Introduction
Ketamine is a phencyclidine derivative first used as
an intravenous anesthetic agent in 1965 (1) and approved by the US Food and Drug
Administration in 1970 for use in producing analgesia and amnesia,
with rapid recovery (2,3). It is predominantly an
N-methyl-D-aspartate (NMDA) receptor antagonist, producing a state
that is similar to catalepsy, termed dissociative anesthesia, in
which sensory inputs appear to reach the cortical sensory areas but
are not perceived, owing to suppression of association areas
(4). In addition, ketamine affects
non-NMDA glutamine receptors, as well as opioid, nicotinic,
monoaminergic and muscarinic cholinergic receptors (5). While it is used medically as an
anesthetic agent, the use of ketamine as a recreational drug in
Taiwan has increased over the last 10 years, and it is emerging as
an increasingly popular choice among young drug users, particularly
in dance clubs (6,7). There is a prevailing misconception
among this population that as a short-acting psychotropic agent,
ketamine is not as harmful as other drugs, for example heroin, and
that it has a broad margin of safety and a low potential for
dependence. However, it has been reported that the majority of
ketamine abusers (79%) display features of physiological dependence
following a year of regular ketamine abuse, and 54% reported
withdrawal symptoms on termination of use (8).
Ketamine abuse can also lead to severe lower urinary
tract symptoms, complicated by decreased bladder capacity and
hematuria. Ketamine-associated cystitis was first reported in case
reports by Chu et al (9)
and Shahani et al (10) in
2007 and was recently comprehensively reviewed by Wei et al
(11). While the incidence of
ketamine-associated lower urinary tract symptoms is difficult to
assess, it is thought to be at least 30% among abusers (12). It has been suggested that the
presence of ketamine and its active metabolites, including
norketamine and dehydronorketamine, in the urine may damage the
urinary tract mucosa (10). A
recent study in rats reported that ketamine treatment results in
bladder hyperactivity, along with ulcerated urothelium and
mononuclear cell infiltration (13). These alterations were accompanied
by significant increases in the expression levels of
cyclooxygenase-2 (COX-2) and two nitric oxide synthase (NOS)
isoforms [inducible NOS (iNOS) and endothelial NOS (eNOS)], which
were determined by western blot analysis, in addition to a
significant increase in the number of COX-2-positive cells, as
determined by immunohistochemistry (13). The authors suggested that these
pathological changes, together with the upregulation of
inflammatory proteins, may have an important role in
ketamine-induced ulcerative cystitis in rats. The aim of the
present study was to assess the histopathological features and the
degree of inflammation in the bladder urothelium, vessel walls, and
smooth muscle of patients with ketamine-induced cystitis, to
determine if the expression levels of inflammatory markers, such as
those described above (13), had
correlated with the degree of inflammation.
Materials and methods
Patients and sample collection
This study was approved by the Institutional Review
Board of the Tri-Service General Hospital (Taipei, Taiwan). A total
of 23 patients at the Tri-Service General Hospital with a
self-reported history of ketamine abuse and a confirmed diagnosis
of cystitis were included in this retrospective study. Each patient
presented with lower urinary tract symptoms such as urgency,
nocturia and frequency. Urine and blood samples were collected from
each patient for analysis. The urine test panel included strip
glucose, urine protein, urine bilirubin, urobilinogen, pH, occult
blood, acetone in urine, strip white blood cells (WBCs), nitrite,
clarity, specific gravity and color and sediments [urine red blood
cells (RBCs), urine WBCs, epithelial cells, urine casts, bacteria,
crystals, yeast, spermatozoa, Trichomonas, dysmorphic RBCs
and mucus]. Blood analysis included WBC count, RBC count,
hemoglobin, hematocrit, mean corpuscular volume (MCV), mean
corpuscular hemoglobin (MCH), mean corpuscular hemoglobin
concentration (MCHC = MCH/MCV), platelet count and differential
count (neutrophils, lymphocytes, monocytes, eosinophils and
basophils). Paraffin-embedded urothelial tissues were obtained by
urothelial biopsy 1 week following the urine and blood sample
collection. Marked urothelial atypia with nuclear enlargement was
evident in association with urothelial ulceration. Normal
urothelial tissue was collected from nearby tissue without symptoms
of inflammation. All participants provided written informed
consent. The institutional review boards of the hospital approved
the study protocol.
