Introduction
Idiopathic pulmonary fibrosis (IPF) is a fatal and
highly destructive chronic interstitial lung disease, and the
survival time following initial diagnosis is just 2–5 years
(1). To date, no effective
therapeutic strategy for IPF, except lung transplantation, has been
developed (2). Therefore,
investigation into the pathological mechanisms underlying IPF is
urgently required.
Amongst the factors associated with IPF, fibroblasts
have been demonstrated to have a critical role (3). In IPF, fibroblast accumulation
results in the irreversible destruction of lung architecture, and
the differentiation of fibroblasts induces deposition of
extracellular matrix (ECM) proteins, as well as α-smooth muscle
actin (α-SMA), under fibrotic conditions (3). The elucidation of fibroblast function
is therefore critical for understanding the molecular mechanisms
underlying IPF. Accumulating evidence has demonstrated that
transforming growth factor-β (TGF-β), sphingosine-1-phosphate (S1P)
and lysophosphatidic acid (LPA) are involved in IPF (3–8).
Recent studies indicated that TGF-β, LPA and S1P induced
differentiation of lung fibroblasts, which was followed by
increased expression of ECM proteins, such as fibronectin (FN)
(4–6,9).
Notably, during LPA stimulation of lung fibroblasts, TGF-β
expression increased via LPA receptor type 2-dependent pathways
(5). However, the crosstalk
between the LPA and TGF-β signaling pathways in lung fibroblast
differentiation has remained elusive.
IQ motif containing guanosine triphosphatase
activating protein 1 (IQGAP1), an effector of CDC42, is a
multidomain molecule implicated in the modulation of cell
architecture and the regulation of exocytosis in multiple types of
human cancer (10). In particular,
IQGAP1 has been demonstrated to be expressed and localized in human
cancer tissues (11–14). Recently, IQGAP1 was found to
suppress TGF-β/TGF-β receptor 2 (RII)-dependent myofibroblastic
differentiation in the tumor microenvironment (15). An in vitro study also
indicated that IQGAP1 knockdown increased TβRII stability, and
TGF-β1 induced the transdifferentiation of pericytes to
myofibroblasts (15). Amongst
patients with interstitial lung disease (ILD), the expression of
IQGAP1 is increased in the lung fibroblasts of scleroderma patients
with ILD, and IQGAP1 has crucial functions in the regulation of
endothelial and epithelial cell migration (16). However, identification of IQGAP1
expression in pulmonary fibroblasts during fibrogenesis has
remained elusive.
The present study therefore examined the expression
of IQGAP1 in mouse and human fibroblasts, in order to characterize
its expression under fibrotic conditions.
Materials and methods
Reagents and kits
Bleomycin sulfate (BLM) was purchased from Hospira
Inc. (Lakeforest, IL, USA). LPA (18:1) was obtained from Avanti
Polar Lipids (Alabaster, AL, USA). Human TGF-β1 protein was
obtained from PeproTech, Inc. (Rocky Hill, NJ, USA). Cell lysis
buffer was from Cell Signaling Technology Inc. (Danvers, MA, USA).
Horseradish peroxidase-linked goat anti-mouse immunoglobulin G
(IgG) (cat. no. 170-6516) and goat anti-rabbit IgG (cat. no.
170-6515) secondary antibodies were purchased from Bio-Rad
Laboratories, Inc. (Hercules, CA, USA). The control chicken
anti-goat polyclonal IgG (cat. no. BAF019) and neutralizing chicken
polyclonal anti-TGF-β1 (cat. no. AF-101-NA) antibodies were
obtained from R&D Systems (Minneapolis, MN, USA). Rabbit
polyclonal anti-FN (cat. no. sc-9068) and rabbit polyclonal
anti-IQGAP1 (cat. no. sc-10792) antibodies were obtained from Santa
Cruz Biotechnology, Inc. (Dallas, TX, USA). Mouse monoclonal
anti-α-smooth muscle actin (α-SMA; cat. no. A2547), mouse
monoclonal anti-β-actin (cat. no. A5316) and anti-Bay 11-7082
(NF-κB inhibitor; cat. no. B5556) antibodies were obtained from
Sigma-Aldrich (St. Louis, MO, USA).
Mouse model of IPF
The experimental model of pulmonary fibrosis was
designed as described previously (5,6).
C57/BL6 mice (50 male mice, 8 weeks-old, weighing ~25 g) purchased
from Jackson Laboratory (Bar Harbor, ME, USA) underwent
bleomycin-induced fibrosis. The mice were housed under a controlled
12:12 h light:dark cycle. The air temperature was maintained at
22°C and the mice were given ad libitum access to food and
water. The mice were anesthetized with a 3 ml/kg mixture of 25
mg/kg of ketamine in 2.5 ml of xylazine (NCE Biomedical Co. Ltd.
