Introduction
Lung cancer is the leading cause of
cancer-associated mortality among males and the second leading
cause among females worldwide (1).
The incidence of lung cancer is increasing in females worldwide and
is the main cause of cancer-associated mortality among females in
Europe and the USA, exceeding breast and cervical cancer (2–10).
Lung adenocarcinoma is currently the most common pathological type
of lung cancer (11,12) and is the primary type of lung
cancer in females, adolescents and non-smokers.
Livin, a novel member of the inhibitor of apoptosis
(IAP) protein family, is not detected in the majority of normal
tissues, however, is highly expressed in various types of human
malignancy (13–30). Increased activity of this protein
may be used as a reliable prognostic factor for initial and late
resistance to chemotherapeutic drugs in certain types of human
tumor (31–37). Inhibition of livin gene expression
may effectively promote tumor cell apoptosis and raise the
sensitivity of tumor cells to different treatments in vitro
(35,36). Although several studies have
demonstrated that livin may be used as an effective target for
tumor therapy (38–40), few studies have focused on human
lung adenocarcinoma. Therefore, the present study aimed to
investigate the treatment effect of livin expression inhibition in
lung adenocarcinoma.
In the present study, two different methods were
used to investigate the tumor-suppressing effect of livin in human
lung adenocarcinoma. Firstly, small interfering (si)RNA technology
was used to downregulate livin expression, which was confirmed by
reverse transcription quantitative polymerase chain reaction
(RT-qPCR) and western blot analysis. In addition, cell
proliferation was assessed using an MTT assay in vitro.
Secondly, inhibition of livin expression was induced through the
synergistic inhibitory effect between flavopiridol and tumor
necrosis factor-related apoptosis-inducing ligand (TRAIL).
Furthermore, the effect of the inhibition of livin expression on
SPC-A1 tumor cell proliferation and sensitivity to the chemotherapy
drug cisplatin was investigated. The combination of chemotherapy
and downregulation of livin expression may contribute to the
treatment of human lung adenocarcinoma drug-resistant tumor
cells.
Materials and methods
Materials
The human lung adenocarcinoma SPC-A1 cell line was
acquired from Nanjing KeyGen Biotech., Co., Ltd. (Nanjing, China).
The primers for livin siRNA and control siRNA were synthesized by
Beijing Genomics Institute (Beijing, China). All antibodies were
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA).
Drugs and reagents
Dulbecco’s modified Eagle’s medium (DMEM) and fetal
bovine serum (FBS) were purchased from Gibco-BRL (Carlsbad, CA,
USA). TRIzol reagent and Lipofectamine 2000 were obtained from
Invitrogen Life Technologies (Carlsbad, CA, USA). Reverse
Transcriptase SYBR Green Master mixture was acquired from Takara
Bio, Inc. (Otsu, Japan). The bicinchoninic acid protein assay kit
and ECL-Plus kit were purchased from Thermo Scientific (Rockford,
IL, USA). The MTT cell proliferation assay kit was purchased from
Sangon Biotech (Shanghai, China) and TRAIL was purchased from Merck
Millipore (Darmstadt, Germany). In addition, flavopiridol was
obtained from Sigma-Aldrich (St. Louis, MO, USA) and Z-VAD-FMK was
purchased from R&D Systems (Minneapolis, MN, USA).
Cell culture and transfection
SPC-A1 cells were cultured in DMEM medium
supplemented with 10% heat-inactivated FBS. All were placed in a
humidified incubator, containing 5% CO2 at 37°C. SPC-A1
cells were replated at 2×105 cells/well in six-well
plates once they had reached exponential phase. When the cell
density reached 40–50%, cells were transfected with Lipofectamine
2000 with a pcDNA3.1 expression vector (Invitrogen Life
Technologies) and cultured at 37°C and 5% CO2 for 24 h.
The clone in which the livin-siRNA was transfected was termed the
livin-siRNA group, the group transfected with the negative control
vector was termed the negative control (NC) group and SPC-A1 cells
were termed the control (CON) group. The following RNA silencing
sequences were used: siRNA-livin790, forward
5′-GAGAGGUCCAGUCUGAAAG-3′ and reverse 5′-CUUUCAGACUGGACCUCUC-3′ and
siRNA-livin180, forward 5′-CCUAAAGACAGUGCCAAGU-3′ and reverse
5′-ACUUGGCACUGUCUUUAGG-3′.
