facilitates ischemic
limb recovery. Am J Physiol Heart Circ Physiol. 301:H1872–H1881.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kanady JD, Dellinger MT, Munger SJ, Witte
MH and Simon AM: Connexin37 and Connexin43 deficiencies in mice
disrupt lymphatic valve development and result in lymphatic
disorders including lymphedema and chylothorax. Dev Biol.
354:253–266. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Morel S and Kwak BR: Roles of connexins in
atherosclerosis and ischemia-reperfusion injury. Curr Pharm
Biotechnol. 13:17–26. 2012. View Article : Google Scholar
|
|
9
|
Guo SX, Yang ZY, Wang RX, Yang Y, Cao HM
and Zhang T: Association between C1019T polymorphism of the
connexin37 gene and coronary heart disease in patients with
in-stent restenosis. Exp Ther Med. 5:539–544. 2013.PubMed/NCBI
|
|
10
|
Boerma M, Forsberg L, Van Zeijl L, et al:
A genetic polymorphism in connexin 37 as a prognostic marker for
atherosclerotic plaque development. J Intern Med. 246:211–218.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yeh HI, Chou Y, Liu HF, Chang SC and Tsai
CH: Connexin37 gene polymorphism and coronary artery disease in
Taiwan. Int J Cardiol. 81:251–255. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Han YL, Xi SY, Zhang XL, Yan CH, Yang Y
and Kang J: Association of C1019T polymorphism in the Connexin37
gene and coronary artery disease in Chinese Han population.
Zhonghua Yi Xue Za Zhi. 87:100–104. 2007.(In Chinese). PubMed/NCBI
|
|
13
|
Wong CW, Christen T, Pfenniger A, James RW
and Kwak BR: Do allelic variants of the connexin37 1019 gene
polymorphism differentially predict for coronary artery disease and
myocardial infarction? Atherosclorosis. 191:355–361. 2007.
View Article : Google Scholar
|
|
14
|
Yamada Y, Izawa H, Ichihara S, et al:
Prediction of the risk of myocardial infarction from polymorphisms
in candidate genes. N Engl J Med. 347:1916–1923. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Listi F, Candore G, Lio D, et al:
Association between C1019T polymorphism of connexin37 and acute
myocardial infarction: a study in patients from Sicily. Int J
Cardiol. 102:269–271. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Seifi M, Fallah S, Ghasemi A, Aghajani H,
Razaghi M and Danaei N: Mutations of the connexin 37 and 40
gap-junction genes in patients with acute myocardial infarction.
Clin Lab. 59:343–348. 2013.PubMed/NCBI
|
|
17
|
Pfenniger A, Wong C, Sutter E, et al:
Shear stress modulates the expression of the atheroprotective
protein Cx37 in endothelial cells. J Mol Cell Cardiol. 53:299–309.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kumari SS, Varadaraj K, Valiunas V, et al:
Functional expression and biophysical properties of polymorphic
variants of the human gap junction protein connexin37. Biochem
Biophys Res Commun. 274:216–224. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schecter AD, Rollins BJ, Zhang YJ, et al:
Tissue factor is induced by monocyte chemoattractant protein-1 in
human aortic smooth muscle and THP-1 cells. J Biol Chem.
272:28568–28573. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Moghadasian MH: Experimental
atherosclerosis: a historical overview. Life Sci. 70:855–865. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Reinhard K, Rougier JS, Ogrodnik J and
Abriel H: Electrophysiological properties of mouse and
epitope-tagged human cardiac sodium channel Na v1.5 expressed in
HEK293 cells. F1000Res. 2:482013.
|
|
22
|
Yang JM, Wang Y, Qi LH, et al:
Combinatorial interference of toll-like receptor 2 and 4
synergistically stabilizes atherosclerotic plaque in apolipoprotein
E-knockout mice. J Cell Mol Med. 15:602–611. 2011. View Article : Google Scholar
|
|
23
|
Chapman MJ, Mills GL and Ledford JH: The
distribution and partial characterization of the serum
apolipoproteins in the guinea pig. Biochem J. 149:423–436.
1975.PubMed/NCBI
|
|
24
|
Qi LH, Wang Y, Gao F, et al: Enhanced
stabilization of atherosclerotic plaques in apolipoprotein
E-knockout mice by combinatorial Toll-like receptor-1 and -2 gene
silencing. Hum Gene Ther. 20:739–750. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Thompson PL, Nidorf SM and Eikelboom J:
Targeting the unstable plaque in acute coronary syndromes. Clin
Ther. 35:1099–1107. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mallavia B, Recio C, Oguiza A, et al:
Peptide inhibitor of NF-κB translocation ameliorates experimental
atherosclerosis. Am J Pathol. 182:1910–1921. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zaina S and Lund G: Atherosclerosis: cell
biology and lipoproteins - panoramic views of DNA methylation
landscapes of atherosclerosis. Curr Opin Lipidol. 24:369–370. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Menghini R, Casagrande V and Federici M:
MicroRNAs in endothelial senescence and atherosclerosis. J
Cardiovasc Transl Res. 6:924–930. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Maraghechi P, Hiripi L, Toth G, Bontovics
B, Bosze Z and Gocza E: Discovery of pluripotency-associated
microRNAs in rabbit preimplantation embryos and embryonic stem-like
cells. Reproduction. 145:421–437. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Reed KE, Westphale EM, Larson DM, Wang HZ,
Veenstra RD and Beyer EC: Molecular cloning and functional
expression of human connexin37, an endothelial cell gap junction
protein. J Clin Invest. 91:997–1004. 1993. View Article : Google Scholar : PubMed/NCBI
|