Wuhan, Hubei, China), followed by treatment with saline or
bleomycin sulfate (1.5 U/kg of body weight, ~0.03 U/animal) in
saline by intratracheal injection in a total volume of 50
μl. A total of 21 days after bleomycin administration 34
mice were sacrificed using CO2 euthanasia, and the lungs
were harvested for isolation of lung fibroblasts. All animal
protocols conformed to the standards of Xuzhou Medical College
(Huaian, China) and were in accordance with Chinese animal
operation regulations. The present study was approved by the Ethics
Committee of Xuzhou Medical College.
Isolation of mouse primary fibroblasts
and lung fibroblast culture
Mouse lung fibroblasts were isolated as described
previously (5,6). The WI-38 human lung fibroblast cell
line was purchased from the American Type Culture Collection
(Manassas, VA, USA). Mouse primary lung fibroblasts and WI-38 cells
were cultured in six-well dishes using Dulbecco’s modified Eagle’s
medium (Life Technologies, Carlsbad, CA, USA), supplemented with
10% fetal bovine serum (Life Technologies).
Neutralizing antibodies or NF-κB
inhibitor treatment
WI-38 cells (~90% confluence) were serum-starved for
24 h, and subsequently treated with neutralized control IgG or
anti-TGF-β antibody (5 μg/ml) for 1 h. The starved WI-38
cells were then incubated with 10 μM NF-κB inhibitor (Bay
11-7082) for 1 h. Subsequently, the fibroblasts were treated with
TGF-β (5 ng/ml) or LPA (10 μM) for 48 h. Finally, the cells
were lysed with cell lysis buffer (cat. no. 9803S; Cell Signaling
Technology Inc.) and western blot analysis was used to evaluate
protein expression.
Western blot analysis
Western blot analysis was used to evaluate protein
expression and was performed as described previously (5). Cell lysates (20–30 μg protein,
1.5 mg/ml) were cleared by centrifugation at 10,000 × g for 10 min
and boiled with Laemmli sample buffer (Sigma-Aldrich) for 5 min.
The protein was assayed using the Pierce™ Bicinchoninic Acid
Protein Assay kit (Thermo Fisher Scientific, Inc., Rockford, IL,
USA). The cell lysates were separated by 10% or 4–20% SDS-PAGE and
transferred to polyvinylidene fluoride (PVDF) membranes (Bio-Rad
Laboratories, Inc.). The membranes were then blocked with
tris-buffered saline-Tween (TBST) containing 5% bovine serum
albumin (Bio-Rad Laboratories, Inc.), prior to incubation with the
primary antibodies (1:2,000 dilutions) overnight at 4°C.
Subsequently, the PVDF membranes were washed with TBST buffer and
incubated with secondary antibodies for 2 h at room temperature
(1:2,000 dilutions). All primary and secondary antibodies used were
described in the reagents and kits section. The blots were
visualized using an enhanced chemiluminescence kit (cat. no.
ab65623; Abcam, Cambridge, MA, USA). The integrated density of
pixels on each membrane was quantified and normalized to actin
using Image Quant software version 5.2 (Molecular Dynamics,
Sunnyvale, CA, USA).
Immunofluorescence microscopy
Immunofluorescence microscopy was used to examine
protein expression as described previously (5). Briefly, mouse lung fibroblasts were
incubated with the primary antibodies at a 1:200 dilution in
blocking buffer (Bio-Rad Laboratories, Inc.) for 1 h at room
temperature. Subsequently, the cells were incubated with the
secondary antibodies with Alexa Fluor (Life Technologies) at a
1:200 dilutions in blocking buffer for 1 h at room temperature.
Slides were examined under a Nikon Eclipse TE 2000-S fluorescence
microscope (Nikon Corp., Tokyo, Japan) and recorded with a 60× oil
immersion objective lens.
Statistical analysis
The data from at least three independent sets of
experiments are displayed as the mean ± standard error of the mean.
For data analysis, one-way analysis of variance or a two-tailed
Student’s t-test in SPSS version 22.0 (IBM, Armonk, NY, USA) was
used. P<0.05 was considered to indicate a statistically
significant difference between values (17,18).
Results
IQGAP1 expression is decreased in the
lung fibroblasts of BLM-treated mice
As shown in Fig.