RT-qPCR
Total RNA was isolated with TRIzol reagent and
reverse-transcribed to synthesize cDNA. cDNA was subsequently
amplified by SYBR-Green based qPCR using the following primers:
Livin, forward 5′-GGAGAGAGGTCCAGTCTGAAAGT-3′ and reverse
5′-ACCTTGCACGTCCTCTCCTC-3′ and Homosapiens histone
acetyltransferase (HBOA), forward 5′-ATCAAAGAAATCAGTCAGGAGACG-3′
and reverse 5′-CTCTTTGGCTATCCACTCATCAAT-3′. The 25 μl
fluorescent PCR reaction mix contained: 8.5 μl
ddH2O, 12.5 μl 2X SYBR Premix Ex Taq II, 2
μl cDNA and 1.0 μl forward and reverse primers (10
μmol/l), respectively. The cycling parameters were as
follows: 40 cycles, including denaturation at 95°C for 5 sec,
annealing at 60°C for 30 sec and extension at 72°C for 30 sec.
Melting curve analysis was used to confirm the primer specificity.
The comparative cycle threshold (Ct) method was used for
calculation of livin mRNA expression. The calculation methods were
as follows: ΔCt=Ct (Livin) − Ct (HBOA), ΔΔCt=ΔCt (Treatment) − ΔCt
(Control). ΔΔCt mean values were compared between groups. The
relative mRNA expression was expressed in 2−ΔΔCt.
Western blot analysis
SPC-A1 cells were harvested after 48 h of
transfection and extracted using lysis buffer. Cell extracts were
separated on 15% sodium dodecyl sulfate polyacrylamide gel
electrophoresis. Separated protein bands were electrotransferred
onto polyvinylidene fluoride (PVDF) membranes (Millipore, Bedford,
MA, USA). Finally, 5% skimmed milk powder was used to block the
PVDF membranes for 1 h. The membrane was incubated overnight at 4°C
with a 1:100 dilution of mouse monoclonal anti-livin primary
antibody (sc-166390; Santa Cruz Biotechnology, Inc.). The following
day, goat anti-mouse Immunoglobulin G horesradish
peroxidase-conjugated secondary antibodies (sc-2005; Santa Cruz
Biotechnology, Inc.) were added at a dilution of 1:2,000 and the
mixture was incubated for 2 h at room temperature. PVDF membranes
were washed in phosphate-buffered saline four times. The ECL-Plus
kit was used to visualize the immunoreactive bands. Relative
protein level was normalized by β-actin concentration. Three
separate experiments were performed in duplicate for each
treatment.
Cell proliferation assay
Cell viability was assessed using an MTT assay.
Cells were cultured in 24 well plates at a concentration of
5x104 cells per well and allowed to adhere. SPC-A1 cells
were termed the CON group. The group transfected with the negative
control vector was termed the NC group. The group transfected with
the livin-siRNA vector was termed the livin-siRNA group. The group
initially transfected with livin-siRNA and then treated with 1.2
μg/ml cisplatin was termed the livin-siRNA+cisplatin group.
The FP group was treated with 100 nmol/l flavopiridol and the T
group was treated with 100 ng/ml TRAIL. The cisplatin treatment
group was treated with 1.2 μg/ml cisplatin. The F+T
treatment group was treated with 100 nmol/l flavopiridol and 100
ng/ml TRAIL. The FP+T+cisplatin treatment group was treated with
100 nmol/l FP, 100 ng/ml TRAIL and 1.2 μg/ml cisplatin. The
Z-VAD-FMK group was treated with flavopiridol, 50 μmol/l
Z-VAD-FMK and TRAIL. Following treatment for 24 h, 100 μl
MTT (0.5 mg/ml) was added to the cells and the mixture was
incubated for 4 h at 37°C. Subsequently, the supernatant was
removed, dimethyl sulfoxide was used to dissolve the resultant
formazan crystals and the absorbance value was read at 570 nm using
a Smartspec 3000 spectrophotometer (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
Statistical analysis
SPSS 13.0 (SPSS, Inc., Chicago, IL, USA) was used
for the data analysis. All experiments were repeated three times
and data are presented as the mean ± standard deviation.
Differences between groups were analyzed using one-way analysis of
variance. P<0.05 was considered to indicate a statistically
significant difference.
