Introduction
Prostate cancer is one of the most common types of
cancer in the USA, and has become the second leading cause of
cancer-related mortality among males (1). The presence of invasive cells and of
metastasis are the primary factors that contribute to the prognosis
of patients with prostate cancer (2). Gene expression microarray technology,
which can observe the expression of thousands of genes within a
single experiment, has been widely used in the study of cancer.
Gene expression profiles have shown that numerous molecular
pathways are activated persistently in metastatic processes, and
multiple genes are significantly altered during tumor progression
(3). Thus, identification of the
functions of these pathways and the genes involved in them, may
provide important information for use in the development of
diagnostic tools and therapies for cancer.
The aquaporins (AQPs) are a large family of small
membrane transport proteins that transport either water alone, or
water together with small solutes, such as glycerol (4). Thirteen homologous members of the AQP
family, which have 25–60% homology in protein sequence, have been
identified in mammalian cells. These AQPs may be further classified
into two groups. The first group, includes AQP1, 2, 4, 5 and 8,
which selectively transport water alone. The second group includes
AQP3, 7, 9 and 10, which transport water and small solutes,
including glycerol (5). Recent
evidence has shown that AQPs are upregulated in a number of tumors,
such as cervical and colorectal cancer (6,7).
Studies have shown that AQPs exert a significant impact on cancer
metastasis and progression. It has been reported that silencing of
AQP1 reduces tumor growth and angiogenesis in mice (8). In addition, knockdown of AQP4 was
shown to lead to inhibition of cell invasion in human glioma cells
(9), whereas overexpression of
AQP8 promoted invasion of cervical cancer cells (10). Studies have also demonstrated that
the co-expression of AQP3 and AQP5 in esophageal squamous cell
carcinoma correlates with aggressive tumor progression and a poor
prognosis (11). Furthermore,
silencing of AQP3 has been shown to improve the efficacy of
cryotherapy in prostate cancer treatment (12). However, the role of AQP3 in the
invasion of prostate cancer cells remains unclear. In the present
study, the expression of AQP3 in prostate cancer cells was screened
and validated. In addition, AQP3 expression was silenced by small
interfering RNA (siRNA) in order to investigate the involvement of
AQP3 in prostate cancer cell motility and invasion and the possible
underlying molecular mechanisms.
Materials and methods
Materials
A rabbit monoclonal antibody against AQP3 (cat no.
sc-20811) and a mouse monoclonal antibody against β-actin (cat no.
sc-8432) were obtained from Santa Cruz Biotechnology, Inc. (Dallas,
TX, USA). An extracellular signal-regulated kinase 1/2 (ERK1/2)
antibody (cat no. 1240S) and a phospho-ERK1/2 antibody (cat no.
1150S) were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA). U0126, a specific inhibitor of
mitogen-activated protein kinase 1/2 (MEK1/2), was obtained from
Sigma-Aldrich (St. Louis, MO, USA).
Cell culture
Cell lines were obtained from the Cell Bank of
Chinese Academy of Medical Sciences (Beijing, China). The human
PrEC prostate epithelial cell line was grown in PrEMB medium
(Clonetics-Biowhittaker, Walkersville, MD, USA) supplemented with
10% fetal bovine serum (FBS; Sigma-Aldrich). Human LNCap, DU-145,
PC-3 and 22RV1 prostate cancer cell lines obtained from the Cell
Resource Center of Chinese Academy of Medical Sciences (Beijing,
China) were grown in RPMI-1640 (Sigma-Aldrich) supplemented with
10% FBS. All cell lines were cultured in a CO2 incubator
with 5% CO2 at 37°C.
cDNA microarray
Total RNA was extracted from PrEC, DU-145 and PC-3
cells. Fluorescently-labeled cDNA was obtained using the Illumina
TotalPrep RNA Amplification kit (Ambion Life Technologies, Austin,
TX, USA). Hybridization reactions were conducted using Human HT-12
v4 BeadChip (Illumina, San Diego, CA, USA), according to the
manufacturer’s instructions. The BeadChips were imaged using an
Illumina BeadArray reader (Illumina), and the raw data were
normalized using an averaging algorithm. The cDNA microarray was
conducted by Gene Tech Co., Ltd. (Shanghai, China). The expression
of gene transcripts in PrEC cells were defined as 1, and the
expression of transcripts in the DU-145 and PC-3 cells was
subsequently compared with that of the PrEC cells. Variations
>two-fold were taken as a significant difference in gene
expression between cell lines. Gene ontology analysis and pathway
analysis were then conducted in order to further cluster and
analyze these differentially expressed genes.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Cells were harvested and total RNA was isolated
using TRIzol® reagent (Invitrogen Life Technologies,
Carlsbad, CA, USA). RNA (2 μg) was reverse-transcribed into cDNA
using M-MLV Reverse Transcriptase (Promega Corporation, Madison,
WI, USA). qPCR was then conducted on the cDNA under the following
conditions: 95°C for 5 min; 95°C for 15 sec and 60°C for 1 min, for
40 cycles. Primers used in the qPCR were as follows: Forward:
CCGTGACCTTTGCCATGTG and reverse: CGAAGTGCCAGATTGCATCATAA for AQP3;
forward: AGACCTGGGCAGATTCCAAAC and reverse: CGGCAAGTCTTCCGAGTAGT
for MMP-3; and forward: CTGGAACGGTGAAGGTGACA and reverse:
AAGGGACTTCCTGTAACAATGCA for β-actin. The 2−ΔΔCt method
was used to quantify the expression of AQP3 and MMP-3.