1A–C, the expression of α-SMA and FN was markedly higher in
lung fibroblasts isolated from BLM-challenged mice than that in the
fibroblasts of mice without BLM-challenge. However, the IQGAP1
expression levels were significantly lower in lung fibroblasts from
BLM-challenged mice than in those from control mice (Fig. 1A and D). Immunofluorescent staining
also indicated that the expression levels of IQGAP1 were lower in
lung fibroblasts from BLM-challenged mice, than in those of the
control mice (Fig. 1E).
 | Figure 1α-SMA, FN and IQGAP1 expression in
mouse lung fibroblasts. (A) Representative western blot of the
expression of FN, α-SMA, IQGAP1 and actin in mouse lung
fibroblasts. Quantification of the expression of (B) FN, (C) α-SMA
and (D) IQGAP1. Values are presented as the mean ± standard error
of the mean. *P<0.05, vs. fibroblasts from mice without BLM
challenge (control). (E) Immunoforesent staining of IQGAP1 and
α-SMA in mouse lung fibroblasts (magnification, ×60), α-SMA (red),
IQGAP1 (green) and DAPI (blue). α-SMA, α-smooth muscle actin; FN,
fibronectin; IQGAP1, IQ motif containing guano-sine triphosphatase
activating protein 1; BLM, bleomycin sulfate; PBS,
phophate-buffered saline. |
TGF-β downregulates the expression of
IQGAP1 in WI-38 human lung fibroblast cells
In order to determine whether the expression of
IQGAP1 was associated with lung fibroblast activation and
differentiation, TGF-β -induced IQGAP1 expression was examined in
WI-38 cells. TGF-β treatment (5 ng/ml, 48 h) induced fibroblast
differentiation, characterized by the enhanced expression of α-SMA
and FN (Fig. 2A–C), as well as
significantly inhibiting the expression of IQGAP1 in WI-38 cells
(Fig. 2A and D).
TGF-β inhibits IQGAP1 expression via the
NF-κB pathway
To further examine the potential mechanisms