Results
Protein expression of livin in the human
SPC-A1 cell line
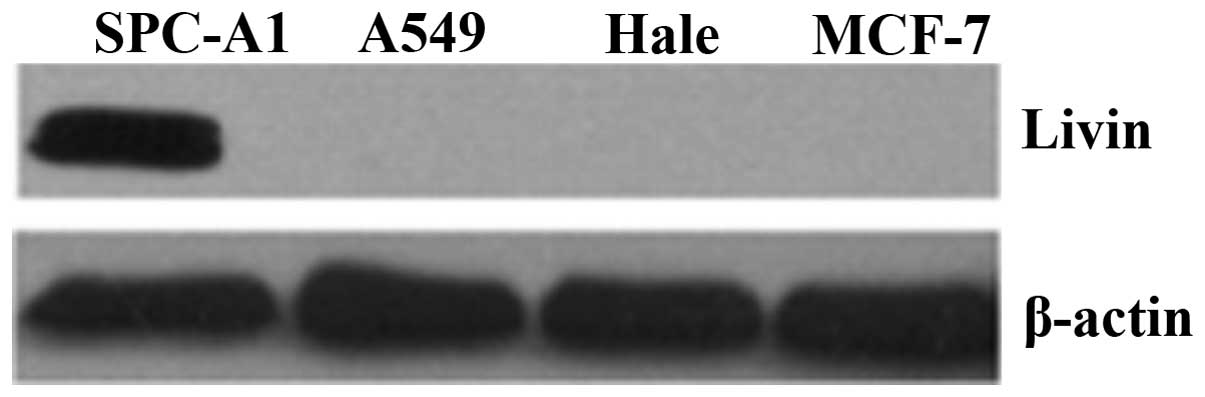
The protein expression of livin was investigated
using western blot analysis in several cell lines (SPC-A1, A549,
Hale and MCF-7). As shown in Fig.
1, livin protein expression was only observed in SPC-A1
cells.
Silencing of livin gene expression by
RNAi in SPC-A1 cells
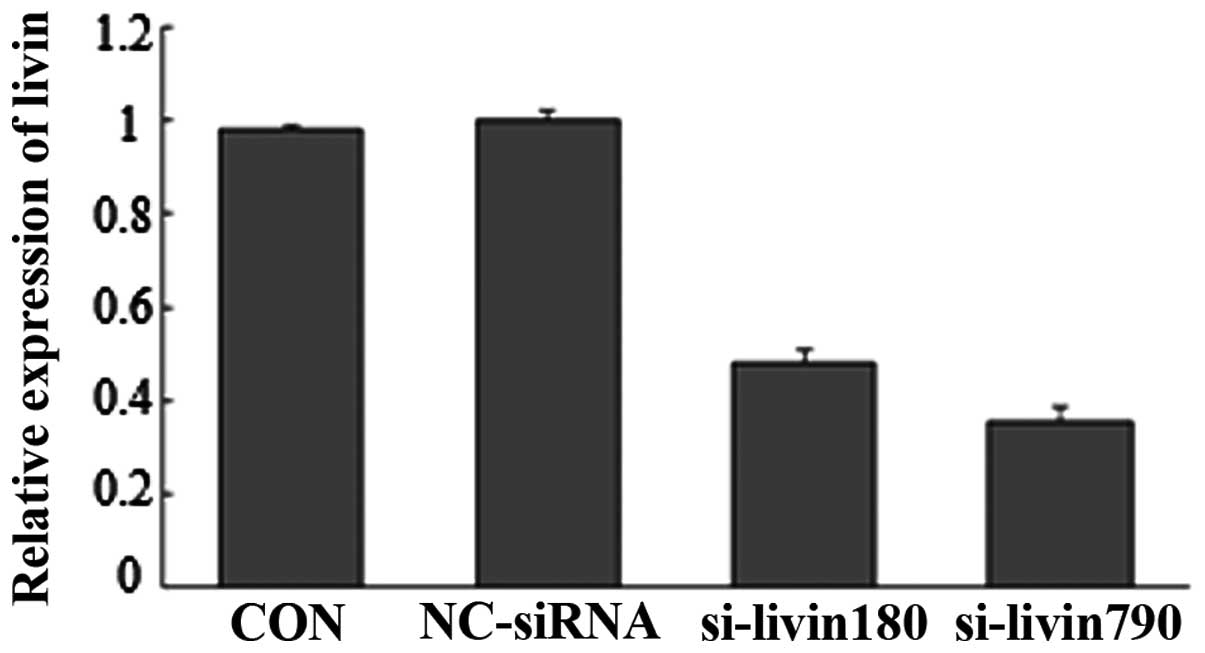
The knockdown efficiency of two candidate siRNA
(siRNA-livin180 and siRNA-livin790) was evaluated using RT-qPCR.