Western blot analysis
Cells were washed with ice-cold phosphate-buffered
saline (PBS) three times and then lysed in radioimmunoprecipitation
assay buffer with protease inhibitor and phosphatase inhibitor
cocktails (Roche Applied Science, Mannheim, Germany). The protein
concentrations of cell lysates were measured using a Bicinchoninic
Protein Assay Reagent kit (Applygen Technologies Inc., Beijing,
China). Total protein was boiled with 2X loading buffer, separated
by 10% SDS-PAGE gel, and transferred to a polyvinylidene difluoride
membrane (Invitrogen Life Technologies). The membrane was then
blocked with 5% non-fat milk in Tris-buffered saline with
Tween® 20 (TBST) for the detection of AQP3, β-actin and
ERK1/2 [or 5% bovine serum albumin (BSA) in TBST for detection of
p-ERK1/2]. The membrane was incubated with the primary antibodies
against AQP3, β-actin, ERK1/2 and p-ERK1/2 overnight at 4°C.
Following 1 h incubation with the mouse polyclonal secondary
antibody (1:3,000; Sigma-Aldrich), the membrane was visualized
using an enhanced chemiluminescence detection system (Applygen
Technologies Inc.). The densitometry of each band was quantified
with Quantity One 4.0 software (Bio-Rad Laboratories, Hercules, CA,
USA).
Small interfering (si)RNA
The AQP3-specific siRNA was synthesized by
Genepharma (Shanghai, China). A scramble siRNA was used as a
control. The sequence of the AQP3 siRNA was CGAUCAAGCUGCCCAUCUU.
DU-145 and PC-3 cells were seeded (1.0×103 cells/ml)
onto a dish or a plate, and incubated at 37°C with 5%
CO2 overnight. Cells were then transfected with siRNA
using Lipofectamine® 2000 (Invitrogen Life
Technologies), according to the manufacturer’s instructions. The
knockdown efficiency was tested 48 h later.
Wound healing assay
Cells were seeded onto a 6-well plate at a density
of 8.0×105 cells/ml. Cells were further incubated for 24
h until they had reached ~90% confluence. A 200-μl filtered tip was
used to create an artificial wound on the confluent cell monolayer.
Cells were washed with PBS three times and then cultured in fresh
medium without FBS. Images were captured at 0 and 24 h with a Sony
CCD-TR56 (Sony Corporation, Tokyo, Japan) under a microscope (BX51,
Olympus, Tokyo, Japan). Five random fields were analyzed in each
group and all groups were assayed in triplicate.
Invasion assay
An invasion assay was performed using a 24-well
Transwell plate (Costar, San Diego, CA, USA). Briefly, cells were
trypsinized and suspended in 200 μl serum-free medium at a
concentration of 1×105 cells/ml. The upper chambers were
coated with Matrigel (Sigma-Aldrich). Cells were then layered in
the upper chambers, while RPMI-1640 medium containing 30% FBS was
placed in the lower chamber. Following incubation in the
CO2 incubator for 24 h, cells that had invaded the lower
chamber of the wells were fixed with 4% formaldehyde and stained
with crystal violet (Boster Biological Tech Ltd., Wuhan, China).
Images from seven visual fields of each well were captured (Sony
CCD-TR56) and counted randomly under a light microscope (BX51,
Olympus). The mean value for each group was then calculated.
MMP-3 ELISA assay
A matrix metalloproteinase-3 (MMP-3) ELISA assay was
performed according to the manufacturer’s instructions, in order to
assess the level of MMP-3 protein in the culture supernatant.
Briefly, following transfection with siRNAs or incubation with
U0126 for 24 h, cell supernatant was collected and subjected to the
ELISA assay, using an MMP-3 ELISA kit (Calbiochem, Darmstadt,
Germany). BSA was used to create a standard curve. Absorbance
values were read at 450 nm, and the concentration of MMP-3 was
determined by comparing the absorbance values against those of the
standard curve.
Statistical analysis
All experiments were repeated three times and data
are expressed as the mean ± standard deviation. Statistical
analysis was performed with GraphPad Prism software 5.0 (GraphPad
Software, Inc., La Jolla, CA, USA) using Student’s t-test or
analysis of variance. P<0.05 was considered to indicate a
statistically significant difference.