underlying the TGF-β-induced inhibition of IQGAP1 expression in
lung fibroblasts, WI-38 cells were treated with NF-κB inhibitor
(Bay 11-7082; 10 μM) for 1 h prior to TGF-β-challenge. As
indicated in Fig. 3, TGF-β
challenge induced upregulation of the expression of α-SMA and FN,
and NF-κB inhibitor treatment markedly attenuated the effects of
TGF-β. Notably, the inhibitory role of TGF-β in IQGAP1 expression
in WI-38 cells was also significantly restored following the
inhibition of NF-κB pathways (Fig. 3A
and D). Therefore, these results indicated that TGF-β inhibited
IQGAP1 expression via the NF-κB signaling pathways.
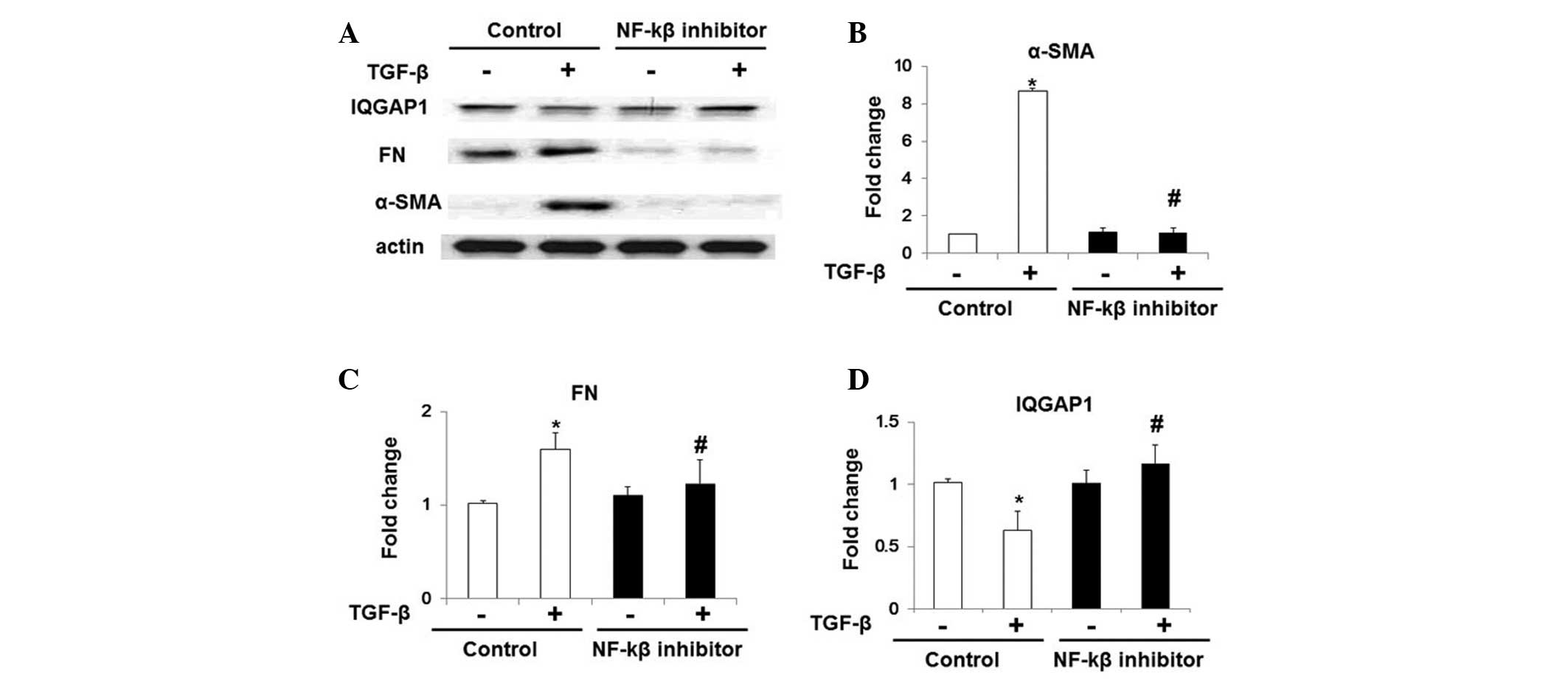 | Figure 3NF-κB pathway inhibition restores
TGF-β-inhibited IQGAP1 expression in WI-38 cells. NF-κB inhibitor
(bay 11-7082; 10 μM, 1 h)-treated WI-38 cells were
challenged with TGF-β (5 ng/ml, 48 h), and protein expression
levels were analyzed by western blot analysis. (A) Representative
western blot of protein expression of α-SMA, FN, actin and IQGAP1.
Quantification of the protein expression levels of (B) FN, (C)
α-SMA and (D) IQGAP1 in WI-38 cells with or without
TGF-β-challenge. Values are expressed as the mean ± standard error
of the mean. *P<0.05, vs. WI-38 cells without TGF-β treatment;
#P<0.05, vs. WI-38 cells without NF-κB inhibitor
challenge but with TGF-β treatment. NF-κB, nuclear factor-κB;
TGF-β, transforming growth factor-β; IQGAP1, IQ motif containing
guanosine triphosphatase activating protein 1; α-SMA, α-smooth
muscle actin; FN, fibronectin. |
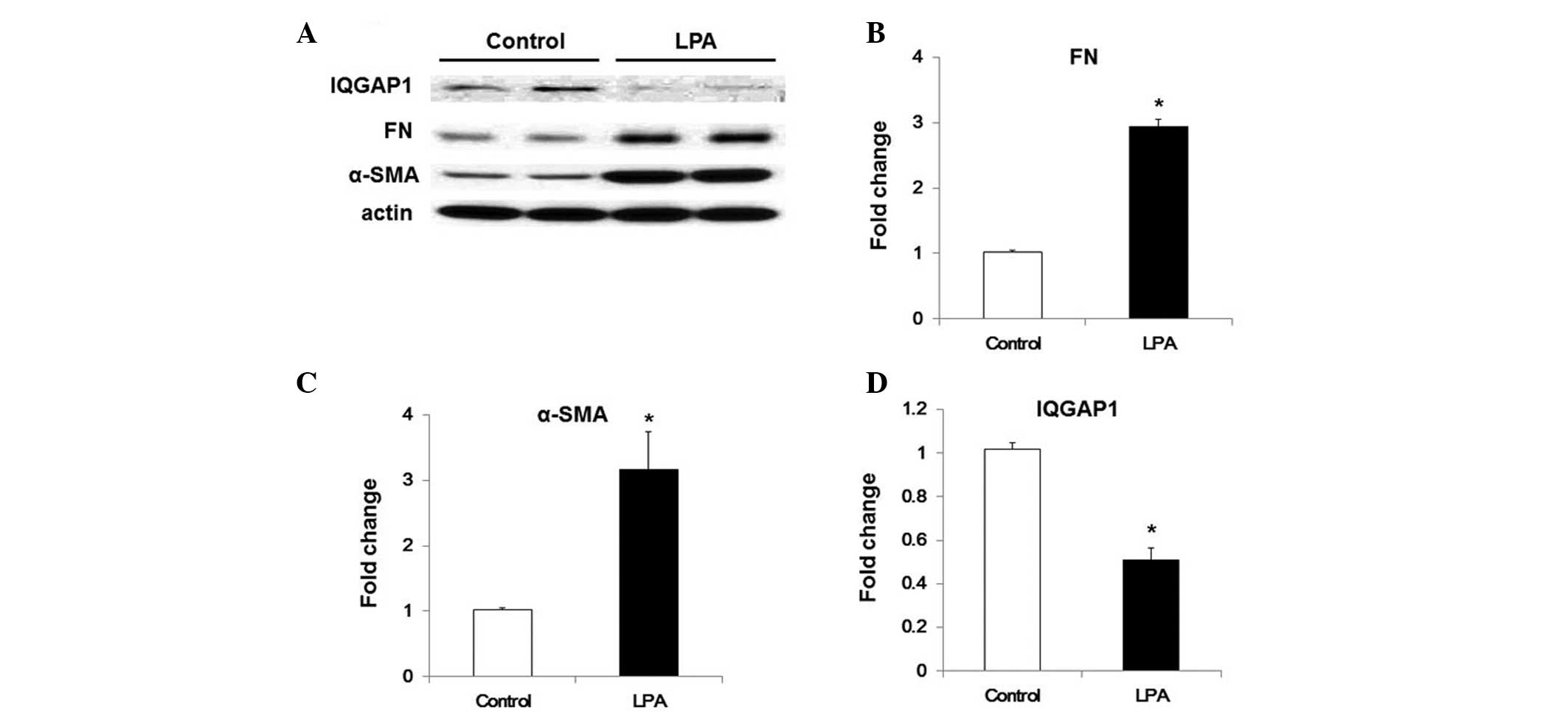
LPA downregulates the expression of IQGAP
in WI38 cells via TGF-β-dependent pathways
LPA has been demonstrated to be involved in lung
fibrogenesis, particularly by inducing the activation and
recruitment of fibroblasts (4,5).
