Introduction
In previous years, implanted prosthodontic dentures
have been applied as an effective way to repair either defective or
absent dentition. The surface characteristics and morphology of
implanted dentures affect the cell survival, adhesion,
proliferation and differentiation of dental tissues on denture
surfaces (1). Biologically inert
titanium (Ti) is widely used in dental implants due to the direct
contact between the implant material and the bone (1,2).
However, as a bioinert material, Ti cannot be biologically
integrated with bone tissue (3).
One approach to improve integration has been to roughen endosseous
areas of dental implants in order to increase the total surface
area available for osseous apposition. Surface roughness has been
demonstrated to alter osteoblast attachment, proliferation,
differentiation and extracellular matrix (ECM) production (4).
Another limitation of Ti implants is the short delay
between implantation and when they are able to impart bactericidal
capabilities, which may lead to the formation of dental plaques
surrounding implanted dentures (5). Several strategies have been developed
to overcome this surface-associated limitation of Ti implants
(6–10), including surface modification by
plasma immersion ion implantation and deposition (PIIID) (11–13).
PIIID generates surface layers that can integrate with specific
substrates and this technique has been used to incorporate zinc
(Zn) into the surfaces of Ti implants (14). Zn is a necessary element for
cellular activities and is specifically important in the
development and maintenance of bone structures (15).
In the current study, a PIIID technique was used to
implant Zn ions onto the smooth surfaces of pure Ti disks. The
physical structure and chemical composition of the modified
Zn-PIIID-Ti surfaces were evaluated by scanning electron microscopy
(SEM) and X-ray photoelectron spectroscopy (XPS), respectively and
then the biocompatibility of the Zn-PIIID-Ti surfaces with regard
to osteoblast function in vitro was examined.
Materials and methods
Preparation and surface characterization
of Ti disks
Pure Ti (grade 4) disks, 10 mm in diameter and 1 mm
thick were polished on one side to a mirror-like finish and then
sequentially subjected to ultrasonication in acetone, absolute
alcohol and deionized water. The clean disks were then air-dried
and stored in a desiccator. PIIID to implant and deposit Zn ions
was performed at the State Key Laboratory of Advanced Welding
Production Technology of the Harbin Institute of Technology
(Harbin, China) using the following parameters: Pulse voltage (V)
of 20 kv; pulse width (τ/) of 300 μs; Zn-ion pulsed cathodic
arc width of 300 μs and working pressure (P) of
1×10−1 Pa. The source of the Zn ions was a pulsed
cathodic arc. Ti disks were prepared with four different
implantation times (T) of 20 min (Zn-Ti-20 min group), 40 min
(Zn-Ti-40 min group), 60 min (Zn-Ti-60 min group) and 80 min
(Zn-Ti-80 min group). Commercially pure Ti (cp-Ti) disks without Zn
implantation were used in the control group.
The physical structure of modified Ti disks was
observed by SEM (Hitachi S-520; Hitachi, Ltd., Tokyo, Japan) and
their chemical composition was characterized by XPS using 300 W Al
Kα radiation and an ESCALab 220i-XL electron spectrometer (VG
Scientific, Ltd., East Grinstead, UK). Binding energies were
referenced to the C1s line at 284.8 eV from trace carbon and the
base pressure was 3×10−9 mbar.
Cell culture
MG-63 human osteosarcoma cells (purchased from the
Cancer Institute and Hospital, Chinese Academy of Medical Sciences,
Beijing, China) were grown in minimum essential medium with 10%
fetal calf serum (HyClone Laboratories, Inc., Logan, UT, USA), 100
IU/ml penicillin, 100 IU/ml streptomycin (North China
Pharmaceutical Group Corporation, Shijiazhuang, China) and 2 mM
L-glutamine in a humidified atmosphere of 5% CO2 at
37˚C. Cells were seeded onto surfaces of Zn-modified Ti disks in
the Zn-Ti-20, −40, −60 and −80 min groups as well as control disks
in 24-well plates at a seeding concentration of 2×104
cells/ml.