Assessment of inflammation
Paraffinized sections (0.6 μm) from each patient
(3–10 sections/patient) were stained with Gill’s hematoxylin V
(MUTO, Tokyo, Japan) and 1% eosin alcohol solution (MUTO; H&E).
The degree of inflammation was assessed according to a
semi-quantitative scale. H&E-stained slides were examined under
a light microscope (Olympus BX51; Olympus, Tokyo, Japan) in a
double-blind manner by two histopathologists. Inflammation was
scored as follows: mild inflammation, <5 mononuclear
inflammatory cells in a 10×10 grid (area, 0.25 mm2;
magnification, ×200); moderate inflammation, mononuclear
inflammatory cells scattered throughout the tissue but background
stromal connective tissue clearly visible; severe inflammation,
mononuclear inflammatory cells densely infiltrating the tissues.
The degree of inflammation in each case was assessed throughout the
specimen. Although lymphoid follicles with germinal centers were
encountered, these were not assessed.
Immunohistochemical staining
Paraffinized sections (0.6 μm) were generated for
immunohistochemistry from patients (3–10 sections/patient) with
sufficient specimens from the urothelial biopsy. When >1 block
was available, we used the same block used for histopathologic
diagnosis. Sections were mounted on silanized glass slides, stored
in the dark at 58°C, and subjected to immunohistochemical analysis
within 1 week. Following paraffin removal and rehydration, the
sections were subjected to antigen retrieval by microwave heating
in 10 mM citrate buffer, pH 6.0 (Merck Eurolab, Copenhagen,
Denmark) twice, for 5 min each time. Endogenous peroxidase activity
was blocked by incubation in 5% H2O2 in
distilled water for 20 min at room temperature, followed by 30 min
incubation at room temperature with primary antibodies targeting
COX-2 (monoclonal, anti-mouse; Thermo Labsystems, Santa Rosa, CA,
USA), iNOS (monoclonal, anti-mouse; Thermo Labsystems), and matrix
metallopeptidase-9 (MMP-9; monoclonal, anti-mouse; Abcam,
Cambridge, UK) (14), as well as
cancer-related markers, including mammalian target of rapamycin
(mTOR; monoclonal, anti-mouse; Thermo, Rockford, IL, USA) and
phosphorylated 40S ribosomal protein S6 (Phos-S6; monoclonal,
anti-mouse; Immunotech, Marseille, France). All of the antibodies
were diluted to 1:200 in antibody diluent (Dako, Glostrup, Denmark)
(15–17). Sections were subsequently incubated
with secondary antibody [EnVision™ FLEX/HRP + Rabbit/Mouse
(LINKER)] for 20 min at room temperature. Intervening washes were
performed in EnVision FLEX wash buffer for 5 min each. For COX-2,
antigen-antibody complex was visualized with 3,3′-diaminobenzendine
(EnVision FLEX DAB + Chromogen). For iNOS, MMP-9, mTOR and Phos-S6,
the antigen-antibody complex was visualized using the
3-amino-9-ethylcarbazole (AEC) color system (MUTO), and sections
were counterstained with Mayer’s hematoxylin (MUTO). The stained
sections without primary antibodies were used as a negative
control. Slides were coverslipped with Glycergel Mounting Medium
(Dako) and examined under an Olympus BX51 light microscope in a
double-blind manner by two histopathologists. The staining
intensity of the urothelium, vessel walls and smooth muscle was
scored as follows: 0, no staining; 1, mild staining; 2, moderate
staining; 3, intense staining. In addition the percentage of
positive cells within a 10 × 10 cm grid (area, 0.25 mm2;
magnification, ×200) was calculated (18). The percentage of positive cells was
counted regardless of staining intensity.
Statistical analysis
Statistical analysis was performed using SPSS
statistics software version 15.0 (SPSS, Inc., Chicago, IL, USA).