Recently, LPA was revealed to enhance TGF-β expression levels in
human lung fibroblasts, potentially via LPA receptor type
2-associated pathways (5). The
present study therefore evaluated the effects of LPA on IQGAP1
expression in WI-38 cells. LPA treatment was demonstrated to induce
an increase in α-SMA and FN expression levels, and inhibit IQGAP1
expression in WI38cells (Fig. 4).
In order to determine whether LPA-induced TGF-β secretion was
associated with the effects of LPA on IQGAP1 expression in WI-38
cells, cells were pre-incubated with chicken anti-TGF-β1 antibody
(5 ng/ml) for 1 h. As shown in Fig.
5, anti-TG-Fmarkedly inhibited LPA-induced fibroblast
differentiation, and significantly increased IQGAP1 expression
under LPA treatment (Fig. 5).
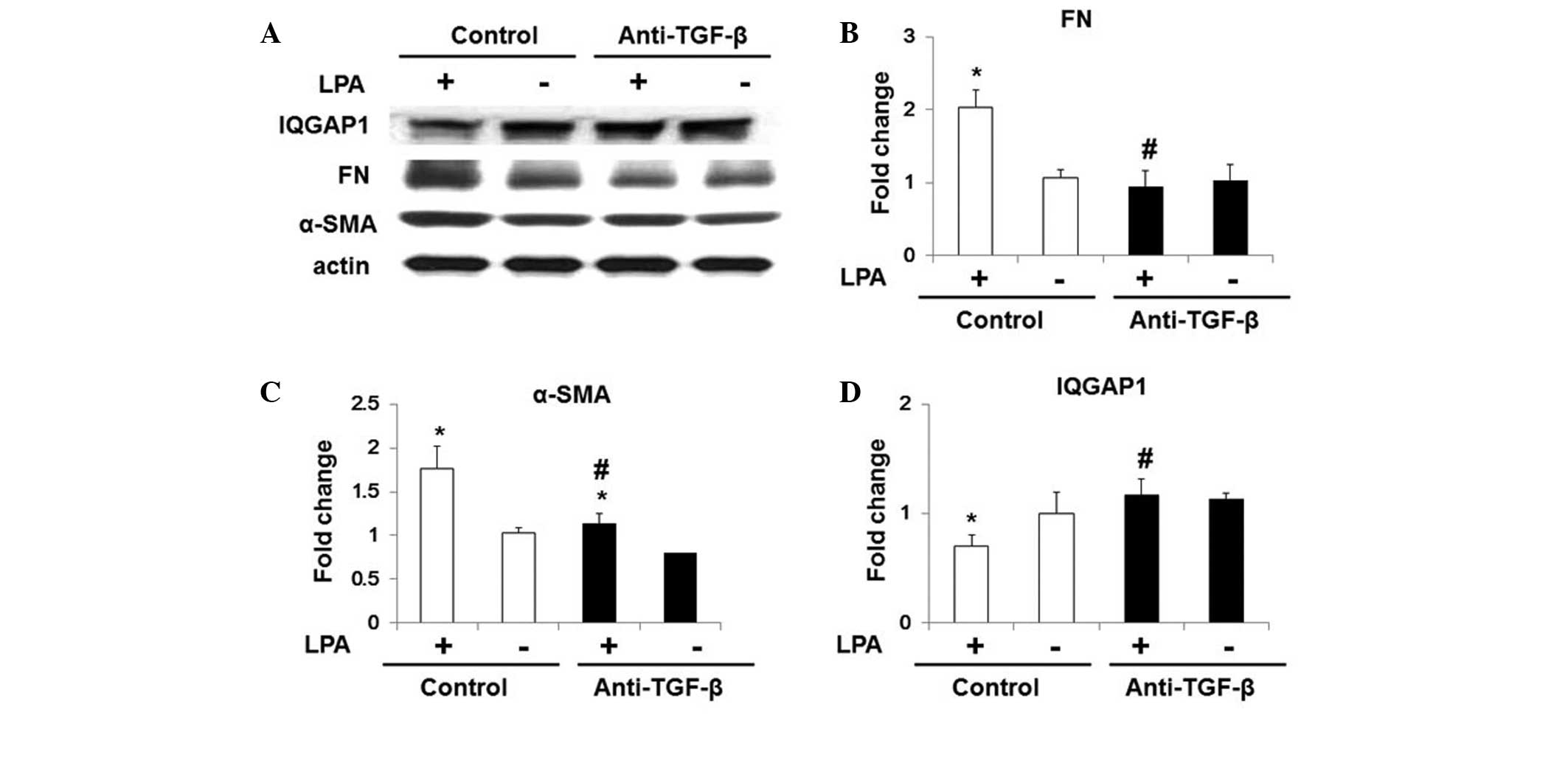 | Figure 5Anti-TGF-β antibody restores LPA
induced inhibition of IQGAP1 expression in WI-38 cells. Starved
WI-38 cells were treated with control immunoglobulin G and
anti-TGF-β antibody (5 μg/ml) for 1 h, and further
challenged with 18:1 LPA (0, 10, 20 or 30 μM) for 48 h. (A)
Representative western blot for protein expression of α-SMA, FN,
actin and IQGAP1. Quantification of the protein expression of (B)
FN, (C) α-SMA and (D) IQGAP1 in WI-38 cells. Values are expressed
as the mean ± standard error of the mean. *P<0.05, vs. WI-38
cells with control antibody but without LPA challenge;
#P<0.05, vs. LPA-challenged WI-38 cells pre-treated
with control antibody. TGF-β, transforming growth factor-β; LPA,
lysophosphatidic acid; IQGAP1, IQ motif containing guanosine
triphosphatase activating protein 1; FN, fibronectin; α-SMA,
α-smooth muscle actin. |
Discussion
IPF is a chronic lung disease, for which there is
currently a lack of effective treatment. Lung transplantation is
the only effective clinical therapeutic approach for IPF (1,2,19).
Inflammatory cells, epithelial cells and fibroblasts are involved
in pulmonary fibrogenesis, and the expression of various bioactive
ligands are altered during the fibrogenesis (3,20).
TGF-β and LPA have been demonstrated to be the key factors