SEM observation of cell attachment
The morphology of attached cells was observed by SEM
(Hitachi S-520; Hitachi, Ltd.). Following culture for 48 h, the
Zn-modified and control Ti disks with attached cells were washed
with phosphate-buffered saline (PBS) and the cells were fixed
overnight in 2.5% glutaraldehyde. Following fixation, the samples
were sequentially dehydrated (10 min each) in increasing
concentrations of ethanol (20, 50, 70, 90 and 100%). Following
dehydration, they were immersed in isoamyl acetate (a critical
point drying fluid) for 1.5 min and finally sputter-coated with a
thin layer of gold/palladium for viewing by SEM.
Quantification of cell attachment and
proliferation
Cell attachment to the Zn-modified and control Ti
disks was quantified using acridine orange (AO) staining. Following
a specific incubation time (6, 24 or 48 h), samples were fixed in
95% ethanol and stained with 4×104 mg/ml AO for 1 min.
Following rinsing with PBS, the samples were examined under a
fluorescence microscope (FV1000; Olympus, Tokyo, Japan) and the
attached cells were counted in randomly selected 2 mm2
areas.
Flow cytometry for cell cycle
analysis
After 48 h, MG-63 cells were collected and prepared
for flow cytometry (FACSCalibur, Becton Dickinson Immunocytometry
Systems, San Jose, CA, USA). A total of 10,000 cells were counted
per sample and the fractions of cells in the G1, S and G2 phases of
the cell cycle were determined. This experiment was performed in
triplicate.
Statistical analysis
Data are expressed as the mean ± standard deviation.
Statistical analyses were performed using SPSS 12.0 software (SSPS,
Inc., Chicago, IL, USA). One-way analysis of variance was conducted
to assess differences among all quantitative indices. P<0.05 was
considered to indicate a statistically significant difference.
Results
Structure and chemical composition of
pure and Zn-modified Ti surfaces
SEM images of the unmodified cp-Ti surfaces revealed
tiny fissures and fuzzy grain edges with smooth shapes (Fig. 1). By contrast, multiple
homogeneously and randomly distributed granular masses were
apparent on the Zn-PIIID-Ti surfaces with increasing Zn exposure
time. The Zn appeared to have penetrated into the base material and
deposited onto the surface. For longer implantation and deposition