The results revealed that the livin mRNA level in cells transfected
with siRNA-livin790 and siRNA-livin180 were significantly decreased
to 52 and 64.4%, respectively (Fig.
2). The most significant silencing effect was observed with
siRNA-livin790, therefore, the siRNA-livin790 transfection group
was used in the follow-up experiment.
Synergy between flavopiridol and TRAIL
affects livin expression
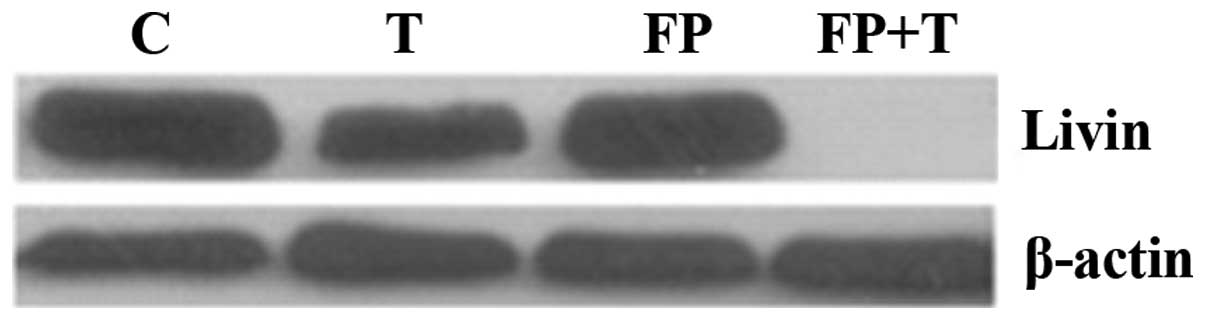
The synergistic inhibitory effect between
flavopiridol and TRAIL on livin protein expression was evaluated by
western blot analysis. Compared with either flavopiridol or TRAIL
alone, combining flavopiridol with TRAIL significantly decreased
the protein expression level of livin (Fig. 3). No significant difference was
identified between flavopiridol and TRAIL treatment groups.
Effects of inhibitor Z-VAD-FMK on livin
protein expression
The result of western blot analysis demonstrated
that the synergistic inhibitory effect between flavopiridol and
TRAIL may effectively inhibit the protein expression of livin,
while the caspase inhibitor Z-VAD-FMK reversed this inhibitory
effect. As shown in Fig. 4,
expression of livin protein in the T+FP+Z-VAD treatment group was
significantly higher than the T+FP group and no significant
difference was identified between the control group and T+FP+Z-VAD
treatment group.
Effect of livin inhibition on cell
proliferation
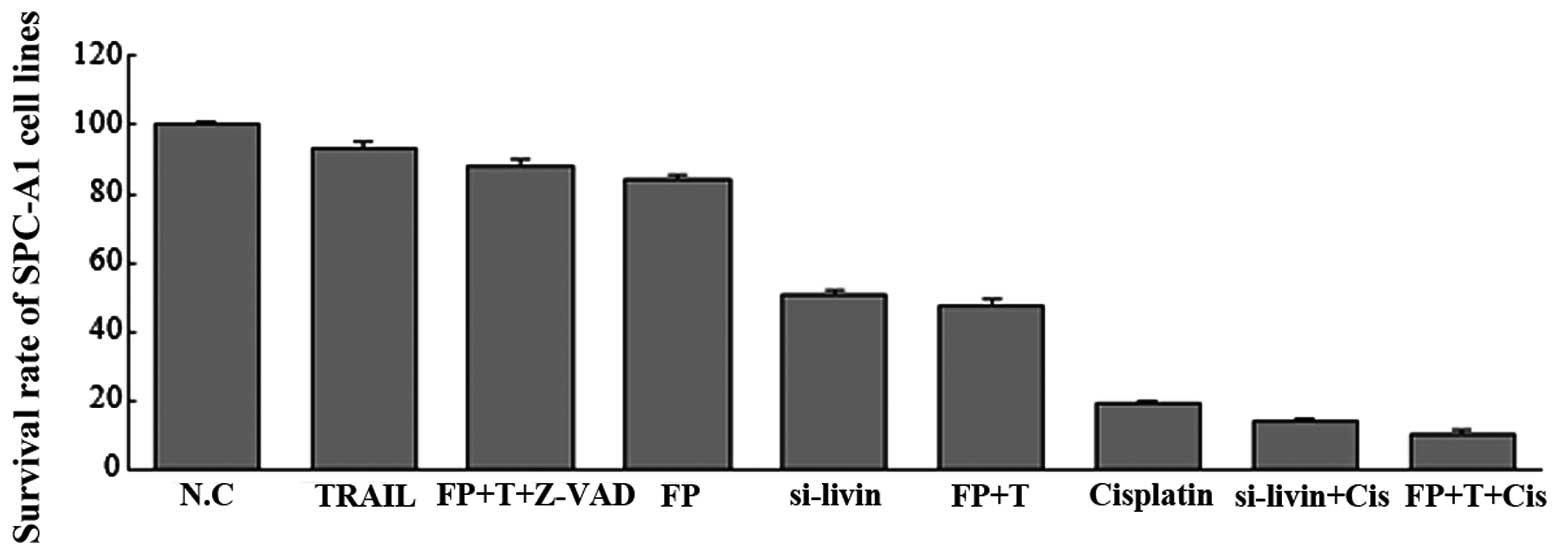
To elucidate the effect of livin inhibition on tumor
growth in SPC-A1 cells, an MTT assay was used to evaluate the tumor
cell proliferative activities. The survival rate of each group was
as follows: Flavopiridol group, 84.30±1.34%; TRAIL group,
93.40±1.56%; F+T combination group, 48.02±1.35%; siRNA-livin group,
50.88±1.14%; cisplatin group, 19.30±0.89%; siRNA-livin+cisplatin
group, 14.37±0.81%; FP+T+cisplatin group, 10.86±0.87% and the
Z-VAD-FMK group, 88.16±1.64%. The data were normalized to the
control group (Fig. 5). As
illustrated in Fig. 5, suppression
of livin resulted in a significant decrease in the proliferation
rate of SPC-A1 cells at 48 h and this suppression effect
unexpectedly enhanced the sensitivity of cells to the chemotherapy