Results
Overexpression of AQP3 is present in
prostate cancer cells
In order to screen the genes that may be involved in
the progression of prostate cancer, a cDNA microarray was produced
with PrEC, DU-145 and PC-3 cells. Genes expressed in PrEC cells
were used as controls. The expression of 685 genes was
significantly changed in DU-145 and PC-3 cells compared with PrEC
cells (Fig. 1A). Among these genes
with significantly altered levels of expression, AQP3 was found to
be upregulated in DU-145 and PC-3 cells. In order to validate the
cDNA microarray data, qPCR was performed in PrEC, DU-145 and PC-3
cells. The results demonstrated that AQP3 was overexpressed in
DU-145 and PC-3 cells compared with PrEC cells (Fig. 1B). Furthermore, increased levels of
the AQP3 protein in LNCap, DU-145, PC-3 and 22RV1 cells was also
observed by western blot analysis (Fig. 1C). These data indicate that AQP3
may be involved in prostate cancer development and progression.
AQP3 is involved in prostate cancer cell
motility
Tumor motility and invasion are essential to the
progression and metastasis of prostate cancer (13). Given that AQP3 expression was found
to be upregulated in prostate cancer cells, the role of AQP3 in the
motility and invasion of prostate cancer cells was further
investigated. DU-145 and PC-3 cells were initially transfected with
AQP3 siRNA or scramble control siRNA, and the knockdown efficiency
was observed using RT-qPCR and western blot analysis (Fig. 2A and B). A wound healing assay was
then conducted with AQP3 siRNA-transfected and control cells. The
results showed that silencing of AQP3 inhibited the motility of
DU-145 and PC-3 cells, indicating an involvement of AQP3 in the
regulation of prostate cancer cell motility (Fig. 2C and D).
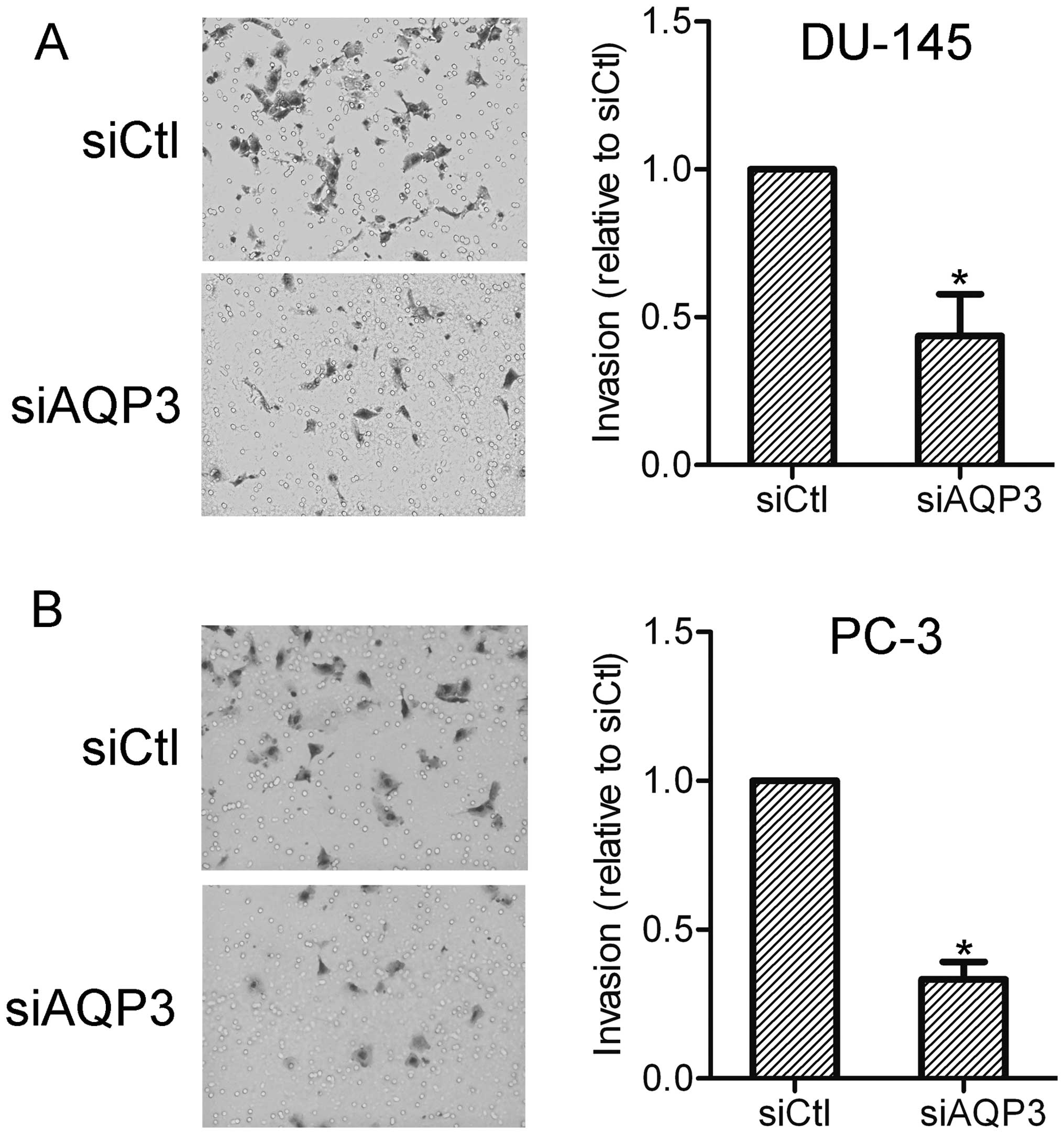
AQP3 contributes to the invasion of
prostate cancer cells
The effect of AQP3 on prostate cancer cell invasion
was further investigated using an invasion assay. The results
showed that AQP3 siRNA-transfected cells exhibited lower invasion
capabilities compared with the control cells. This suggests that
AQP3 expression affects the invasiveness of prostate cancer cells
(Fig. 3).