The patient demographic and clinical characteristics are presented
as the mean ± standard deviation (SD) for continuous data and n (%)
for categorical data. Morphological data are presented as n (%) by
inflammatory stage. The differences among the inflammatory stages
were compared using Fisher’s exact test. Spearman correlation
analysis was performed to identify correlations between the
morphologic data and inflammatory stage. Results are presented as
the coefficient of correlation (r) and the corresponding P-value.
Statistical assessments were two-tailed and significance was set at
P<0.05.
Results
A total of 23 subjects (18 males and 5 females) were
analyzed in this study. Demographic and clinical characteristics
are summarized in Table I. The
average age was 21.8±3.7 years (mean ± SD). Duration of ketamine
usage ranged from 2 to 6 years, however, there were 12 patients for
whom this information was not available. The morphology of the
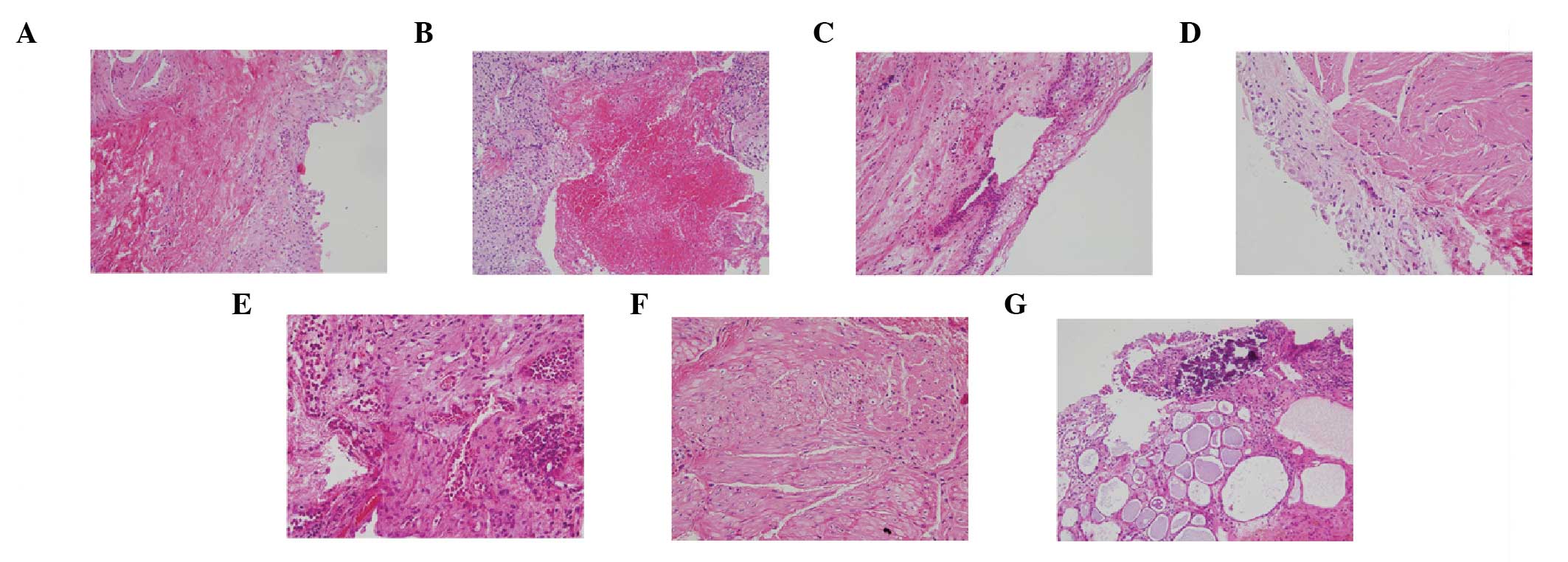
bladder in ketamine-induced cystitis was highlighted by staining
with H&E, as shown in Fig. 1.