drug cisplatin.
Discussion
The anti-apoptotic effect of the IAP family is
possibly regulated through inhibition of the caspase signaling
cascade, inhibiting the cell death receptor tumor necrosis factor
receptor-mediated apoptosis signaling pathway and interaction with
nuclear factor-κB. The IAP family has several family members,
including x-linked inhibitor of apoptosis protein, baculoviral IAP
repeat-containing protein 1 and 3, cellular IAP1, Apollon (Bruce),
IAP-like protein-1, Survivin and livin. The structure of IAP family
members is highly conserved, containing Cys/His baculovirus IAP
repeats and a COOH-terminal ring finger, which has E3 ubiquitin
ligase activity and is critical in the regulation of proliferation
and apoptosis (41).
Livin, a novel member of the IAP family, is
important in apoptosis, cell proliferation and cell cycle control
(20). It has been demonstrated to
be expressed in transformed cells and multiple types of malignant
tumor, including neuroblastoma (20) as well as carcinomas of the bladder
(21,22), lung (23), nasopharynx (24), kidney (25), liver (26), colon/rectum (27), skin (28), bone (29) and stomach (30). Silencing livin leads to apoptosis
induction, cell cycle arrest and proliferation inhibition in
malignant tumor cells (42–50).
The present study investigated the clinical
significance of livin in SPC-A1 cells and examined the potential of
using RNA interference to knock down livin expression, including
the subsequent effects on tumor growth in SPC-A1 cells in
vitro. The present results demonstrated that, following
transfection of the livin gene-silencing vector, the expression of
the livin gene was significantly decreased, SPC-A1 cell
proliferation was significantly reduced and the therapeutic effect
of the chemotherapy drug cisplatin was markedly improved. The
livin-mediated signaling pathway remains to be elucidated, although
a number of studies have demonstrated that silencing livin promotes
tumor cell apoptosis by regulating mitomycin, tumor necrosis
factor-α, caspase-3 and caspase-9 (45) and mediates gastric tumor cell
invasion via MAPK signaling (51).
The livin-mediated signaling pathway requires further
investigation.