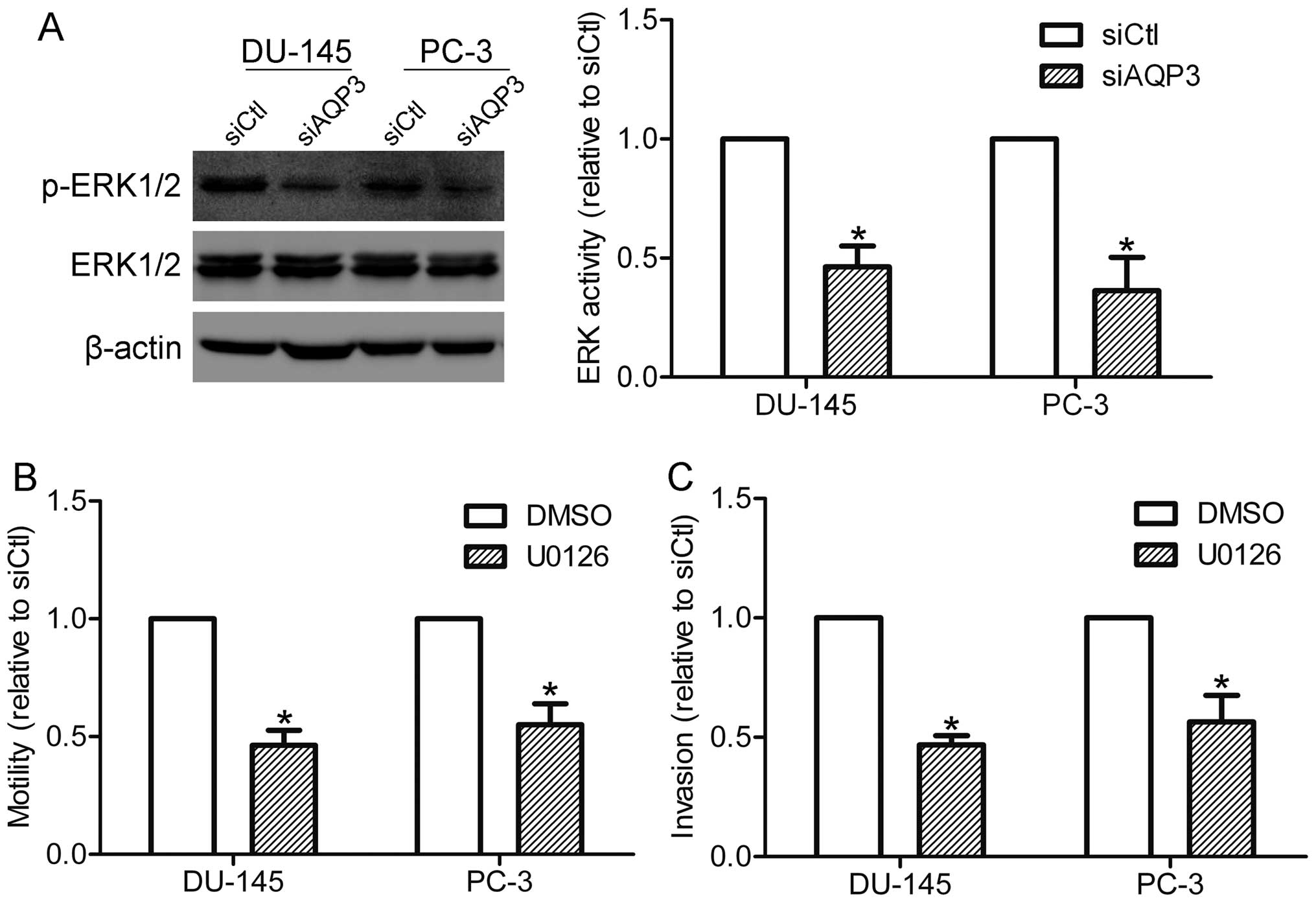
ERK pathway is required for AQP3-mediated
motility and invasion
In order to explore which pathways may be involved
in AQP3-mediated motility and invasion of prostate cancer cells,
the activation of ERK1/2 in DU-145 and PC-3 cells was detected. As
shown in Fig. 4A, the
phosphorylation of ERK1/2 was markedly suppressed in AQP3
siRNA-transfected cells compared with that in control cells,
suggesting that AQP3 is involved in the activation of the ERK
pathway in prostate cancer cells. The function of the ERK pathway
in AQP3-mediated motility and invasion was also investigated. A
MEK1/2 inhibitor, U0126 (20 μM), was used to specifically suppress
the activation of the ERK pathway. Notably, U0126 treatment was
shown to inhibit the motility and invasion of DU-145 and PC-3 cells
(Fig. 4B and C), supporting the
hypothesis that the ERK pathway is required for AQP3-mediated
motility and invasion.