Mucosal histologic features of ketamine-associated cystitis
included the following in variable numbers of patients (Fig. 1): denuded urothelial mucosa with a
thinner layer of epithelial cells, ulceration, changes in clarity,
increased collagen deposition, smooth muscle degeneration in the
stromal tissue, vessel proliferation, calcification, and
inflammation with variable numbers of neutrophils, eosinophils, and
mast cells. Inflammation was mild for 2 patients (8.7%), moderate
for 15 (65.2%) and severe for 6 (26.1%). All of the patients
received a diagnosis of urothelial atypia, and the majority (22/23)
showed intravascular eosinophil accumulation. In addition,
lymphatic duct proliferation was noted in only one patient.
 | Table IDemographic characteristics of the 23
subjects and the morphological features of their urinary
bladder. |
Table I
Demographic characteristics of the 23
subjects and the morphological features of their urinary
bladder.
| Characteristic | n
(%)a |
|---|
| Age
(years)a | 21.8±3.7 |
| Gender |
| Male | 18 (78.3) |
| Female | 5 (21.7) |
| Denuded urothelial
mucosa |
| Yes | 13 (56.5) |
| No | 10 (43.5) |
| Ulceration |
| Yes | 10 (43.5) |
| No | 13 (56.5) |
| Urothelial
atypia |
| Yes | 23 (100) |
| No | 0 |
| Intravascular
eosinophil accumulation |
| Yes | 22 (95.7) |
| No | 1 (4.3) |
| Collagen
deposition |
| Yes | 15 (65.2) |
| No | 8 (34.8) |
| Smooth muscle
degeneration |
| Yes | 7 (30.4) |
| No | 16 (69.6) |
| Vessel
proliferation |
| Yes | 12 (52.2) |
| No | 11 (47.8) |
| Calcification |
| Yes | 3 (13.0) |
| No | 20 (87.0) |
| Mucosal clear
change |
| Yes | 2 (8.7) |
| No | 21 (91.3) |
| Inflammatory
stage |
| Mild | 2 (8.7) |
| Moderate | 15 (65.2) |
| Severe | 6 (26.1) |
Urine analysis
Seven patients showed no protein in the urine, two
showed equivocal results, five showed 1+ proteinuria,
eight showed 2+, and one showed 3+. Only one
patient had urine bilirubin. The urine pH range was 5.5–7.5.
Testing for occult blood was negative in seven patients, equivocal
in two, 1+ in one, 2+ in three, and
3+ in ten. The results of testing for RBCs were <10
in nine patients, 10–100 in four and >100 in ten. The results of
testing for WBCs were <10 in 13 patients, 10 to 100 in seven,
and >100 in three. Testing for bacteria was negative in 17
patients, equivocal in one, 1+ in three and
2+ in two.
Blood analysis leukocytosis was present
in three patients, and six were anemic
All 23 patients had a normal platelet range. Four
patients showed neutrophilia, one showed neutropenia, and seven
showed lymphocytopenia. Five patients showed an increase in the
levels of monocytes, and one showed a decrease in the levels of
monocytes. One patient showed eosinophilia.
Immunohistochemical staining for
inflammation markers
Immunostaining in tissues with different degrees of
inflammation (mild, moderate and severe) revealed staining for all
five of the inflammation markers (Fig.
2). Quantification of the immunohistochemical staining of
sections from certain patients is presented in Tables II and III. Table
II displays immunohistochemical staining intensity (0–3) by
inflammation level (mild, moderate and severe) in the urothelium,
vessel walls, and smooth muscle. COX-2 staining differed
significantly between the degrees of inflammation (mild, moderate
and severe) in the urothelium and smooth muscle (P=0.027
urothelium; P=0.020 smooth muscle). In the urothelium, for example,
only one specimen stained 3+ and one specimen stained
2+ for COX-2 in the tissue with mild inflammation.
However, all four specimens from the tissue with severe
inflammation stained 3+ for COX-2 (Table II). In addition, iNOS staining
differed significantly between the degrees of inflammation in
smooth muscle (P=0.029). No significant difference was found
between the degrees of inflammation for MMP-9, Phos-S6 or mTOR
staining.
 | Table IIImmunostaining for inflammation
markers and histologic assessment of inflammation in the
urothelium, vessel wall and smooth muscle. |
Table II
Immunostaining for inflammation
markers and histologic assessment of inflammation in the
urothelium, vessel wall and smooth muscle.