times, the interstices among the distributed granular masses
increased. The size and shape of the masses were also increasingly
more homogeneous and the edges of the grains became much sharper,
but no significant differences were observed in the number of minor
fissures among the samples prepared with different exposure times
(P>0.05). SEM images revealed that the Ti surfaces exhibited a
rough ‘honeycomb’ structure in which the diameter was 60–100 nm
with PIIID processing. The ‘honeycomb’ structure deepened with
increasing processing time and surfaces in the Zn-Ti-80 min group
demonstrated a 200 nm deep structure with grade 9 smoothness and
0.4 μm roughness (Fig.
1F).
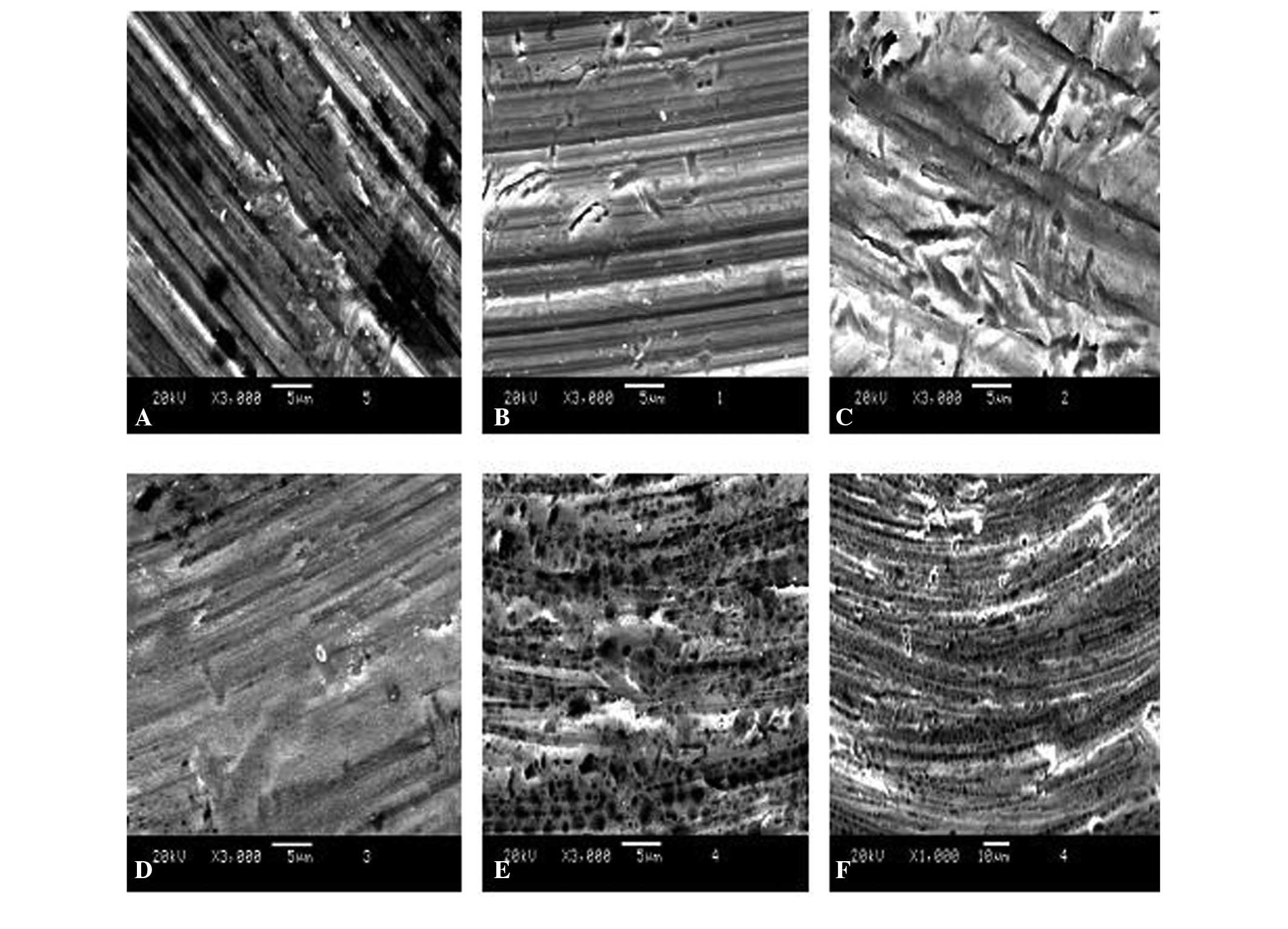 | Figure 1Scanning electron microscope images of
unmodified and Zn-modified Ti surfaces. (A) Commercially pure-Ti,
(B) Zn-Ti-20 min, (C) Zn-Ti-40 min, (D) Zn-Ti-60 min, (E) Zn-Ti-80
min and (F) Zn-Ti-80 min surfaces. (A-E) Magnification ×3,000,
scale bar=5 μm; (F) magnification ×1,000, scale bar=10
μm. TI, titanium; Zn, zinc. |
The XPS results revealed alterations in the surface
layer chemical composition upon Zn-PIIID (Fig. 2). The O1s peak relative intensity
significantly decreased following PIIID. An additional peak
corresponding to Zn-containing materials was also observed,
although its intensity was extremely weak. XPS analysis also was
used to obtain relative atomic concentrations of carbon, Ti and Zn
on the surface of each group (Table
I). In the Zn-Ti-20, −40, −60 and −80 min groups, the
percentages of Zn were 1.04±0.08, 1.32±0.06, 1.35±0.04 and
2.14±0.06, respectively.
 | Table IX-ray photoelectron spectroscopy
elemental percentages for Zinc-plasma immersion ion implantation
and deposition on titanium surfaces. |
Table I
X-ray photoelectron spectroscopy
elemental percentages for Zinc-plasma immersion ion implantation
and deposition on titanium surfaces.
| Element | cp-Ti | Zn-Ti-20 min | Zn-Ti-40 min | Zn-Ti-60 min | Zn-Ti-80 min |
|---|
| Ti | 62.76±3.24 | 65.44±3.98 | 64.61±3.55 | 61.61±3.61 | 57.51±3.47 |
| O | 37.24±1.26 | 33.52±1.33 | 34.08±1.19 | 37.04±1.38 | 40.35±1.41 |
| Zn | 0.00 | 1.04±0.08a | 1.32±0.06a,b | 1.35±0.04a,b | 2.14±0.06a,b,c |
MG-63 morphological alterations on pure
and Zn-modified Ti surfaces
As shown in Fig.