TRAIL, as a member of the tumor necrosis factor gene
superfamily, selectively induces apoptosis in numerous transformed
cells, excluding normal cells. The synergistic inhibitory effect
between TRAIL and chemotherapy drugs has been examined in ovarian
carcinoma (52), the combination
resulted in increased sensitivity to TRAIL, promoted induction of
apoptosis, reduced drug dosage and decreased normal tissue
toxicity. Through mediating cell cycle arrest and apoptosis in
breast cancer cells, flavopiridol has made important contributions
to increasing drug effectiveness and identifying new drug targets
(53).
The synergistic inhibitory effect between
flavopiridol and TRAIL has been demonstrated in SPC-A1 cells
(54). The present study aims to
further confirm this synergistic inhibitory effect on apoptosis
promotion and examine whether this combination may enhance
chemosensitivity. The current results revealed that the expression
of livin protein was significantly reduced in the flavopiridol and
TRAIL combination treatment group. The survival rate of SPC-A1
cells in the combination treatment group was significantly compared
the groups treated with flavopiridol or TRAIL alone. Additionally,
the survival rate was lowest in the group treated with a
combination of flavopiridol, TRAIL and cisplatin.
In conclusion, the RNA silencing and the synergistic
inhibitory effect between flavopiridol with TRAIL was able to
effectively inhibit the expression of livin, significantly decrease
SPC-A1 tumor cell proliferation and significantly enhance
sensitivity to the chemotherapy drug cisplatin. These findings
suggest that livin may be used as a novel target for tumor gene
therapy.
Acknowledgments
This study was supported by a grant from the Natural
Science Foundation of Yunnan Province (grant no. 2010ZC131).
References
|
1
|
Novaes FT, Cataneo DC, Ruiz RL Jr, et al:
Lung cancer: histology, staging, treatment and survival. J Bras
Pneumol. 34:595–600. 2008.In English and Portuguese. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thomas L, Doyle LA and Edelman MJ: Lung
cancer in women: emerging differences in epidemiology, biology, and
therapy. Chest. 128:370–381. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Novello S and Vavalà T: Lung cancer and
women. Future Oncol. 4:705–716. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Devesa SS, Bray F, Vizcaino AP, et al:
International lung cancer trends by histologic type: male:female
differences diminishing and adenocarcinoma rates rising. Int J
Cancer. 117:294–299. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Egleston BL, Meireles SI, Flieder DB, et
al: Population-based trends in lung cancer incidence in women.
Semin Oncol. 36:506–515. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ondrusova M, Muzik J, Hunakova L, et al:
Trends in the lung cancer incidence and mortality in the Slovak and
Czech Republics in the contexts of an international comparison.
Clin Transl Oncol. 14:659–666. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Eilstein D and Eshai K: Lung and breast
cancer mortality among women in France: future trends. Cancer
Epidemiol. 36:e341–e348. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Thompson CA, Waldhör T, Schernhammer ES,
et al: Smoking and lung cancer: current trends in Austria. Wien
Klin Wochenschr. 124:493–499. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Youlden DR, Cramb SM and Baade PD: The
international epidemiology of lung cancer: geographical
distribution and secular trends. J Thorac Oncol. 3:819–831. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Malvezzi M, Bertuccio P, Levi F, et al:
European cancer mortality predictions for the year 2012. Ann Oncol.
23:1044–1052. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stahel RA: Adenocarcinoma, a molecular
perspective. Ann Oncol. 18(Suppl 9): 147–149. 2007. View Article : Google Scholar
|
|
12
|
Kadara H, Kabbout M and Wistuba II:
Pulmonary adenocarcinoma: a renewed entity in 2011. Respirology.
17:50–65. 2012. View Article : Google Scholar
|
|
13
|
Li J, Chen P, Li XQ, et al: Elevated
levels of survivin and livin mRNA in bronchial aspirates as markers
to support the diagnosis of lung cancer. Int J Cancer.
132:1098–1104. 2013. View Article : Google Scholar
|
|
14
|
Hartman ML and Czyz M: Anti-apoptotic
proteins on guard of melanoma cell survival. Cancer Lett.