| Urothelium | Vessel wall | Smooth muscle |
|---|
|
|
|
|
|---|
| | Inflammation | | | Inflammation | | | Inflammation | |
|---|
| |
| | |
| | |
| |
|---|
| Staining
intensitya | No. of
samplesb | Mild (n=2) | Moderate (n=15) | Severe (n=6) | P-value | No. of
Samplesb | Mild (n=2) | Moderate (n=15) | Severe (n=6) | P-value | No. of
Samplesb | Mild (n=2) | Moderate
(n=15) | Severe (n=6) | P-value |
|---|
| COX-2 | | | | | 0.027c | | | | | 1.000 | | | | | 0.020d |
| 0 | 0 | 0 | 0 | 0 | | 1 | 0 | 1 | 0 | | 1 | 0 | 1 | 0 | |
| 1 | 1 | 0 | 1 | 0 | | 5 | 1 | 3 | 1 | | 3 | 0 | 1 | 2 | |
| 2 | 6 | 1 | 5 | 0 | | 4 | 0 | 3 | 1 | | 6 | 1 | 1 | 4 | |
| 3 | 6 | 1 | 1 | 4 | | 8 | 1 | 4 | 3 | | 8 | 1 | 7 | 0 | |
| MMP-9 | | | | | 0.626 | | | | | 0.384 | | | | | 0.335 |
| 0 | 0 | 0 | 0 | 0 | | 1 | 0 | 1 | 0 | | 1 | 0 | 1 | 0 | |
| 1 | 4 | 1 | 2 | 1 | | 0 | 0 | 0 | 0 | | 3 | 0 | 1 | 2 | |
| 2 | 2 | 0 | 2 | 0 | | 6 | 1 | 2 | 3 | | 9 | 1 | 5 | 3 | |
| 3 | 9 | 0 | 6 | 3 | | 14 | 1 | 10 | 3 | | 7 | 1 | 6 | 0 | |
| iNOS | | | | | 0.643 | | | | | 0.808 | | | | | 0.029c |
| 0 | 0 | 0 | 0 | 0 | | 3 | 1 | 2 | 0 | | 3 | 1 | 1 | 1 | |
| 1 | 0 | 0 | 0 | 0 | | 4 | 0 | 2 | 2 | | 8 | 0 | 3 | 5 | |
| 2 | 2 | 0 | 2 | 0 | | 4 | 0 | 2 | 2 | | 7 | 1 | 6 | 0 | |
| 3 | 11 | 2 | 5 | 4 | | 7 | 1 | 4 | 2 | | 0 | 0 | 0 | 0 | |
| Phos-S6 | | | | | 0.201 | | | | | 0.774 | | | | | 0.327 |
| 0 | 0 | 0 | 0 | 0 | | 2 | 1 | 1 | 0 | | 6 | 1 | 4 | 1 | |
| 1 | 2 | 1 | 1 | 0 | | 0 | 0 | 0 | 0 | | 3 | 1 | 0 | 2 | |
| 2 | 5 | 1 | 4 | 0 | | 7 | 1 | 4 | 2 | | 3 | 0 | 2 | 1 | |
| 3 | 5 | 0 | 2 | 3 | | 4 | 0 | 2 | 2 | | 0 | 0 | 0 | 0 | |
| mTOR | | | | | 1.000 | | | | | 0.778 | | | | | 0.335 |
| 0 | 0 | 0 | 0 | 0 | | 2 | 1 | 1 | 0 | | 0 | 0 | 0 | 0 | |
| 1 | 1 | 0 | 0 | 1 | | 4 | 0 | 3 | 1 | | 1 | 0 | 1 | 0 | |
| 2 | 5 | 1 | 2 | 2 | | 5 | 1 | 2 | 2 | | 5 | 0 | 2 | 3 | |
| 3 | 1 | 0 | 0 | 1 | | 3 | 0 | 1 | 2 | | 6 | 1 | 3 | 2 | |
 | Table IIIPercentage of cells immunopositive
for inflammation markers and histologic assessment of inflammation
in the urothelium, vessel wall and smooth muscle. |
Table III
Percentage of cells immunopositive
for inflammation markers and histologic assessment of inflammation
in the urothelium, vessel wall and smooth muscle.