AQP3 upregulates the expression and
secretion of MMP-3 via the ERK pathway
MMP-3 is an important member of the MMP family that
is secreted by cancer cells and is known to be involved in invasion
and metastasis of cancer cells (14). Therefore, the expression and
secretion of MMP-3 was compared between control cells and AQP3
siRNA-transfected cells. RT-qPCR analysis showed that silencing of
AQP3 downregulated the expression of MMP-3 mRNA in DU-145 and PC-3
cells (Fig. 5A). In accordance
with this finding, the ELISA assay demonstrated that MMP-3
secretion was reduced in AQP3 siRNA-transfected cells compared with
control cells (Fig. 5B). These
data suggest that AQP3 regulates the expression and secretion of
MMP-3 in prostate cancer cells. The role of the ERK pathway in
AQP3-mediated MMP-3 expression was further investigated through
inhibition of the ERK pathway by U0126. The results showed that
U0126 treatment decreased the expression and secretion of MMP-3 in
prostate cancer cells (Fig. 5C and
D). As AQP3 regulates the ERK pathway and MMP-3 secretion in
DU-145 and PC-3 cells, the results suggest that AQP3 increases
MMP-3 expression and secretion of prostate cancer cells via
regulation of the ERK pathway.
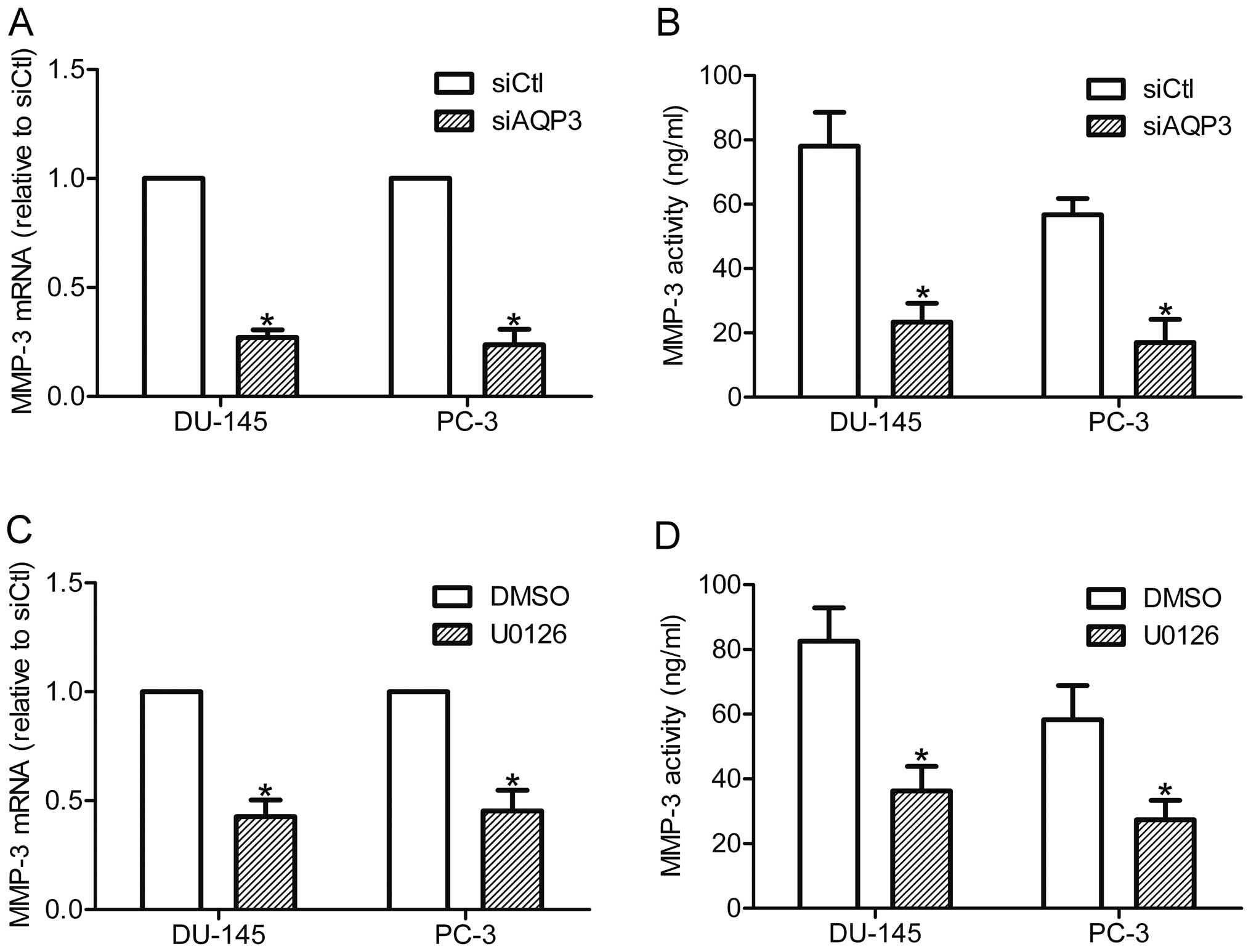 | Figure 5AQP3 increased MMP-3 expression and
secretion by activating the ERK pathway. DU-145 and PC-3 cells were
transfected with siAQP3 or siCtl, and further incubated in the
CO2 incubator for 48 h. (A) MMP-3 mRNA expression was
examined by RT-qPCR. (B) MMP-3 secretion was detected by an ELISA
assay. DU-145 and PC-3 cells were incubated with U0126 (20 μM) or
DMSO for 12 h, MMP-3 expression and secretion were examined by (C)
RT-qPCR and (D) ELISA assay. *P<0.05, compared with
control. AQP3, aquaporin 3; MMP-3, matrix metalloproteinase; ERK,
extracellular signal-regulated kinase; siAQP3, cells transfected
with AQP3-specific small interfering RNA; siCtl, cells transfected
with control siRNA; RT-qPCR, reverse transcription-quantitative
polymerase chain reaction; DMSO, dimethyl sulfoxide. |
Discussion
The AQP family is composed of 13 homologous members
in mammalian cells. AQP3 has been shown to be upregulated in human
gastric carcinoma (15). In the
present study, AQP3 was shown to be overexpressed in prostate
cancer cells, which indicates an involvement of AQP3 in prostate
cancer progression. Previous studies have shown that AQP3 is