331:24–34. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ye L, Song X, Li S, et al: Livin-α
promotes cell proliferation by regulating G1-S cell cycle
transition in prostate cancer. Prostate. 71:42–51. 2012. View Article : Google Scholar
|
|
16
|
Guo H, Gao YT, Zhang Q, et al: Expression
and clinical significance of livin protein in hepatocellular
carcinoma. Dis Markers. 35:489–496. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bavykin AS, Korotaeva AA, Poyarkov SV, et
al: Double siRNA-targeting of cIAP2 and LIVIN results in synergetic
sensitization of HCT-116 cells to oxaliplatin treatment. Onco
Targets Ther. 6:1333–1340. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu M, Xia LP, Fan LJ, et al: Livin and
caspase-3 expression are negatively correlated in cervical squamous
cell cancer. Eur J Gynaecol Onco. l34:152–155. 2013.
|
|
19
|
Li F, Yin X, Luo X, et al: Livin promotes
progression of breast cancer through induction of
epithelial-mesenchymal transition and activation of AKT signaling.
Cell Signal. 25:1413–1422. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dasgupta A, Alvarado CS, Xu Z, et al:
Expression and functional role of inhibitor-of-apoptosis protein
livin (BIRC7) in neuroblastoma. Biochem Biophys Res Commun.
400:53–59. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gazzaniga P, Gradilone A, Giuliani L, et
al: Expression and prognostic significance of LIVIN, SURVIVIN and
other apoptosis-related genes in the progression of superficial
bladder cancer. Ann Oncol. 14:85–90. 2003. View Article : Google Scholar
|
|
22
|
Xi RC, Sheng YR, Chen WH, et al:
Expression of survivin and livin predicts early recurrence in
non-muscle invasive bladder cancer. J Surg Oncol. 107:550–554.
2013. View Article : Google Scholar
|
|
23
|
Tanabe H, Yagihashi A, Tsuji N, et al:
Expression of survivin mRNA and livin mRNA in non-small-cell lung
cancer. Lung Cancer. 46:299–304. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xiang Y, Yao H, Wang S, et al: Prognostic
value of Survivin and Livin in nasopharyngeal carcinoma.
Laryngoscope. 116:126–130. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kempkensteffen C, Hinz S, Christoph F, et
al: Expression of the apoptosis inhibitor livin in renal cell
carcinomas: correlations with pathology and outcome. Tumor Biol.
28:132–138. 2007. View Article : Google Scholar
|
|
26
|
Augello C, Caruso L, Maggioni M, et al:
Inhibitors of apoptosis proteins (IAPs) expression and their
prognostic significance in hepatocellular carcinoma. BMC Cancer.
9:1252009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xi RC, Biao WS and Gang ZZ: Significant
elevation of survivin and livin expression in human colorectal
cancer: inverse correlation between expression and overall
survival. Onkologie. 34:428–432. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lazar I, Perlman R, Lotem M, et al: The
clinical effect of the inhibitor of apoptosis protein livin in
melanoma. Oncology. 82:197–204. 2012. View Article : Google Scholar
|
|
29
|
Li X, Fan S, Li L, et al: RNA
interference-mediated knockdown of Livin suppresses cell
proliferation and invasion and enhances the chemosensitivity to
cisplatin in human osteosarcoma cells. Int J Oncol. 43:159–168.
2013.PubMed/NCBI
|
|
30
|
Liang YZ, Fang TY, Xu HG, et al:
Expression of CD44v6 and Livin in gastric cancer tissue. Chin Med J
(Engl). 125:3161–3165. 2012.
|
|
31
|
Choi J, Hwang YK, Sung KW, et al:
Expression of Livin, an antiapoptotic protein, is an independent
favorable prognostic factor in childhood acute lymphoblastic
leukemia. Blood. 109:471–477. 2007. View Article : Google Scholar
|
|
32
|
Nedelcu T, Kubista B, Koller A, et al:
Livin and Bcl-2 expression in high-grade osteosarcoma. J Cancer Res
Clin Oncol. 134:237–244. 2008. View Article : Google Scholar
|
|
33
|
Yang YL, Lin SR, Chen JS, et al:
Expression and prognostic significance of the apoptotic genes
BCL2L13, Livin, and CASP8AP2 in childhood acute lymphoblastic
leukemia. Leuk Res. 34:18–23. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu HB, Kong CZ, Zeng Y, et al: Livin may
serve as a marker for prognosis of bladder cancer relapse and a
target of bladder cancer treatment. Urol Oncol. 27:277–283. 2009.