| | Urothelium | Vessel wall | Smooth muscle |
|---|
| |
|
|
|
|---|
| Marker | Inflammation | No. of
samplesa | % Positive
cellsb | r-valuec | P-value | No. of
samplesa | % Positive
cellsb | r-valuec | P-value | No. of %
samplesa | Positive
cellsb | r-valuec | P-value |
|---|
| COX-2 | | | | 0.194 | 0.526 | | | 0.049 | 0.851 | | | −0.088 | 0.736 |
| Mild | 2 | 80.0 | | | 2 | 40.0 | | | 2 | 50.0 | | |
| Moderate | 7 | 61.4 | | | 10 | 52.0 | | | 9 | 47.8 | | |
| Severe | 4 | 80.0 | | | 5 | 48.0 | | | 6 | 41.7 | | |
| MMP-9 | | | | 0.209 | 0.454 | | | −0.105 | 0.658 | | | 0.020 | 0.933 |
| Mild | 1 | 90.0 | | | 2 | 45.0 | | | 2 | 55.0 | | |
| Moderate | 10 | 77.0 | | | 12 | 70.0 | | | 13 | 65.4 | | |
| Severe | 4 | 87.5 | | | 6 | 51.7 | | | 5 | 60.0 | | |
| iNOS | | | | 0.315 | 0.295 | | | 0.159 | 0.572 | | | −0.002 | 0.994 |
| Mild | 2 | 85.0 | | | 1 | 30.0 | | | 2 | 40.0 | | |
| Moderate | 7 | 74.3 | | | 8 | 50.0 | | | 10 | 39.0 | | |
| Severe | 4 | 87.5 | | | 6 | 51.7 | | | 5 | 36.0 | | |
| Phos-S6 | | | | 0.815 | 0.001d | | | 0.286 | 0.394 | | | 0.112 | 0.833 |
| Mild | 2 | 20.0 | | | 4 | 67.5 | | | 1 | 10.0 | | |
| Moderate | 7 | 50.0 | | | 1 | 60.0 | | | 2 | 25.0 | | |
| Severe | 3 | 83.3 | | | 6 | 53.3 | | | 3 | 20.0 | | |
| mTOR | | | | 0.250 | 0.588 | | | −0.263 | 0.409 | | | 0.020 | 0.951 |
| Mild | 1 | 30.0 | | | 1 | 70.0 | | | 1 | 30.0 | | |
| Moderate | 2 | 60.0 | | | 6 | 70.0 | | | 6 | 41.7 | | |
| Severe | 4 | 65.0 | | | 5 | 46.0 | | | 5 | 39.0 | | |
Table III shows
the percentage of immunostaining positive cells by degree of
inflammation. Results of the Spearman correlation analysis
indicated a positive correlation between the percentage of
Phos-S6-positive cells and the degree of inflammation in the
urothelium (r=0.815; P=0.001) but no in other tissues. No section
from normal tissue and negative control showed immunostaining
positive cells.
Discussion
The aim of the present study was to evaluate the
histopathological features and inflammation status of samples from
patients with ketamine-induced cystitis to determine if markers of
inflammation were expressed. The results revealed histopathological
features consistent with those previously reported (7,9,10–12),
including marked intravascular eosinophil accumulation and variable
denuded urothelial mucosa and ulceration; the majority of patients
(65.2%) showed moderate inflammation, with the remainder showing
severe (26.1%) or mild (8.7%) inflammation. Samples showed positive
immunostaining for all 5 of the inflammation markers assessed, with
COX-2 staining differing significantly between the three degrees of
inflammation in the urothelium and smooth muscle, and iNOS staining
differing significantly with inflammation level in smooth
muscle.