3A, MG-63 cells typically have irregular polygon shapes, where
the ratio of the macro axis to the minor axis is usually greater
than those of other cells growing in the culture flask. SEM
analysis revealed that the in vitro morphology of MG-63
cells was altered on Zn-PIIID-Ti surfaces (Fig. 3B). Compared with the predominately
round cells observed on the cp-Ti disks (Fig. 3C), cells grown on the Zn-PIIID-Ti
disks exhibited a relatively improved spreading and fattening, with
an increased cell to substrate contact ratio (Fig. 3D). Increased Zn implantation
resulted in a greater density of attached cells. The cells secreted
considerable quantities of ECM protein that covered the granular
surface (Fig. 3E) and appeared to
be actively proliferating and differentiating (Fig. 3F). Cells on the Zn-PIIID-Ti
surfaces also had extensive networks of cytoplasmic processes that
extended to the underlying substrate and connected to neighboring
cells, whereas cells on the cp-Ti surfaces had far fewer and
shorter fibrillar extensions.
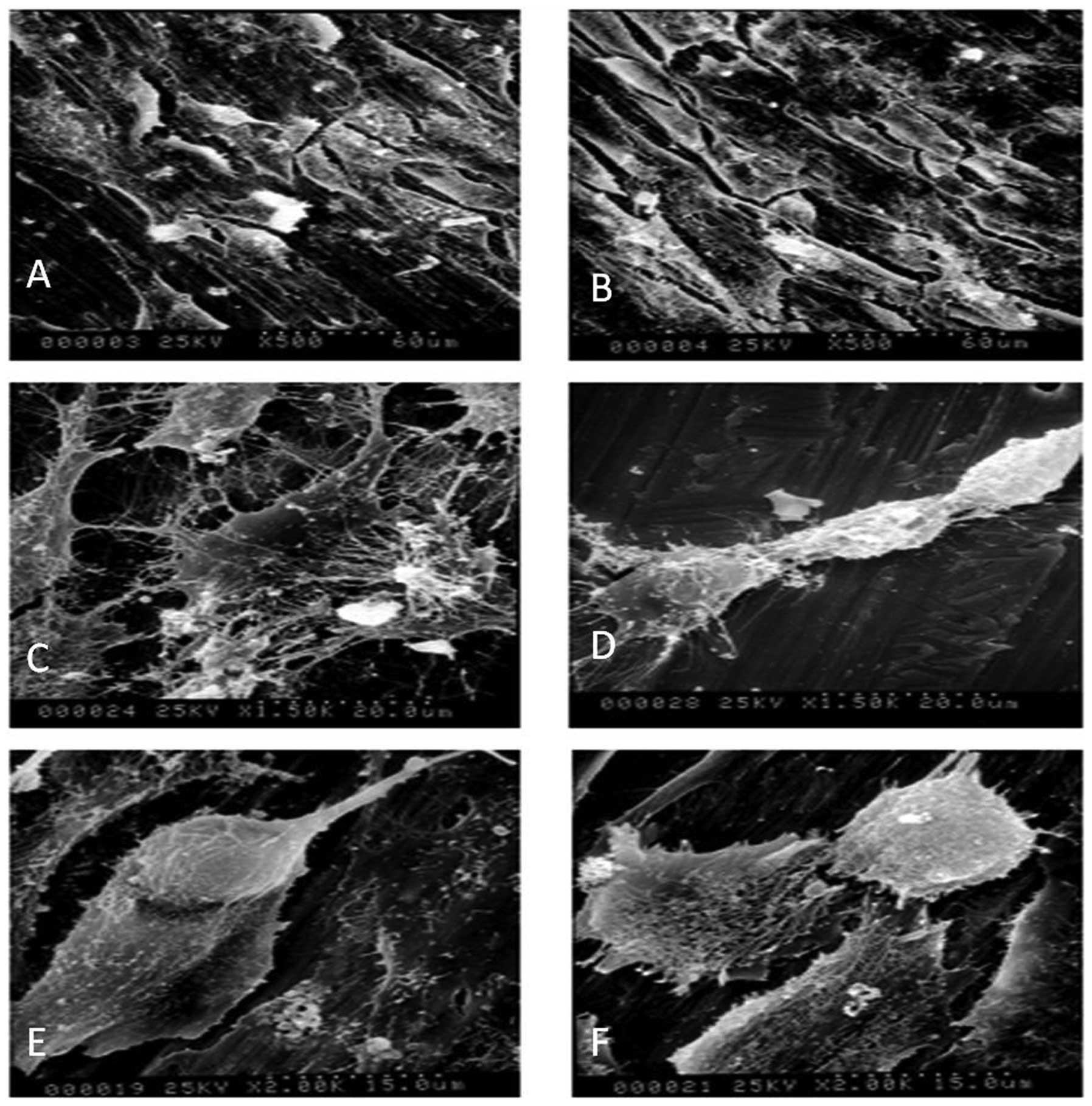 | Figure 3Scanning electron microscope images
demonstrating the morphology of MG-63 cells on unmodified and
Zn-modified Ti surfaces. (A and C) MG-63 cells on cp-Ti; (B and
D-F) MG-63 cells on Zn-Ti-20 min surfaces. (C) MG-63 cells were
primarily rounded on unmodified Ti surfaces, whereas on the (D)
Zn-modified Ti surfaces, cells assumed fusiform shapes with
well-spread pseudopodia and extensive networks of cytoplasmic
processes. (D) Secreted extracellular matrix was visible covering
the substrate granules and (E) cells undergoing division were
observed. (A and B) Magnification, ×500. (C and D) Magnification,
×1,500. (E and F) Magnification, ×2,000. Cp, commercially pure; TI,
titanium; Zn, zinc. |
Cell proliferation on pure and
Zn-modified Ti surfaces\
The initial attachment of cells is crucial to
subsequent cell spreading, proliferation and differentiation on
substrates. As shown in Fig. 4 by
fluorescence microscopy, the number of attached and proliferating
MG-63 cells increased with culture time for all samples. At 6 h
after cell seeding, there were no significant differences
identified in cell proliferation among the five samples