View Article : Google Scholar
|
|
35
|
Wang X, Xu J, Ju S, et al: Livin gene
plays a role in drug resistance of colon cancer cells. Clin
Biochem. 43:655–660. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sun JG, Liao RX, Zhang SX, et al: Role of
inhibitor of apoptosis protein Livin in radiation resistance in
nonsmall cell lung cancer. Cancer Biother Radiopharm. 26:585–592.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Crnković-Mertens I, Muley T, Meister M, et
al: The anti-apoptotic livin gene is an important determinant for
the apoptotic resistance of non-small cell lung cancer cells. Lung
Cancer. 54:135–142. 2006. View Article : Google Scholar
|
|
38
|
Chang H and Schimmer AD: Livin/melanoma
inhibitor of apoptosis protein as a potential therapeutic target
for the treatment of malignancy. Mol Cancer Ther. 6:24–30. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Crnković-Mertens I, Wagener N, Semzow J,
et al: Targeted inhibition of Livin resensitizes renal cancer cells
towards apoptosis. Cell Mol Life Sci. 64:1137–1144. 2007.
View Article : Google Scholar
|
|
40
|
Liu B, Han M, Wen JK, et al: Livin/ML-IAP
as a new target for cancer treatment. Cancer Lett. 250:168–176.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Abd-Elrahman I, Hershko K, Neuman T, et
al: The inhibitor of apoptosis protein Livin (ML-IAP) plays a dual
role in tumorigenicity. Cancer Res. 69:5475–5480. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yu L and Wang Z: Effects of Livin gene RNA
interference on apoptosis of cervical cancer HeLa cells and
enhanced sensitivity to cisplatin. J Huazhong Univ Sci Technolog
Med Sci. 29:625–630. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang TS, Ding QQ, Guo RH, et al:
Expression of livin in gastric cancer and induction of apoptosis in
SGC-7901 cells by shRNA-mediated silencing of livin gene. Biomed
Pharmacother. 64:333–338. 2010. View Article : Google Scholar
|
|
44
|
Yang D, Song X, Zhang J, et al:
Therapeutic potential of siRNA-mediated combined knockdown of the
IAP genes (Livin, XIAP, and Survivin) on human bladder cancer T24
cells. Acta Biochim Biophys Sin (Shanghai). 42:137–144. 2010.
View Article : Google Scholar
|
|
45
|
Yang D, Song X, Zhang J, et al:
Suppression of livin gene expression by siRNA leads to growth
inhibition and apoptosis induction in human bladder cancer T24
cells. Biosci Biotechnol Biochem. 74:1039–1044. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liu H, Wang S, Sun H, et al: Inhibition of
tumorigenesis and invasion of hepatocellular carcinoma by
siRNA-mediated silencing of the livin gene. Mol Med Rep. 3:903–907.
2010.
|
|
47
|
Yuan B, Ran B, Wang S, et al: siRNA
directed against Livin inhibits tumor growth and induces apoptosis
in human glioma cells. J Neurooncol. 107:81–87. 2012. View Article : Google Scholar
|
|
48
|
Chen F, Yang D, Wang S, et al: Livin
regulates prostate cancer cell invasion by impacting the NF-κB
signaling pathway and the expression of FN and CXCR4. IUBMB Life.
64:274–283. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Liu X, Wang A, Gao H, et al: Expression
and role of the inhibitor of apoptosis protein livin in
chemotherapy sensitivity of ovarian carcinoma. Int J Oncol.
41:1021–1028. 2012.PubMed/NCBI
|
|
50
|
Wang XT, Xie YB and Xiao Q: siRNA
targeting of Cdx2 inhibits growth of human gastric cancer MGC-803
cells. World J Gastroenterol. 18:1903–1914. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ou JM, Ye B, Qiu MK, et al: Knockdown of
Livin inhibits growth and invasion of gastric cancer cells through
blockade of the MAPK pathway in vitro and in vivo. Int J Oncol.
44:276–284. 2014.
|
|
52
|
Trinh DT, Shibata K, Hirosawa T, et al:
Diagnostic utility of CD117, CD133, SALL4, OCT4, TCL1 and
glypican-3 in malignant germ cell tumors of the ovary. J Obstet
Gynaecol Res. 38:841–848. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Jin T, Branch DR, Zhang X, et al:
Examination of POU homeobox gene expression in human breast cancer
cells. Int J Cancer. 81:104–112. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
He YQ, Zhuang li, Liu SY, et al: Mechanism
of the apoptosis induction by Flavopiridol synergizes TRAIL in
SPC-A1 cell. Chinese Journal of Cancer Prevention and Treatment.
20:730–733. 2013.In Chinese.
|