underlying fibrogenesis, and to regulate the activation and
differentiation of fibroblasts (4,5,6).
Therefore, investigation into the effects of TGF-β and LPA on
fibrogenesis are essential for the elucidation of the pathological
mechanisms underlying IPF.
IQGAP1, which is one of three IQGAP homologs, is
expressed in all human organs (21,22).
Increasing evidence has indicated that IQGAP1 regulates cell
migration and adhesion, extracellular signals and cytokinesis in
multiple types of cell (23,24).
An oncological study revealed that IQGAP1 expression and
localization are frequently altered in tumor tissues, and its
expression is correlated with cancer progression (10). According to western blot and
quantitative polymerase chain reaction analyses, the expression of
IQGAP1 is also increased in various types of cancer cell (23,24).
Detailed analysis of this effect indicated that IQGAP1 was
associated with the β-catenin and extracellular signal-regulated
kinase (ERK) signaling pathways. It also demonstrated that IQGAP1
also binds with mitogen-activated protein kinase kinase, B-Raf and
ERK to regulate their sequential signaling cascades, resulting in
tumorigenesis in humans and experimental animals (25,26).
Investigation in liver cancer indicated that IQGAP1 inhibited the
TGF-β/TβRII/myofibroblast differentiation signaling pathway and
blocked tumor growth in the tumor tissues. These effects are
associated with the binding of IQGAP1 to TβRII, inducing the
ubiquitination and degradation of hepatic stellate cells (15). A recent study indicated that PDGF
stimulation rapidly promotes the association of IQGAP1 with PDGF
receptor-β (PDGFR), and overexpression of IQGAP1 enhances PDGFR
autophosphorylation (27). Thus
suggesting that through interaction with PDGFR and focal adhesions
signaling proteins, IQGAP1 promotes activation of PDGFR in focal
adhesions, and contributes to vascular smooth muscle cell migration
and neointimal formation after injury (27). In addition, IQGAP1 increases
bronchial epithelial cell proliferation and wound closure via the
phosphorylation of IQGAP1 (28).