(P>0.05). However, after 24 h, a higher density of cells was
present on the Zn-PIIID-Ti surfaces compared with that on the cp-Ti
surface (P<0.05). This trend became more pronounced for higher
levels of Zn ion implantation and deposition, whereas no
significant difference in the quantified numbers of cells was noted
between the Zn-Ti-60 min and Zn-Ti-80 min groups (P>0.05;
Fig. 5).
MG-63 cell cycle distribution on pure and
Zn-modified Ti surfaces
After 48 h in culture, flow cytometric analysis
revealed that the percentage of cells in S phase was 28.62% in the
cp-Ti group (Fig. 6A), 30.1% in
the Zn-Ti-20 min group (Fig. 6B),
31.9% in the Zn-Ti-40 min group (Fig.
6C), 34.2% in the Zn-Ti-60 min group (Fig. 6D) and 36.3% in the Zn-Ti-80 min
group (Fig. 6E). These percentages
for each of the five groups were significantly different from each
other (P<0.01) within this gradual increase in the S phase from
the Zn-Ti-20 min group to the Zn-Ti-80 min group. These results
demonstrated that the modified Zn-PIIID-Ti surfaces may inhibit
MG-63 cell apoptosis as well as promote proliferation.
Discussion
Previous studies have reported that increased
surface roughness of Ti implants improves t he rate of
osseointegration and biomechanical fixation (16–18).
Surface roughness in the nanometer range is important in the
absorption of proteins and adhesion of osteoblastic cells and thus
affects the rate of osseointegration (19). However, surface roughness at this
scale is difficult to achieve using chemical treatments. In
addition, the optimal surface nanotopography for selective
adsorption of proteins that enable adhesion of osteoblastic cells
and rapid bone apposition remains to be elucidated. Therefore,
other roughening methods with more predictable outcomes are
required.
PIIID overcomes the limitations of regular ion beam
implantation and is suitable for processing samples with complex
shapes (20). Following PIIID
processing, the anti-corrosion, anti-abrasion and anti-fatigue
properties of materials are significantly improved and therefore,
it has gained attention worldwide and is now used in several
biomedical applications (11–13).
In the present study, PIIID was used to implant and deposit Zn ions
within and onto the smooth surface of cp-Ti.