required for EGF-enhanced pancreatic cancer cell migration
(16), and is important for cell
proliferation in esophageal and oral squamous cell carcinoma
(17). Clinical studies have
reported that AQP3 expression is associated with lymph node
metastasis in gastric and colorectal carcinoma (15,18).
However, little is currently known regarding the function of AQP3
in prostate cancer. The present study demonstrated that AQP3 may
regulate the motility and invasion of prostate cancer cells,
further confirming the association between AQP3 and a number of
types of malignancy.
AQP3 has been implicated in the regulation of
numerous signaling pathways. It has been reported that AQP3 induces
the activation of the phosphoinositide 3-kinase/protein kinase B
signaling pathway in human gastric carcinoma cells (19). Knockdown of AQP3 markedly
suppresses the p38 MAPK pathway in keratinocytes (20). In the current study, AQP3 was shown
to promote the activation of ERK1/2 in DU-145 and PC-3 prostate
cancer cells. The ERK signaling pathway, which has been widely
investigated in a number of cancer types, contributes to regulation
of diverse cellular processes, such as proliferation, survival,
differentiation, invasion and metastasis (21). The present study showed that
blocking ERK1/2 activation attenuated prostate cancer cell motility
and invasion, suggesting a possible role for the ERK pathway in
AQP3-mediated motility and invasion.
It is now well-established that the MMP family of
proteins enhance tumor invasion and metastasis via degradation of
the extracellular matrix (22). As
the prominent member of the MMP family, MMP-3 expression has been
reported to be associated with metastasis and a poor prognosis in a
number of tumors, including prostate cancer (23,24).
Studies have shown that AQP3 positively regulates MMP-9 expression
in SGC7901 human gastric carcinoma cells (19). The present study demonstrated that
AQP3 upregulates the expression and secretion of MMP-3 in prostate
cancer cells. The ERK pathway has been reported to be respond to
the expression of MMPs in numerous types of cancer cells (25,26).
In the present study, the results showed that inhibition of the ERK
pathway by U0126 treatment decreased the expression and secretion
of MMP-3 in prostate cancer cells, suggesting that the ERK pathway
is involved in AQP3-mediated MMP-3 expression and secretion in
prostate cancer cells.