However, to the best of our knowledge, the expression of IQGAP1
during pulmonary fibrosis has not previously been investigated, and
the potential roles of IQGAP1 in fibroblasts have remained elusive.
In the present study, the expression of IQGAP1 was evaluated in
human and mouse lung fibroblasts during pulmonary fibrosis. The
results suggested that the expression of IQGAP1 was markedly
decreased in pulmonary fibroblasts under fibrotic conditions. These
studies therefore suggested that IQGAP1 expression was correlated
with the differentiation of lung fibroblasts and lung fibrosis.
Various bioactive lipid compounds have been
implicated in inflammation and pulmonary fibrosis (5,6,17,18,29–33).
In particular, S1P and LPA induce fibroblast recruitment and
differentiation during pulmonary fibrogenesis (4–6). LPA
also induces the expression of TGF-β in fibroblasts from human lung
tissue in a dose-dependent manner, through activation of LPA
receptor type 2 (5). The present
study indicated that LPA also inhibited IQGAP1 expression via TGF-β
secretion, which suggested that there was crosstalk between the LPA
and TGF-β signaling pathways in the regulation of IQGAP1 expression
in lung fibroblasts during IPF.
In conclusion, the results of the present study
revealed that TGF-β inhibited the expression of IQGAP1 via
activation of the NF-κB signaling pathway, and suggested that
targeting IQGAP1 to inhibit pulmonary fibrosis may present
potential novel therapeutic strategies for the treatment of
IPF.
References
|
1
|
Huang LS and Natarajan V: Sphingolipids in
pulmonary fibrosis. Adv Biol Regul. 57C:55–63. 2015. View Article : Google Scholar
|
|
2
|
Marks JH: Update in pulmonary medicine.
Adolesc Med State Art Rev. 24:307–329. 2013.PubMed/NCBI
|
|
3
|
Wynn TA: Integrating mechanisms of
pulmonary fibrosis. J Exp Med. 208:1339–1350. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tager AM, LaCamera P, Shea BS, et al: The
lysophosphatidic acid receptor LPA (1) links pulmonary fibrosis to
lung injury by mediating fibroblast recruitment and vascular leak.
Nat Med. 14:45–54. 2008. View
Article : Google Scholar
|
|
5
|
Huang LS, Fu P, Patel P, Harijith A, Sun
T, Zhao Y, Garcia JG, Chun J and Natarajan V: Lysophosphatidic acid
receptor 2 deficiency confers protection against bleomycin-induced
lung injury and fibrosis in mice. Am J Respir Cell Mol Biol.
49:912–922. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huang LS, Berdyshev E, Mathew B, et al:
Targeting sphingosine kinase 1 attenuates bleomycin-induced
pulmonary fibrosis. FASEB J. 27:1749–1760. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Natarajan V, Dudek SM, Jacobson JR, et al:
Sphingosine-1-phosphate, FTY720 and sphingosine-1-phosphate
receptors in the pathobiology of acute lung injury. Am J Respir
Cell Mol Biol. 49:6–17. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bartram U and Speer CP: The role of
transforming growth factor beta in lung development and disease.
Chest. 125:754–765. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rancoule C, Pradere JP, Gonzalez J, Klein
J, Valet P, Bascands JL, Schanstra JP and Saulnier-Blache JS:
Lysophosphatidic acid-1-receptor targeting agents for fibrosis.
Expert Opin Investig Drugs. 20:657–667. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Johnson M, Sharma M and Henderson BR:
IQGAP1 regulation and roles in cancer. Cell Signall. 21:1471–1478.
2009. View Article : Google Scholar
|
|
11
|
Nabeshima K, Shimao Y, Inoue T and Koono
M: Immunohistochemical analysis of IQGAP1 expression in human
colorectal carcinomas: its overexpression in carcinomas and
association with invasion fronts. Cancer Lett. 176:101–109. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Clark EA, Golub TR, Lander ES and Hynes
RO: Genomic analysis of metastasis reveals an essential role for
RhoC. Nature. 406:532–535. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brown MD and Sacks DB: IQGAP1 in cellular
signaling: bridging the GAP. Trends Cell Biol. 16:242–249. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Takemoto H, Doki Y, Shiozaki H, Imamura H,
Utsunomiya T, Miyata H, Yano M, Inoue M, Fujiwara Y and Monden M:
Localization of IQGAP1 is inversely correlated with intercellular
adhesion mediated by e-cadherin in gastric cancers. Int J Cancer.
91:783–788. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu C, Billadeau DD, Abdelhakim H, Leof E,
Kaibuchi K, Bernabeu C, Bloom GS, Yang L, Boardman L, Shah VH and
Kang N: IQGAP1 suppresses TbetaRII-mediated myofibroblastic
activation and metastatic growth in liver. J Clin Invest.