According to XPS analysis, with increasing Zn ion
implantation and deposition time, carbon, Ti and Zn signals
increased on the Zn-PIIID-Ti surfaces, whereas oxygen signals
decreased. The significant Zn content on the surface reached its
maximum with the 80 min deposition time. Following implantation and
deposition, the surfaces contained ZnO and TiO2. SEM
imaging revealed that following Zn-PIIID, dispersed lumps had
formed on the originally smooth Ti surface and this may explain why
certain Zn ions were implanted within the substrate matrix in the
form of interstitial atoms, while other Zn ions deposited directly
onto the Ti surface. Notably, the physical structure of the
Zn-Ti-80 min sample surface was the most homogeneous among assessed
surfaces, indicating that the size of granular masses became more
uniform with increasing PIIID processing time. SEM, in turn
revealed that Ti surfaces exhibited a rough ‘honeycomb’ structure
with a diameter range of 60–100 nm upon PIIID processing. The
‘honeycomb’ structure continually deepened with increasing PIIID
processing time and reached a depth of 200 nm with grade 9
smoothness and 0.4 μm roughness in the Zn-Ti-80 min group.
Thus, the present study demonstrated that the PIIID technique for
Ti surface modification offers an easy and effective way to
increase the roughness of the surfaces of Ti implants and the
degree of roughness achieved is more significant, with a deeper
nanostructure, than that generated using other methods (6,21,22).
MG-63 cells were seeded onto pure and Zn-modified Ti
surfaces in order to assess the effects of Zn implantation and
deposition on the biocompatibility of the Ti surfaces. SEM images
revealed pseudopodia extending from osteoblasts residing on the
Zn-modified Ti surfaces, indicating active cellular metabolism.
Cells extended multiple long and slender pseudopodia that inserted
between the granules of the Zn-modified Ti surfaces and formed
anchoring structures for strong adhesion. The present study also
revealed that the density of attached cells on the Zn-modified
surfaces was significantly greater than that observed on the
unmodified Ti surfaces and the secretion of ECM proteins followed
this trend as well, with ECM proteins covering the granules of
Zn-modified Ti surfaces. The current findings are in agreement with
the electron microscopy images presented in previous studies
(14,23,24)
of murine bone marrow cells grown on hydroxyapatite-coated cp-Ti
surfaces following 2 h incubation.
Based on the collective results of the present
study, it is concluded that Ti substrates modified using Zn PIIID
exhibit improved biocompatibility with the MG-63 cell line compared
with cp-Ti and thus may be applicable in a wide variety of dental
implants. Experiments to determine the in vivo performance
of these Zn-modified Ti substrates are underway. Based on the
present results demonstrating the biocompatibility of these
substrates as well as previous findings that Zn-modified Ti
surfaces discourage bacterial adhesion (14), it is expected that Zn-modification
of Ti may enhance the success rate of dental implants.
Acknowledgments
This study was supported by the Beijing Natural
Science Foundation (grant no. 7112124) and the Natural Science
Foundation of Hebei Province (grant no. H2013209040).
References
|
1
|
Rudy RJ, Levi PA, Bonacci FJ, Weisgold AS
and Engler-Hamm D: Intraosseous anchorage of dental prostheses: an
early 20th century contribution. Compend Contin Educ Dent.
29:220–222. 2008.PubMed/NCBI
|
|
2
|
Elias CN, Oshida Y, Lima JH and Muller CA:
Relationship between surface properties (roughness, wettability and
morphology) of titanium and dental implant removal torque. J Mech
Behav Biomed Mater. 1:234–242. 2008. View Article : Google Scholar
|
|
3
|
Condie R, Bose S and Bandyopadhyay A: Bone
cell-materials interaction on Si microchannels with bioinert
coatings. Acta Biomater. 3:523–530. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hara T, Matsuoka K, Matsuzaka K, Yoshinari
M and Inoue T: Effect of surface roughness of titanium dental
implant placed under periosteum on gene expression of bone
morphogenic markers in rat. Bull Tokyo Dent Coll. 53:45–50. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Oga M, Arizono T and Sugioka Y: Bacterial
adherence to bioinert and bioactive materials studied in vitro.
Acta Orthop Scand. 64:273–276. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li F and Zhu L: Effect of
surfactant-induced cell surface modifications on electron transport
system and catechol 1,2-dioxygenase activities and phenanthrene
biodegradation by Citrobacter sp. SA01. Bioresour Technol.
123:42–48. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nieciecka D, Nawara K, Kijewska K, et al:
Solid-core and hollow magnetic nanostructures: Synthesis, surface
modifications and biological applications. Bioelectrochemistry.