The mechanism of ketamine-induced ulcerative
cystitis remains to be elucidated. Results from animal models
(13) and the pathological data of
the current study suggest that the urothelium may play a role in
the pathogenesis of ketamine-induced cystitis. The etiology of
ketamine-induced cystitis may be the direct toxic effect of
ketamine and its active metabolite on the bladder mucosa (7). Ketamine induces significant
submucosal hemorrhage, enhanced macrophage infiltration,
ulceration, reduced urothelium thickness and the degeneration of
smooth muscle. These pathological changes may be the cause of the
ketamine-induced expression of COX-2, iNOS and MMP-9 in the
urothelium, vessel wall and smooth muscle. The present results,
along with those in rats (13),
suggest that inflammatory markers, including COX-2 and iNOS, may be
involved, either as a result of ketamine-induced cystitis or
inducing it. Notably, an increase in the expression of COX-2 and
iNOS has been reported in response to cyclophosphamide-induced
cystitis in rats (19,20). Matrix metalloproteinase (MMP)-2 and
MMP-9, collectively known as the gelatinases, are closely
associated with inflammatory and infectious diseases in a number of
organs (21). However, the current
study did not find a correlation between the MMP-9 expression and
inflammation levels in tissues of ketamine-induced cystitis.
Notably, the histopathological features of
ketamine-induced cystitis in this study showed a marked
intravascular eosinophil accumulation, suggesting that the
inflammatory response may begin from the blood vessels in the
bladder. However, significant differences in the COX-2 and iNOS
staining intensity as well as % immunostaining positive cells were
not observed between tissues from three degrees of inflammation. It
has been suggested that other inflammatory mediators induced by
ketamine may be involved in the induction of intravascular
eosinophil accumulation.
The results of the present study indicated a
positive correlation between the percentage of Phos-S6-positive
cells and inflammation level in the urothelium. The expression of
mTOR and mTOR pathway members, such as Phos-S6, have been reported
in bladder carcinoma (15–17,22).
Oxley et al (23) have
reported that ketamine-induced cystitis is a mimic of carcinoma
in situ. The results of the current study confirmed their
observations that the markers of bladder carcinoma may be observed
in the tissues of patients with ketamine-induced cystitis. However,
it is unclear at this time why there was a positive correlation
between the percentage of Phos-S6-positive cells in the urothelium
and the degree of inflammation.
Limitations of the present retrospective study
include the fact that no samples were analyzed from control
subjects (e.g. non-ulcerative cystitis or healthy tissues from
non-abusers) due to the ethical reasons, and the fact that
immunohistochemical staining was not performed for all subjects. In
addition this study did not assess the ketamine level in urine
samples, or the association between duration of ketamine usage and
severity of ulcerative cystitis (severity of inflammation). The
latter information was not available for 12 subjects. In
conclusion, these results add to the descriptive literature on
histopathologic aspects of ketamine-induced cystitis, emphasizing
the inflammatory nature and a possible role for proteins such as
COX-2, iNOS and Phos-S6. Ketamine and its active metabolites may
have a direct toxic effect on the bladder mucosa; the observed
histopathologic changes may induce or increase expression of COX-2,
iNOS and Phos-S6 in the urothelium and smooth muscle.
Acknowledgements
This study was supported by a grant from Tri-Service
General Hospital, Taiwan, Republic of China (grant nos.
TSGH-C100-145, TSGH-C101-059, TSGH-C102-056 and TSGH-C102-159).
References
|
1
|
Domino EF, Chodoff P and Corssen G:
Pharmacologic effects of CI-581, a new dissociative anesthetic, in
man. Clin Pharmacol Ther. 6:279–291. 1965.PubMed/NCBI
|
|
2
|
Sehdev RS, Symmons DA and Kindl K:
Ketamine for rapid sequence induction in patients with head injury
in the emergency department. Emerg Med Australas. 18:37–44. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Craven R: Ketamine. Anaesthesia. 62:48–53.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bhutta AT: Ketamine: a controversial drug
for neonates. Semin Perinatol. 31:303–308. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Corssen G and Domino EF: Dissociative
anesthesia: further pharmacologic studies and first clinical
experience with the phencyclidine derivative CI-581. Anesth Analg.
45:29–40. 1966. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lankenau SE and Sanders B: Patterns of
ketamine use among young injection drug users. J Psychoactive
Drugs. 39:21–29. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen CH, Lee MH, Chen YC and Lin MF:
Ketamine-snorting associated cystitis. J Formos Med Assoc.