123:1138–1156. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bogatkevich GS, Ludwicka-Bradley A,
Singleton CB, Bethard JR and Silver RM: Proteomic analysis of
CTGF-activated lung fibroblasts: identification of IQGAP1 as a key
player in lung fibroblast migration. Am J Physiol Lung Cell Mol
Physiol. 295:L603–L611. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang LS, Hung ND and SokDEandKim MR:
Lysophosphatidylcholine containing docosahexaenoic acid at the sn-1
position is anti-inflammatory. Lipids. 45:225–236. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang LS, Kang JS, Kim MR and Sok DE:
Oxygenation of arachidonoyl lysophospholipids by lipoxygenases from
soybean, porcine leukocyte, or rabbit reticulocyte. J Agric Food
Chem. 56:1224–1232. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
King TE: Update in pulmonary medicine. Ann
Intern Med. 129:806–812. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wolters PJ, Collard HR and Jones KD:
Pathogenesis of idiopathic pulmonary fibrosis. Annu Rev Pathol.
9:157–79. 2014. View Article : Google Scholar :
|
|
21
|
Weissbach L, Settleman J, Kalady MF,
Snijders AJ, Murthy AE, Yan YX and Bernards A: Identification of a
human rasGAP-related protein containing calmodulin-binding motifs.
J Biol Chem. 269:20517–20521. 1994.PubMed/NCBI
|
|
22
|
Wang S, Watanabe T, Noritake J, Fukata M,
Yoshimura T, Itoh N, Harada T, Nakagawa M, Matsuura Y, Arimura N
and Kaibuchi K: IQGAP3, a novel effector of Rac1 and Cdc42,
regulates neurite outgrowth. J Cell Sci. 120:567–577. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jadeski L, Mataraza JM, Jeong HW, Li Z and
Sacks DB: IQGAP1 stimulates proliferation and enhances
tumorigenesis of human breast epithelial cells. J Biol Chem.
283:1008–1017. 2008. View Article : Google Scholar
|
|
24
|
Dong PX, Jia N, Xu ZJ, Liu YT, Li DJ and
Feng YJ: Silencing of IQGAP1 by shRNA inhibits the invasion of
ovarian carcinoma HO-8910PM cells in vitro. J Exp Clin Cancer Res:
CR. 27:772008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ren JG, Li Z and Sacks DB: IQGAP1
modulates activation of B-Raf. Proc Nat Acad Sci USA.
104:10465–10469. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Roy M, Li Z and Sacks DB: IQGAP1 is a
scaffold for mitogen-activated protein kinase signaling. Mol Cell
Biol. 25:7940–7952. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kohno T, Urao N, Ashino T, Sudhahar V,
Inomata H, Yamaoka-Tojo M, McKinney RD, Fukai T and Ushio-Fukai M:
IQGAP1 links PDGF receptor-beta signal to focal adhesions involved
in vascular smooth muscle cell migration: role in neointimal
formation after vascular injury. Am J Physiol Cell Physiol.
305:C591–C600. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang Y, Wang M, Wang F, Zhu M, Ma Y, Wang
X and Wu R: IQGAP1 promotes cell proliferation and is involved in a
phosphorylation-dependent manner in wound closure of bronchial
epithelial cells. Int J Mol Med. 22:79–87. 2008.PubMed/NCBI
|
|
29
|
Huang LS, Kim MR and Sok DE: Linoleoyl
lysophosphatidylcholine is an efficient substrate for soybean
lipoxygenase-1. Arch Biochem Biophys. 455:119–126. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang LS, Kim MR and Sok DE: Oxygenation
of 1-docosahexaenoyl lysophosphatidylcholine by lipoxygenases;
conjugated hydroperoxydiene and dihydroxytriene derivatives.
Lipids. 42:981–990. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang LS, Kim MR and Sok DE: Regulation of
lipoxygenase activity by polyunsaturated lysophosphatidylcholines
or their oxygenation derivatives. J Agric Food Chem. 56:7808–7814.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huang LS, Kim MR and Sok DE: Enzymatic
reduction of polyunsaturated lysophosphatidylcholine hydroperoxides
by glutathione peroxidase-1. Eur J Lipid Sci Tech. 111:584–592.
2009. View Article : Google Scholar
|
|
33
|
Huang LS, Mathew B, Li H, et al: The
mitochondrial cardiolipin remodeling enzyme lysocardiolipin
acyltransferase is a novel target in pulmonary fibrosis. Am J
Respir Crit Care Med. 189:1402–1415. 2014. View Article : Google Scholar : PubMed/NCBI
|