93:2–14. 2013. View Article : Google Scholar
|
|
8
|
Yamaguchi H, Ino S, Hamano N, Okada S and
Teranaka T: Examination of bond strength and mechanical properties
of Y-TZP zirconia ceramics with different surface modifications.
Dent Mater J. 31:472–480. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Iwai Y, Matsuda Y, Nakatsuka M, Mikami Y
and Kumabe S: A preliminary study of the dental implant therapy -
initial osteogenesis of human mesenchymal stem (HMS0014) cells on
titanium discs with different surface modifications. Okajimas Folia
Anat Jpn. 88:133–140. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qin Z, Huang Y, Liao Q, Zhang Z and Zhang
Y: Effect of surface modifications on ZnO nanorod arrays electrode
for dye-sensitized solar cells. J Nanosci Nanotechnol. 12:463–468.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang L, Huang L, Xie Z, Wang X and Tang B:
Fourth-generation plasma immersion ion implantation and deposition
facility for hybrid surface modification layer fabrication. Rev Sci
Instrum. 79:0233062008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kwok SC, Yang P, Wang J, Liu X and Chu PK:
Hemocompatibility of nitrogen-doped, hydrogen-free diamond-like
carbon prepared by nitrogen plasma immersion ion
implantation-deposition. J Biomed Mater Res A. 70:107–114. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang P, Huang N, Leng YX, et al:
Activation of platelets adhered on amorphous hydrogenated carbon
(a-C:H) films synthesized by plasma immersion ion
implantation-deposition (PIII-D). Biomaterials. 24:2821–2829. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xua JA, Ding G, Li JL, et al: Zinc-ion
implanted and deposited titanium surfaces reduce adhesion of
Streptococccus mutans. Appl Surf Sci. 256:7540–7544. 2010.
View Article : Google Scholar
|
|
15
|
Anna K, Nina P, Yuri K, et al: Coating
zinc oxide submicron crystals on poly(methyl methacrylate) chips
and spheres via ultrasound irradiation. Ultrason Sonochem.
15:839–845. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Le Guéhennec L, Soueidan A, Layrolle P and
Amouriq Y: Surface treatments of titanium dental implants for rapid
osseointegration. Dent Mater. 23:844–854. 2007. View Article : Google Scholar
|
|
17
|
Chung SH, Kim HK, Shon WJ and Park YS:
Peri-implant bone formations around (Ti,Zr)O(2)-coated zirconia
implants with different surface roughness. J Clin Peridontol.
40:404–411. 2013. View Article : Google Scholar
|
|
18
|
Sul YT: Electrochemical growth behavior,
surface properties, and enhanced in vivo bone response of
TiO2 nanotubes on microstructured surfaces of blasted,
screw-shaped titanium implants. Int J Nanomedicine. 5:87–100. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Triplett RG, Frohberg U, Sykaras N and
Woody RD: Implant materials, design and surface topographies: their
influence on osseointegration of dental implants. J Long Term Eff
Med Implants. 13:485–501. 2003. View Article : Google Scholar
|
|
20
|
Sosa NE, Chen C, Liu J, et al: Nanoscale
structure, composition and charge transport analysis of transparent
conducting oxide nanowires written by focused ion beam
implantation. J Am Chem Soc. 132:7347–7354. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Minagar S, Berndt CC, Wang J, Ivanova E
and Wen C: A review of the application of anodization for the
fabrication of nanotubes on metal implant surfaces. Acta Biomater.
8:2875–2888. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Saksø M, Jakobsen SS, Saksø H, et al: Acid
etching and plasma sterilization fail to improve osseointegration
of grit blasted titanium implants. Open Orthop J. 6:376–382. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hempel U, Hefti T, Dieter P and Schlottig
F: Response of human bone marrow stromal cells, MG-63 and SaOS-2 to
titanium-based dental implant surfaces with different topography
and surface energy. Clin Oral Implants Res. 24:174–182. 2013.
View Article : Google Scholar
|
|
24
|
Cosar M, Ozer AF, Iplikcioglu AC, et al:
The results of beta-tricalcium phosphate coated hydroxyapatite
(beta‑TCP/HA) grafts for interbody fusion after anterior cervical
discectomy. J Spinal Disord Tech. 21:436–441. 2008. View Article : Google Scholar : PubMed/NCBI
|




