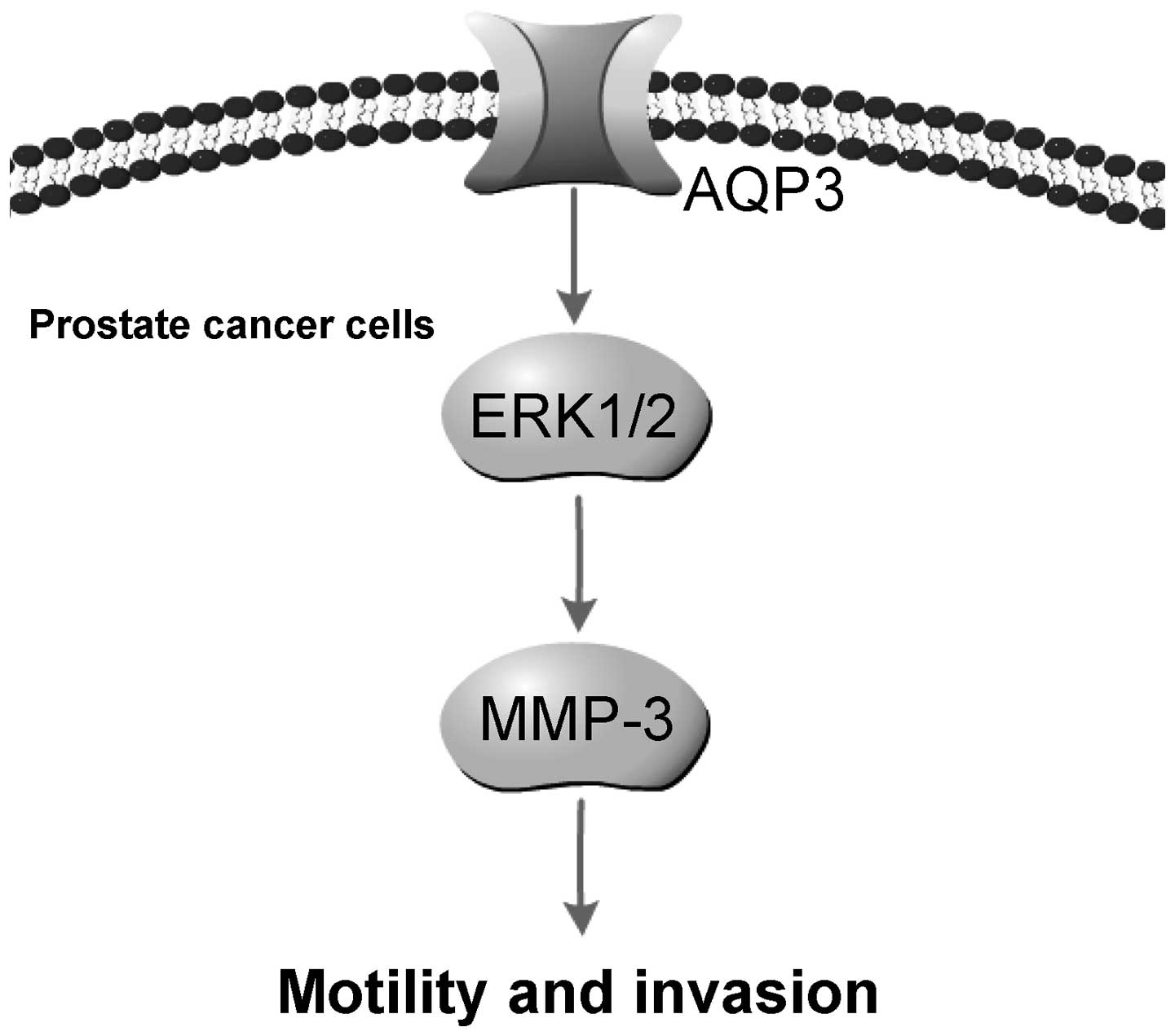
In conclusion, the present study demonstrated that
AQP3 is upregulated in prostate cancer cells. Knockdown of AQP3
suppressed the motility and invasion of DU-145 and PC-3 prostate
cancer cells. Furthermore, AQP3 promoted ERK1/2 activation and
increased the expression and secretion of MMP-3 in prostate cancer
cells. The ERK pathway and MMP-3 are important in prostate cancer
cell invasion and metastasis. Blocking of the ERK pathway decreased
MMP-3 expression and secretion, and attenuated AQP3-mediated
motility and invasion. Therefore, it is possible that AQP3 promotes
prostate cancer cell motility and invasion via regulation of
ERK1/2-mediated MMP-3 secretion (Fig.
6). In vivo studies are required in order to further
determine the effect of AQP3 on tumor cell metastasis.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bubendorf L, Schopfer A, Wagner U, et al:
Metastatic patterns of prostate cancer: An autopsy study of 1,589
patients. Hum Pathol. 31:578–583. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang XY, Hao JW, Zhou RJ, et al:
Meta-analysis of gene expression data identifies causal genes for
prostate cancer. Asian Pac J Cancer Prev. 14:457–461. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Agre P, King LS, Yasui M, et al: Aquaporin
water channels-from atomic structure to clinical medicine. J
Physiol. 542:3–16. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hara-Chikuma M and Verkman AS: Prevention
of skin tumorigenesis and impairment of epidermal cell
proliferation by targeted aquaporin-3 gene disruption. Mol Cell
Biol. 28:326–332. 2008. View Article : Google Scholar :
|
|
6
|
Moon C, Soria JC, Jang SJ, et al:
Involvement of aquaporins in colorectal carcinogenesis. Oncogene.