110:787–791. 2011. View Article : Google Scholar
|
|
8
|
Chen R, Lee AM and Chan R: A study on the
cognitive impairment and other harmful effects from ecstasy and
ketamine abuse. Hong Kong Chinese 2004. Narcotics Division,
Security Bureau. The Government of the Hong Kong Special
Administrative Region; 2004, http://www.nd.gov.hk/pdf/Study%20on%20the%20Cognitive%20Impairment%20and%20Other%20Harmful%20Effects%20Caused%20by%20Ketamine%20Abuse.pdf.
|
|
9
|
Chu PS, Kwok SC, Lam KM, et al: ‘Street
ketamine’-associated bladder dysfunction: a report of ten cases.
Hong Kong Med J. 13:311–313. 2007.PubMed/NCBI
|
|
10
|
Shahani R, Streutker C, Dickson B and
Stewart RJ: Ketamine-associated ulcerative cystitis: a new clinical
entity. Urology. 69:810–812. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wei YB, Yang JR, Yin Z, Guo Q, Liang BL
and Zhou KQ: Genitourinary toxicity of ketamine. Hong Kong Med J.
19:341–348. 2013.PubMed/NCBI
|
|
12
|
Chu PS, Ma WK, Wong SC, et al: The
destruction of the lower urinary tract by ketamine abuse: a new
syndrome? BJU Int. 102:1616–1622. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chuang SM, Liu KM, Li YL, et al: Dual
involvements of cyclooxygenase and nitric oxide synthase
expressions in ketamine-induced ulcerative cystitis in rat bladder.
Neurourol Urodyn. 32:1137–1143. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mohammed MA, Seleim MF, Abdalla MS,
Sharada HM and Abdel Wahab AH: Urinary high molecular weight matrix
metalloproteinases as non-invasive biomarker for detection of
bladder cancer. BMC Urol. 13:252013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schultz L, Chaux A, Albadine R, et al:
Immunoexpression status and prognostic value of mTOR and
hypoxia-induced pathway members in primary and metastatic clear
cell renal cell carcinomas. Am J Surg Pathol. 35:1549–1556. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hansel DE, Platt E, Orloff M, et al:
Mammalian target of rapamycin (mTOR) regulates cellular
proliferation and tumor growth in urothelial carcinoma. Am J
Pathol. 176:3062–3072. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Makhlin I, Zhang J, Long CJ, et al: The
mTOR pathway affects proliferation and chemosensitivity of
urothelial carcinoma cells and is upregulated in a subset of human
bladder cancers. BJU Int. 108:E84–E90. 2011. View Article : Google Scholar :
|
|
18
|
Sappayatosok K, Maneerat Y, Swasdison S,
et al: Expression of pro-inflammatory protein, iNOS, VEGF and COX-2
in oral squamous cell carcinoma (OSCC), relationship with
angiogenesis and their clinico-pathological correlation. Med Oral
Patol Oral Cir Bucal. 14:E319–E324. 2009.PubMed/NCBI
|
|
19
|
Hu VY, Malley S, Dattilio A, Folsom JB,
Zvara P and Vizzard MA: COX-2 and prostanoids expression in
micturition pathways after cyclophosphamide-induced cystitis in the
rat. Am J Physiol Regul Integr Comp Physiol. 284:R574–R585.
2003.
|
|
20
|
Xu X, Cubeddu LX and Malave A: Expression
of inducible nitric oxide synthase in primary culture of rate
bladder smooth muscle cells by plasma from cyclophosphamide-treated
rats. Eur J Pharmacol. 416:1–9. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chakrabarti S and Patel KD: Matrix
metalloproteinase-2 (MMP-2) and MMP-9 in pulmonary pathology. Exp
Lung Res. 31:599–621. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chaux A, Compérat E, Varinot J, et al:
High levels of phosphatase and tensin homolog expression are
associated with tumor progression, tumor recurrence, and systemic
metastases in pT1 urothelial carcinoma of the bladder: a tissue
microarray study of 156 patients treated by transurethral
resection. Urology. 81:116–122. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Oxley JD, Cottrell AM, Adams S and Gillatt
D: Ketamine cystitis as a mimic of carcinoma in situ.
Histopathology. 55:705–708. 2009. View Article : Google Scholar : PubMed/NCBI
|