22:6699–6703. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shi YH, Chen R, Talafu T, Nijiati R and
Lalai S: Significance and expression of aquaporin 1, 3, 8 in
cervical carcinoma in Xinjiang Uygur women of China. Asian Pac J
Cancer Prev. 13:1971–1975. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
HU J and Verkman AS: Increased migration
and metastatic potential of tumor cells expressing aquaporin water
channels. Faseb J. 20:1892–1894. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ding T, Ma YJ, Li W, et al: Role of
aquaporin-4 in the regulation of migration and invasion of human
glioma cells. Int J Oncol. 38:1521–1531. 2011.PubMed/NCBI
|
|
10
|
Shi YH, Rehemu N, Ma H, Tuokan T, Chen R
and Suzuke L: Increased migration and local invasion potential of
SiHa cervical cancer cells expressing Aquaporin 8. Asian Pac J
Cancer Prev. 14:1825–1828. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu SL, Zhang SY, Jiang H, Yang YX and
Jiang Y: Co-expression of AQP3 and AQP5 in esophageal squamous cell
carcinoma correlates with aggressive tumor progression and poor
prognosis. Med Oncol. 30:2013. View Article : Google Scholar
|
|
12
|
Ismail M, Bokaee S, Davies J, Harrington
KJ and Pandha H: Inhibition of the aquaporin 3 water channel
increases the sensitivity of prostate cancer cells to cryotherapy.
Br J Cancer. 100:1889–1895. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gueron G, De Siervi A and Vazquez E:
Advanced prostate cancer: reinforcing the strings between
inflammation and the metastatic behavior. Prostate Cancer Prostatic
Dis. 15:213–221. 2012. View Article : Google Scholar
|
|
14
|
Liu HQ, Song S, Wang JH and Zhang SL:
Expression of MMP-3 and TIMP-3 in gastric cancer tissue and its
clinical significance. Oncology Lett. 2:1319–1322. 2011.
|
|
15
|
Shen L, Zhu ZC, Huang Y, et al: Expression
profile of multiple aquaporins in human gastric carcinoma and its
clinical significance. Biomed Pharmacother. 64:313–318. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu W, Wang K, Gong K, Li X and Luo K:
Epidermal growth factor enhances MPC-83 pancreatic cancer cell
migration through the upregulation of aquaporin 3. Mol Med Rep.
6:607–610. 2012.PubMed/NCBI
|
|
17
|
Kusayama M, Wada K, Nagata M, et al:
Critical role of aquaporin 3 on growth of human esophageal and oral
squamous cell carcinoma. Cancer Sci. 102:1128–1136. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li A, Lu D, Zhang Y, et al: Critical role
of aquaporin-3 in epidermal growth factor-induced migration of
colorectal carcinoma cells and its clinical significance. Oncol
Rep. 29:535–540. 2013.
|
|
19
|
Xu H, Xu Y, Zhang WJ, Shen LZ, Yang L and
Xu ZK: Aquaporin-3 positively regulates matrix metalloproteinases
via PI3K/AKT signal pathway in human gastric carcinoma SGC7901
cells. J Exp Clin Cancer Res. 30:2011. View Article : Google Scholar
|
|
20
|
Hara-Chikuma M and Verkman AS: Aquaporin-3
facilitates epidermal cell migration and proliferation during wound
healing. J Mol Med (Berl). 86:221–231. 2008. View Article : Google Scholar
|
|
21
|
Kohno M and Pouyssegur J: Targeting the
ERK signaling pathway in cancer therapy. Ann Med. 38:200–211. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gialeli C, Theocharis AD and Karamanos NK:
Roles of matrix metalloproteinases in cancer progression and their
pharmacological targeting. Febs J. 278:16–27. 2011. View Article : Google Scholar
|
|
23
|
Jung K, Nowak L, Lein M, Priem F, Schnorr
D and Loening SA: Matrix metalloproteinases 1 and 3, tissue
inhibitor of metalloproteinase-1 and the complex of
metalloproteinase-1/tissue inhibitor in plasma of patients with
prostate cancer. Int J Cancer. 74:220–223. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang M, Dai C, Zhu H, et al: Cyclophilin
A promotes human hepatocellular carcinoma cell metastasis via
regulation of MMP3 and MMP9. Mol Cell Biochem. 357:387–395. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cho SJ, Chae MJ, Shin BK, Kim HK and Kim
A: Akt- and MAPK-mediated activation and secretion of MMP-9 into
stroma in breast cancer cells upon heregulin treatment. Mol Med
Rep. 1:83–88. 2008.PubMed/NCBI
|
|
26
|
Wang Q, Tang H, Yin S and Dong C:
Downregulation of microRNA-138 enhances the proliferation,
migration and invasion of cholangiocarcinoma cells through the
upregulation of RhoC/p-ERK/MMP-2/MMP-9. Oncol Rep. 29:2046–2052.
2013.PubMed/NCBI
|